FDA Approves Pembrolizumab for Patients with Non-Small Cell Lung Cancer
, by NCI Staff
On October 2, the Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab (Keytruda®) to treat patients with metastatic non-small cell lung cancer (NSCLC) whose tumors express a protein called PD-L1 and whose cancers progressed after platinum-based chemotherapy.
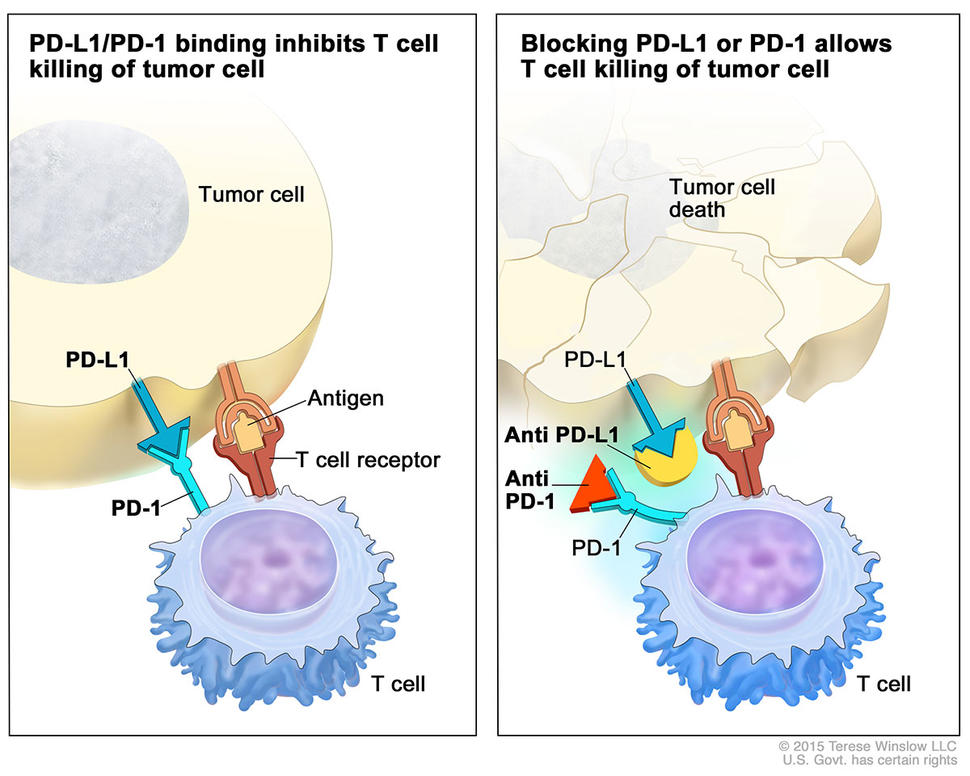
Pembrolizumab targets a protein on immune cells called PD-1, one of a family of so-called checkpoint proteins that can restrain the immune response. When PD-1 ligand, or PD-L1, on tumor cells binds to PD-1 on immune system cells, the PD-1 signaling pathway is activated, inhibiting an immune response. By blocking this interaction, pembrolizumab allows the immune system to recognize and attack tumor cells.
The FDA approval was based on a subgroup analysis of 61 patients in a large clinical trial whose tumors overexpressed PD-L1. After treatment with pembrolizumab, tumor size decreased in 24 patients (41 percent) and the effect lasted up to 9.1 months.
The approval of pembrolizumab for this indication requires the use of a companion diagnostic test called PD-L1 IHC 22C3 pharmDx, which is used to measure the PD-L1 expression levels of a patient's tumor.
"The approval of a companion diagnostic for pembrolizumab is extremely important," said Elad Sharon, M.D., M.P.H., of NCI’s Cancer Therapy Evaluation Program. "This companion diagnostic is the first PD-L1 test to be approved by the FDA, and it will set a new standard for future PD-L1 tests as time goes on. The approval of the companion diagnostic will likely benefit not only patients with lung cancer, as the FDA will likely be able to use it as a common reference standard to examine response rates in different types of cancers."
As required by the FDA, Merck, which manufactures pembrolizumab, is conducting a randomized trial to confirm the safety and efficacy of pembrolizumab.
Other clinical trials are testing pembrolizumab in patients whose tumors express high levels of PD-L1 and in patients whose tumors do not express PD-L1, Dr. Sharon explained.
"PD-1 inhibitors—and related drugs called PD-L1 inhibitors that affect the same pathway—generally have had higher degrees of response and have been more effective in patients whose tumors express PD-L1," he said.
Pembrolizumab is similar to another FDA-approved drug, nivolumab (Opdivo®), which also targets PD-1 on immune system cells. No trials have compared the two drugs head to head, Dr. Sharon said, but both drugs have shown activity in a growing number of cancers and both now have been approved for melanoma and non-small cell lung cancer.
NCI is currently funding trials to test these drugs in a variety of other tumor types. For example, preliminary results from an NCI-sponsored trial of pembrolizumab in patients with Merkel cell carcinoma presented in September at the European Cancer Congress showed that 10 of 14 patients had significant tumor responses, including two patients with complete responses.