Cancer clinical trials lead to the development of new and improved treatments for patients and with rapidly changing science, cancer trials are more important than ever. NCI's Coordinating Center for Clinical Trials (CCCT) plays a central role in coordinating NCI-supported cancer trials that are offered across the U.S. with the goal of accelerating the delivery of effective treatments for patients.
NCI established CCCT in 2006 in response to the 2005 National Cancer Advisory Board (NCAB) Clinical Trials Working Group (CTWG) report. The CTWG recommended the creation of an organization within NCI to coordinate the integration of the entire clinical trials enterprise supported by NCI.
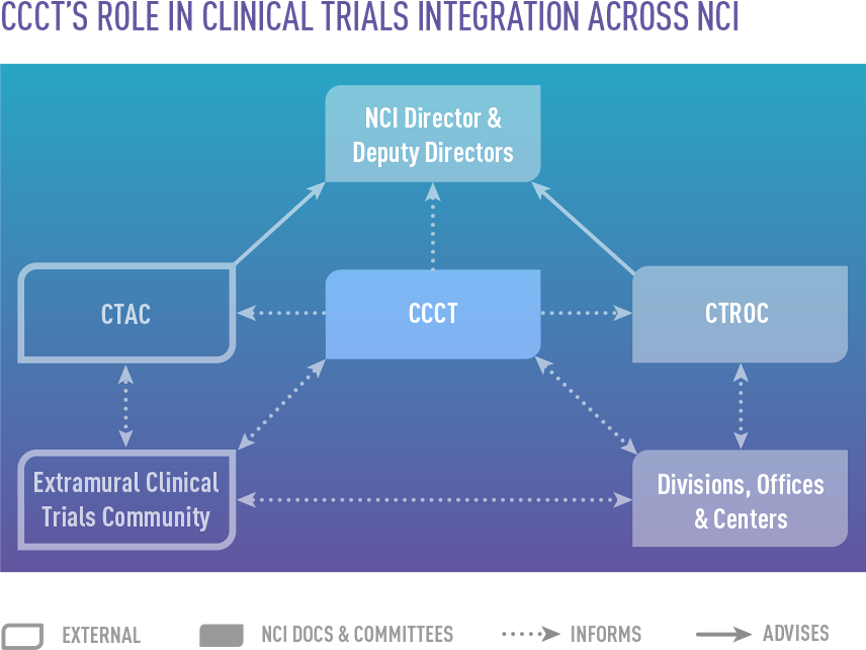
Mission and Activities
CCCT facilitates the integration of NCI's clinical trials programs and associated translational research by encouraging an ongoing exchange of information. NCI clinical trials programs include:
- NCI National Clinical Trials Network (NCTN) and Early Therapeutics Clinical Trials Network (ETCTN) treatment trials for which the Division of Cancer Treatment and Diagnosis (DCTD) is responsible
- NCI Community Oncology Research Program (NCORP) trials for supportive care and cancer care delivery research for which the Division of Cancer Prevention (DCP) and the Division of Cancer Control and Population Science (DCCPS) are responsible
- Trials taking place through NCI's Center for Cancer Research (CCR)
- Trials taking place with NCI support, either in NCI-designated cancer centers or through NCI-funded grants
These are cooperative endeavors that draw upon the strongest components of NCI’s clinical research system and scientific infrastructure.
Major CCCT-managed activities that promote integration of NCI’s clinical trials and translational research programs include:
- Scientific Steering Committees, which prioritize NCI's most important clinical trials
- Biomarker, Imaging, and Quality of Life Studies Funding Program (BIQSFP)
- Clinical Trials Reporting Program (CTRP), a comprehensive database with up-to-date information on all NCI-supported clinical trials
- Clinical and Translational Research Operations Committee (CTROC), an internal oversight committee
- Clinical Trials and Translational Research Advisory Committee (CTAC), which advises NCI leadership on clinical trials and associated translational science