NCI intramural research highlighted at 2014 AACR meeting
- Posted:
240-760-6600
At this year’s annual American Association for Cancer Research (AACR) meeting in San Diego there were plenary talks by two NCI intramural scientists -- Steven Rosenberg, M.D., and Louis Staudt, M.D., Ph.D. Intramural research, which takes place mostly on the NCI main campus in Bethesda, Md, comprises about 15 percent of all NCI-funded research efforts.
The two intramural scientists highlighted the challenges in developing varied and potentially synergistic treatments for aggressive forms of cancer. Each researcher has been focusing primarily on a particularly difficult to treat cancer (melanoma and lymphoma, respectively) for several decades, and their presentations highlight the diligence and tenacity needed to come to a better understanding of the cancer process and to find effective agents to target the diseases they study. They are certainly not undertaking these efforts in a vacuum as the other 85 percent of NCI’s research funding, to extramural scientists, is also supporting similar research efforts.
Dr. Rosenberg, chief of NCI’s Surgery Branch, focused on the curative potential of adoptive T-cell therapy (ACT) in his presentation. This experimental therapy attempts to enhance the cancer-fighting ability of a patient’s immune system using the patient’s own natural or genetically engineered T-lymphocytes, which are cells that play a central role in fighting infection. In one form of this therapy, researchers first harvest T-cells that have invaded a patient’s tumor, called tumor-infiltrating lymphocytes (or TILs). The TILs with the greatest antitumor activity are identified and large populations of those cells are grown in a laboratory. The patients are treated to destroy their own immune cells, and the laboratory-grown TILs are then infused back into the patient, where they attack and destroy cancer cells.
Adoptive T-cell therapy using TILs has been particularly effective in treating metastatic melanoma patients. This treatment approach was first developed in 1988 by Dr. Rosenberg. Its efficiency was enhanced in 2002 based on results from an NCI intramural clinical trial that showed that it was necessary to deplete the patient’s immune system before TIL transfer.
In his plenary talk, Dr. Rosenberg reported newly available long-term follow-up data (a median of 9 years) for 93 patients with metastatic melanoma treated with ACT. In more than half of the patients (52 patients) the cancer regressed, and in 20 of these patients the cancer regressed completely (with all but one remaining in complete remission as of the AACR 2014 meeting).
Dr. Rosenberg also reported results of his recent confirmatory trial involving 101 patients with metastatic melanoma, in which 55 patients treated with ACT-based therapy exhibited cancer regression. This 54 percent response rate exceeds that seen in patients with metastatic melanoma treated with two other types of immunotherapy (ipilimumab and anti-PD1 therapy, which showed response rates of about 10 percent and 35 percent, respectively). Moreover, ACT was effective in patients who were not responsive to these other treatments.
Although the primary focus of much of the research in Dr. Rosenberg’s lab and clinic has focused on melanoma, he is also working on extending TIL and other cell-based therapies to other malignancies. These include B-cell lymphoma, the most common type of non-Hodgkin lymphoma; leukemia; and other lymphomas.
Dr. Rosenberg’s group was the first to report the use of genetically modified T-cells to successfully treat patients with advanced lymphoma. In his plenary talk, he described updated results for 18 adult patients who had indolent and aggressive B-cell lymphomas who had not responded to other therapies. Sixteen patients saw their cancer regress and eight patients experienced a complete regression. The finding that patients who were unable to respond to other treatments responded well to ACT reinforces an overall theme of the need for alternate approaches and synergistic therapies.
