How Genomics Is Shaping Precision Medicine in Oncology
, by Lou Staudt, M.D., Ph.D.
Share your thoughts below in the comments. And on December 8 at 2:00 p.m. ET, be part of our Twitter chat on cancer genomics and precision medicine. Visit our social media events page for more information.
The following is the latest in a series of posts from senior NCI scientists and leaders on NCI’s Annual Plan and Budget Proposal for Fiscal Year 2017, which was officially submitted to the President on September 17, 2015. The proposal provides an overview of NCI’s priorities and key initiatives and the institute’s funding request for the President to consider in formulating his own Fiscal Year (FY) 2017 budget proposal.
In this post, Louis M. Staudt, M.D., Ph.D., director of NCI’s Center for Cancer Genomics, discusses the principles of precision medicine and how they are being applied to improve the treatment of lymphoma.
The phrase “precision medicine” often refers to the emerging practice of using information about a patient’s tumor to diagnose or treat his or her disease. In this approach, physicians select the most appropriate treatments for patients based on their knowledge of the molecular abnormalities, such as genetic mutations, in the patients’ tumors.
The concept of precision medicine is not new. But cancer is fundamentally a disease of the genome, and efforts in precision medicine have accelerated over the past decade with the introduction of newer and cheaper technologies for sequencing DNA.
These technological advances have facilitated research on the biology of cancer cells, leading to the discovery of potential diagnostic markers and therapeutic targets. At the same time, a new generation of clinical trials, guided by tumor profiling and genetic testing, has emerged to begin to translate the basic discoveries into new diagnostic tests and targeted therapies.
As early successes in the field of precision medicine—such as the development of imatinib for chronic myelogenous leukemia—have illustrated, insights into the molecular machinery of a cancer cell can lead to therapies that target tumor cells while largely sparing normal cells. For patients, this selectivity may result in fewer toxic side effects than are associated with traditional treatments such as chemotherapy.
An emerging example from work in my lab at NCI involves the most common type of lymphoma, diffuse large B-cell lymphoma (DLBCL). This research offers a window into how studying differences between cancer cells and normal cells can ultimately lead to new and more effective treatments for patients with cancer.
The story began a few years ago, when my team used genetic tools to identify two molecularly distinct subtypes of DLBCL—the activated B-cell-like (ABC) subtype and the germinal center B-cell-like (GCB) subtype. Until that point, DLBCL had been considered a single disease.
The discovery that DLBCL actually consists of two primary subtypes suggested to us that it might be possible to develop targeted therapies for patients with each form of the disease.
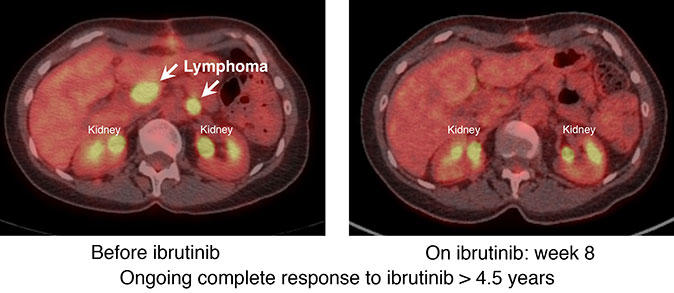
Our research on the signaling pathways involved in the development of different types of DLBCL led to us focus on a drug called ibrutinib (Imbruvica®), which targets an enzyme called Bruton’s tyrosine kinase (BTK). The enzyme plays a pivotal role in signaling by the B-cell receptor, which normal B lymphocytes use to respond to foreign antigens in the environment.
Our laboratory studies showed that B-cell receptor signaling is required for the survival and proliferation of cell line models of the ABC subtype of DLBCL but not models of GCB DLBCL. Treatment of the ABC cell lines with ibrutinib killed them while the GCB lines were unaffected.
These laboratory findings led us to investigate the activity of ibrutinib in patients with specific subtypes of DLBCL. Last summer, we reported results from a clinical trial showing that patients with the ABC subtype of DLBCL were much more likely to respond to the drug than patients with the GCB subtype, as we predicted.
The trial was one of the first clinical studies to demonstrate the importance of precision medicine for patients with lymphoma. The trial also illustrates how insights from basic cancer research—such as the discovery of previously unknown lymphoma subtypes—can be the foundation for a hallmark of precision medicine: developing therapies for patients whose cancers share certain characteristics.
Based on these results, an international phase III study has been launched to determine whether the addition of ibrutinib to standard chemotherapy can improve the cure rate among patients with the ABC subtype of DLBCL. The trial will test standard chemotherapy with or without ibrutinib in patients with DLBCL, excluding the GCB subtype.
The story is far from over. More research is under way to refine our diagnostic categories and to develop additional targeted therapies for patients with different subtypes of lymphoma. Similar work focused on many other types of cancer is taking place in laboratories and at medical centers around the world, often through collaborative research projects.
Many of these projects are focused on an important frontier in precision medicine: the development of combination therapies. Most cancer cells use more than one molecular pathway to promote their malignant proliferation and survival. Often, when only one pathway is blocked by a drug, another pathway takes over to keep the cancer cell alive and dividing rapidly. By combining several targeted drugs into one therapeutic regimen, we can disrupt these “bypass” mechanisms, potentially leading to better results for our patients.
The mantra of precision medicine is “divide and conquer.” That is, divide cancers into molecular subtypes, and treat them with drugs that target the abnormal biological mechanisms that define each subtype. Many of us believe that this rational approach, based on a deep understanding of cancer genetics and mechanisms, will be necessary for the successful conquest of cancer.
Social Media Event
I encourage you to weigh in with your thoughts and questions about precision medicine and its role in fostering progress against cancer. I also encourage you to read the Annual Plan and Budget Proposal and the remaining blog posts in this series.
And on December 8 at 2:00 p.m. ET, please join my colleague, Jean-Claude Zenklusen, Ph.D., Director of the Cancer Genome Atlas (TCGA), for a Twitter chat about cancer genomics and precision medicine, using the hashtag #CancerGenomics. You can find more details on our social media events page.
NCI FY 2017 Annual Plan & Budget Proposal Blog Series
December 10: Precision Medicine Part II: Clinical Trials
January 13: Cancer Prevention: The Best Defense