Cal Tech & UCLA — Nanosystems Biology Cancer Center (NSBCC)
Center Overview
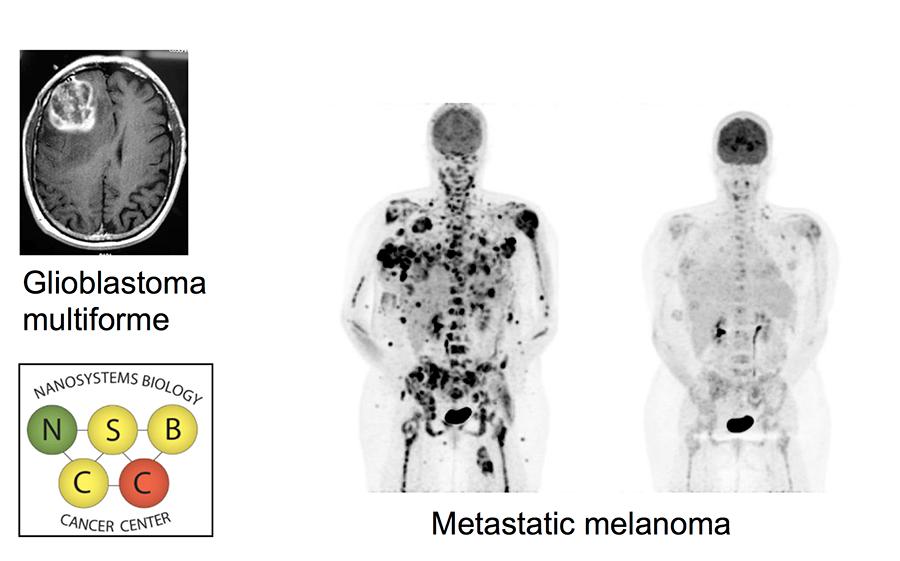
The Nanosystems Biology Cancer Center (NSBCC) is a partnership between the California Institute of Technology, the Jonsson Comprehensive Cancer Center (JCCC) at the University of California Los Angeles (UCLA), and the David Geffen School of Medicine. The NSBCC is led by the renowned scientists, James Heath of CalTech and Mike Phelps of UCLA. Both are supported by a team of co-investigators whom cross the projects from these institutions. The NSBCC was supported by the prior two rounds of CCNE funding, but it enters into this new funding period with a new set of faculty and a mission centered around trying to understand how best to administer combination therapies for cancer patients. The Center's cancer focus is on advanced brain cancers, as a model for a heterogeneous cancer that is effectively untreatable, and melanoma, as a model for understanding and expanding the power of immunotherapy. The NSBCC has four projects and two cores listed below.
Projects
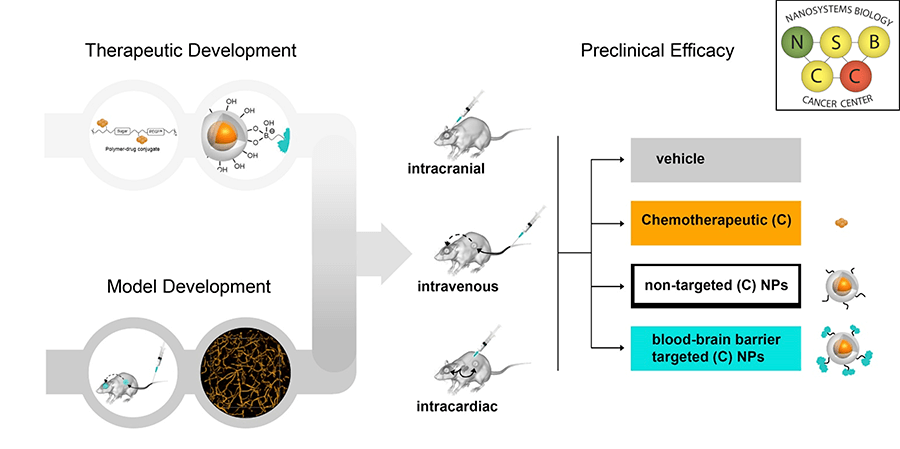
Project 1: Targeted Nanoparticle Therapeutics for Treating Intracranial Disease
Project Investigators: Mark Davis, Ph.D., Timothy Cloughesy, M.D., and David Nathanson, Ph.D.