B-cell associated cancers and the challenges in treating them were the focus of the presentation by Dr. Louis Staudt, head of NCI’s Molecular Biology of Lymphoid Malignancies Section. His talk was on rationally targeted therapy for diffuse large B-cell lymphomas (DLBCLs), which constitute 30 to 40 percent of newly diagnosed lymphomas. In 2000, Dr. Staudt and colleagues, using DNA microarray technology that was relatively advanced for that time, found that this type of lymphoma was actually two distinct diseases. In the past 14 years, his group has developed a better molecular understanding of the disease, and they now know that there are at least three molecular subtypes of DLBCL, each derived from B cells at unique stages in their development. The activated B-cell (ABC) subtype of DLBCL, which accounts for approximately 40 percent of DLBCL cases and has the poorest clinical outcome with current therapy, is a primary focus of current research efforts.
Researchers in Dr. Staudt’s group have recently been looking for ways to target B-cell receptor signaling as a treatment strategy because this signaling appears to be a key part of the disease process. This research identified the enzyme Bruton’s tyrosine kinase (BTK) as a critical component in the B-cell receptor signaling pathway that is required to maintain the survival of ABC lymphoma cells. Based on this molecular research, the investigators chose the drug ibrutinib, a potent inhibitor of BTK, to use in their clinical trials.
In studies led by Staudt and his NCI colleague, Wyndham Wilson, M.D., ibrutinib was first evaluated in patients with ABC DLBCL who enrolled in a pilot trial at NCI. The results showed that use of the single-agent pill form of ibrutinib elicited major anti-lymphoma effects with minimal side effects. A multicenter phase 2 trial of ibrutinib, first reported at AACR in 2012, has shown a high rate of response in ABC but not in another form of DLBCL, as hypothesized, highlighting the need to find other, potentially synergistic, approaches to treating the cancer.
In his plenary talk, Dr. Staudt delved into the molecular mechanisms in ABC DLBCL that may account for the high rate of response to ibrutinib. The tumor cells of some patients have a mutation in the CD79B gene, which codes for a component of the B cell receptor. When this gene is mutated, ABC DLBCL tumors are more responsive to ibrutinib. However, ibrutinib was effective against many tumors that did not have a mutation affecting the B cell receptor, leading Dr. Staudt to propose that this receptor may be triggered by other, perhaps non-genetic, mechanisms in these patients.
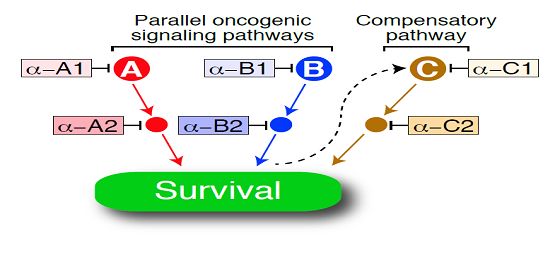
While some patients who received ibrutinib remain in complete remission for more than one year, Dr. Staudt noted that most patients who responded eventually relapse, necessitating combination therapies to improve ibrutinib effectiveness. He emphasized that ibrutinib is likely to be a good cornerstone for combination therapy since it has few, if any, side effects in most individuals treated. The key concept behind combination therapy, illustrated below, is that cancer cells typically have more than one mechanism to abnormally sustain their survival, and successful therapy must block most or all of these survival pathways. In addition, the cancer cell may respond to one drug therapy by devising an “end run” in which a compensatory survival pathway is activated. Combination therapies will have to anticipate these compensatory mechanisms to be successful.
Consequently, Dr. Staudt’s research team has looked for other drugs or substances that might act synergistically with ibrutinib to kill ABC DLBCL cells. They have found that substances known as IRAK4 inhibitors, BET inhibitors, and the drug lenalidomide might all be effective co-agents. Ultimately, this paradigm illustrates the difficulty of developing clinical trials for combination therapies where there are tens or even hundreds of possible combinations to consider. Extensive preclinical research will be needed to identify the most promising combination or combinations of therapies to move forward to clinical trials.
Both Drs. Rosenberg’s and Staudt’s research teams continue to refine their understanding of the diseases they’ve been studying over the past several decades. In addition to these two high-profile plenary talks, NCI researchers and staff provided a variety of insights and perspectives at various discussions during the 5-day AACR meeting.