Case Western Reserve University —Treatment of Glioblastoma Using Chain-Like Nanoparticles
Principal Investigators: Efstathios Karathanasis, Ph.D.
Co-Investigators: James Basilion, Ph.D., Mark Griswold, Ph.D., Ketankumar Ghaghada, Ph.D. (Texas Childrens Hospital), and Jeremy Rich, M.D. (Cleveland Clinic).
Project Summary
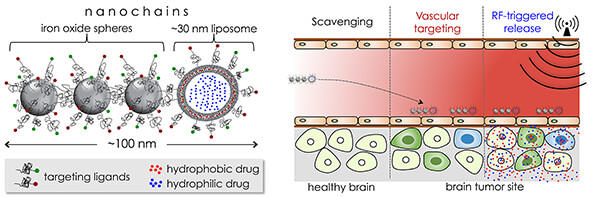
The invasive forms of brain tumors, such as glioblastoma multiforme (GBM) are characteristically diffused with infiltrating edges, resistant to drugs and nearly inaccessible to systemic therapies due to the brain-tumor barrier. To address the challenges of drug delivery and resistance, our objective is to integrate the unique features of a chain-like nanoparticle with the appropriate combination of complementary drugs to enable effective treatment of invasive brain tumors. These nanochains are comprised of iron oxide nanospheres and a drug-loaded liposome chemically linked into a chain-like assembly with high precision. The nanochain possesses a unique ability to scavenge the tumor endothelium. By utilizing effective vascular targeting of the ανβ3 integrin receptor, the nanochains achieve rapid deposition on the vascular bed of glioma sites establishing well-distributed drug reservoirs on the endothelium of brain tumors. After reaching the target sites, an on-command, external low-power radiofrequency field can remotely trigger rapid drug release, due to mechanical disruption of the liposome, facilitating widespread and effective drug delivery across the brain-tumor barrier into regions harboring brain tumor cells.
To address the drug resistance issue, we have identified glioma stem cell (GSC)-specific regulators amenable to pharmacologic targeting. We recently showed that the inducible nitric oxide synthase (iNOS) is a unique signal regulator in GSCs. Due to the flexibility of loading various types of drugs within the nanochain, the nanochain will be loaded with standard chemotherapy and an iNOS inhibitor that eliminates the small fraction of GBM cells that are resistant, and can migrate to cause tumor recurrence. Due to enhanced site-specific targeting and rapid drug release, we hypothesize that guaranteeing the simultaneous delivery of drugs with synergistic activity into hard-to-reach glioma sites will facilitate effective treatment and ultimately eradication of the disease using a safe dose of the drugs.
Project Expertise
This project brings together a team of collaborators from Case Comprehensive Cancer Center, Cleveland Clinic, and Texas Childrens Hospital, with a long prior history of collaboration. The investigators exhibit complementary expertise including cancer nanomedicine (Karathanasis), stem cell biology and neuro-oncology (Rich), cancer pharmacology (Basilion), MR physics (Griswold) and translational nanotechnology (Ghaghada).