Project 1 is led by Mark Davis, a pioneer in the development of nanoparticle therapeutics for cancer, with co-investigators Timothy Cloughesy, M.D., a clinical researcher, and David Nathanson, who has built a library of patient derived mouse models of brain cancer for testing response to therapies. The project is focused on developing nanotherapeutics that can cross the blood-brain barrier by combining state-of-the-art nanotherapeutics, deep clinical experience, and patient derived murine models.
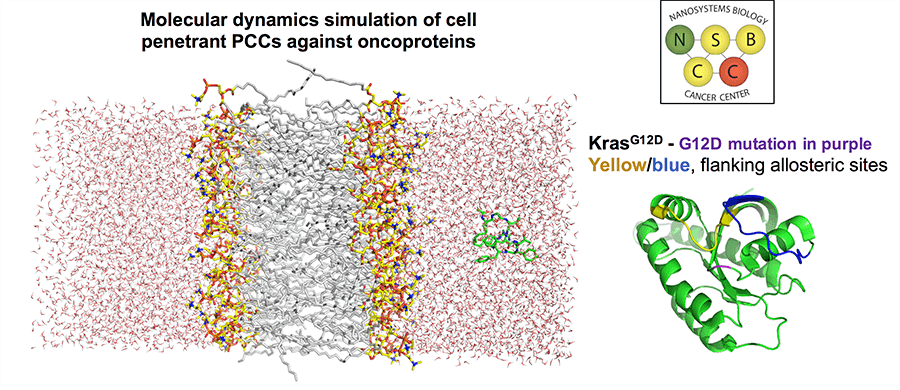
Project 2: Specifically Targeting Oncoproteins with PCC Agent-Loaded Nanoparticles
Project Investigators: James Heath, Ph.D., Mark Davis, Ph.D., Raymond Deshaies, Ph.D., and Johannes Czernin, M.D.
In Project 2, NSBCC PI James Heath will collaborate with Mark Davis, Raymond Deshaies, Ph.D. and Johannes Czernin, M.D. to design molecular and nanotechnologies for targeting specific oncoproteins, with a particular focus on KRASG12D. In previous funding cycles, NSBCC resources were leveraged to help develop protein catalyzed capture agents (PCCs). PCCs can be targeted to bind to specific mutated sites on at least certain oncoproteins, with high selectivity relative to wildtype. This provides the opportunity for drug-targeting only diseased tissues. Here, PCCs will be combined with nanotherapeutics and novel proteolysis targeting chimeric antigen (PROTAC) methods developed by Raymond Deshaies to develop a new class of targeted cancer therapies.
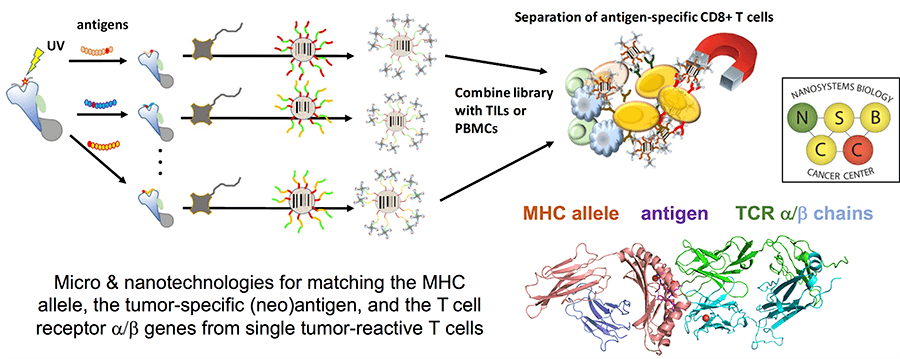
Project 3: Tools for Capturing Immune Cell / Cancer Cell Interactions in Cancer Immunotherapies and Combination Immunotherapies
Project Investigators: Antoni Ribas, Ph.D., and Mikhail Shapiro, Ph.D.
