The National Cancer Act of 1937
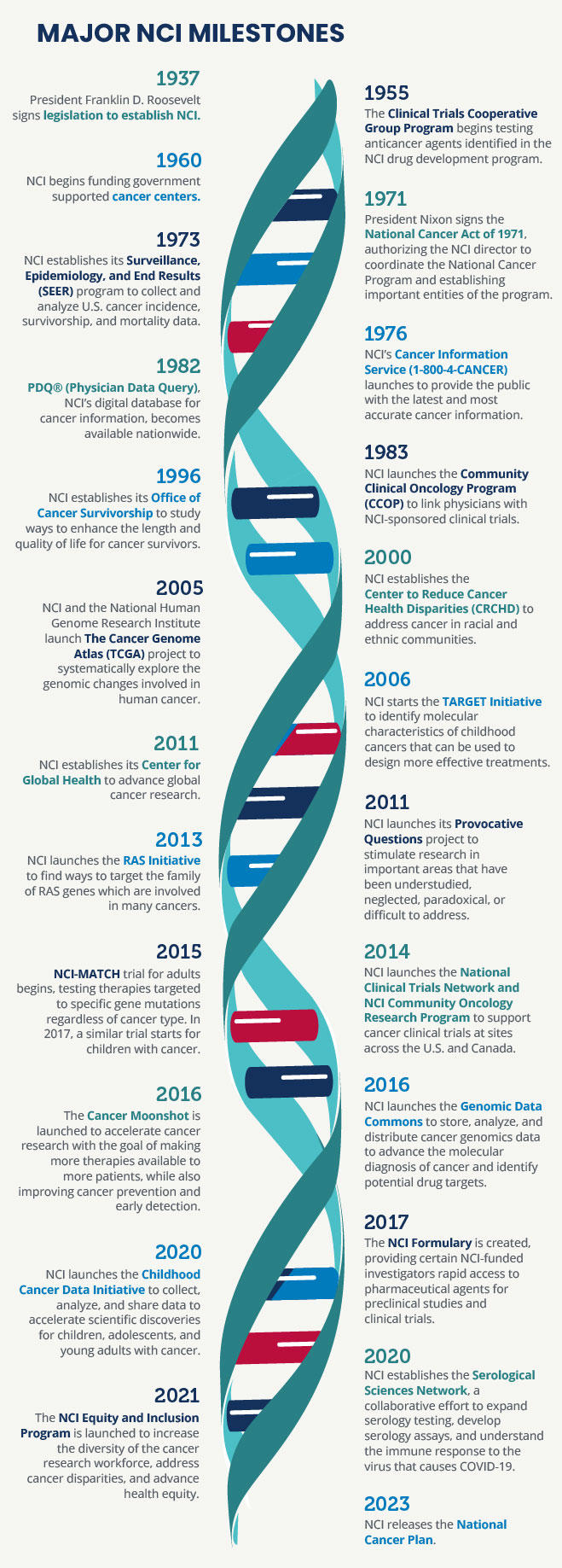
In 1937, Congress established the National Cancer Act of 1937 to provide additional support for cancer research—it was the first time Congress had appropriated funds toward a noncommunicable disease. The act, signed by President Franklin D. Roosevelt, established the National Cancer Institute (NCI) as the federal government's primary agency to address research and training needs for the cause, diagnosis, and treatment of cancer. NCI’s responsibilities included:
- conducting, coordinating, and promoting research and studies relating to the cause, diagnosis, treatment, and prevention of cancer
- reviewing and approving grant applications to support promising cancer research
- providing training and instruction in the diagnosis and treatment of cancer
- assisting and promoting similar research at other public and private institutions
- receiving advice from cancer experts in the United States and abroad
- cooperating with state health agencies in the prevention, control, and eradication of cancer
- collecting, analyzing, and disseminating the results of cancer research conducted in the United States and in other countries
The act called upon NCI to assist and promote similar research at other public and private institutions and merged the Office of Cancer Investigations at Harvard University with a pharmacology division of NIH to create NCI as an independent research institute within the division of the Public Health Service (independent of NIH).
The act also established the National Advisory Cancer Council (now known as the National Cancer Advisory Board [NCAB]), which was authorized to review all research projects for approval before recommending funding by the surgeon general, who was directed to “provide for, foster, and aid in coordinating research related to cancer within NCI and among other agencies and organizations.”
The National Cancer Act of 1971
In 1944, Congress approved the Public Health Service Act of 1944 (42 U.S.C. 201), which substantially consolidated and revised all existing legislation relating to the Public Health Service. This act also had direct effects on NCI because it made the institute an operating division of the National Institutes of Health (NIH). The National Cancer Act of 1971 expands upon these authorities. This act, signed by President Richard Nixon, created the National Cancer Program and:
- Granted the director of NCI broad authority to plan and develop an expanded, intensified and coordinated National Cancer Program that included NCI and related programs, other research institutes and federal and nonfederal programs “in order to more effectively carry out the national effort against cancer."
- Provided the NCI director with direct access to the President of the United States and required the NCI director to submit an annual budget proposal, called the “professional judgement budget,” directly to the President, bypassing the approval of the NIH director or HHS, as required of other NIH institutes.
- Mandated that NCI develop its programs with the advice of a National Cancer Advisory Board (NCAB), a presidentially appointed committee of 18 members, including both distinguished scientists and laypersons from the general public and 12 ex-officio members from other government agencies, and established the President's Cancer Panel (PCP), a three-member panel, which was specifically required to submit an annual report to the President and hold periodic public hearings.
- Provided additional funding for NCI, establishing 15 new cancer research centers, local cancer control programs, and an international cancer research data bank.
- Gave the NCI director additional authorities, in consultation with the NCAB, to:
- create new cancer centers and physician and researcher training programs
- appoint advisory committees, allowing the director to explore new issues/opportunities
- expand the physical location at NIH and other research facilities across the country
- award contracts for research
- collaborate with other federal, state, or local public agencies and private industry
- conduct cancer control activities
- establish an international cancer research data bank that collects, catalogues, stores, and disseminates results of cancer research
- award research grants