Project 3 is led by Antoni Ribas, a pioneer in cancer immunotherapy, and Mikhail Shapiro, an expert in nano-enabled imaging techniques. This project represents a continuation of a cancer immunotherapy theme that has existed within the NSBCC for that past 10 years, but with goals that are informed by the recent dramatic advances in the field. Aim 1 is focused on applying a new class of nanotechologies towards identifying the matched antigen specificity and T cell receptor a/b gene sequence for each T cell that infiltrates into a tumor. Aim 2 is focused on applying new molecular nanotechnologies towards understanding the emergence of resistance that is seen in some patients treated with anti-PD1 checkpoint inhibitor therapy. Aim 3 is a new nanobubble ultrasound imaging technology for monitoring T cell trafficking in vivo. Project collaborators will include James Heath and David Baltimore, Ph.D.
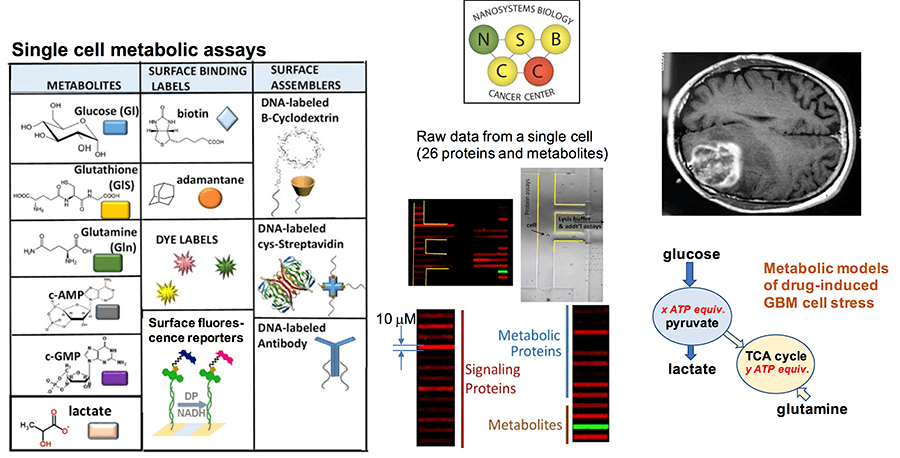
Project 4: A Discovery-Level Functional Proteomics / Metabolomics Platform for Resolving the Heterogeneity of GBM Tumors and Identifying Effective Therapy Combinations
Project Investigators: David Nathanson, Ph.D., James Heath, Ph.D., Wei Wei, Ph.D., Michael Phelps, Ph.D., and Timothy Cloughesy, M.D.
Led by David Nathanson, Project 4 is centered around nanotechnology for resolving the heterogeneity of GBM tumors and identifying effective combination therapies for treating patients. Nathanson will collaborate with Wei Wei, Ph.D., James Heath, Timothy Cloughesy and Michael Phelps to apply nanotechnologies to study and understand GBM patient responses, and to use the results of measurements to guide effective combination therapies. At the heart of the technology platform are very highly multiplexed, integrated metabolic and functional proteomic single cell assay platforms, expanding up to near 100 analytes assayed, and with applications to both studies of intracranial patient-derived murine models, as well as the analysis of resected patient tissue.
Cores
Nano / micro Fabrication and Materials (NM2) Core
Core Investigators: James Heath, Ph.D., and Young Shik Shin, Ph.D.
The NM2 core is a common set of facilities for fabricating the technological platforms of the NSBCC, and providing access to commonly studied cell lines and data bases.
Crump Preclinical Imaging Technology Center
Core Investigator: Michael Phelps, Ph.D.
The imaging core provides services to all four projects. The NSBCC also takes advantage of a number of core facilities within the JCCC and CIT.