KRAS somatic cancer-driver mutations in endometriosis without cancer: towards a molecular classification for endometriosis?
, by Paul J. Yong, Natasha L. Orr, Michael S. Anglesio
Paul J. Yong is an Associate Professor at the University of British Columbia, Research Director at the BC Women’s Centre for Pelvic Pain and Endometriosis, and a Canada Research Chair (Tier 2) in Endometriosis and Pelvic Pain.
Natasha L. Orr is a post-doctoral fellow at the University of British Columbia, supported by a Canadian Institutes of Health Research (CIHR) post-doctoral award.
Michael S. Anglesio is an Assistant Professor at the University of British Columbia, a Michael Smith Foundation Scholar, and a CIHR Early Career Investigator in Maternal, Reproductive, Child & Youth Health.
In 2017, we and others demonstrated that somatic cancer-driver mutations including KRAS are more common than once thought in endometriosis without cancer. Endometriosis is defined as endometrial-like tissue (glandular epithelium/stroma) being present outside of the uterus. Anatomic subtypes of endometriosis are superficial peritoneal endometriosis, deep endometriosis (locally invasive), and ovarian endometriomas (forming cysts). It is an estrogen-dependent inflammatory disease that causes infertility (with recent epidemiologic studies also showing an association with pregnancy complications), as well as pelvic pain (ranging from painful periods, painful sexual activity, and chronic pelvic pain). Conventional treatments involve hormonal suppression of estrogen and surgical excision of endometriosis lesions (with or without hysterectomy or oophorectomy). Staging of disease is done at surgery. Major clinical problems include significant rates of re-operation after endometriosis surgery, and that hormonal suppressive therapies are not curative. In addition to the common presentations with infertility and pelvic pain, endometriosis is also a risk factor for certain ovarian cancer subtypes (particularly endometrioid and clear cell), with endometriosis adjacent to, or contiguous with, ovarian cancer often showing atypical histological/cytological features and along with many (sometimes all) of the same somatic mutations present in the cancer.
An unanswered question has been whether these somatic “cancer-driver” mutations are also present in endometriosis without ovarian cancer. While earlier studies using less sensitive methodologies showed low rates of somatic mutations in endometriosis, the advent of next generation sequencing has allowed more sensitive interrogation of the incidence and potential role of somatic cancer-driver mutations in endometriosis without cancer. In 2017, we and others demonstrated that somatic cancer-driver mutations are more common than once thought in endometriosis without cancer: our initial study found one-third (5/15) of deep endometriosis cases harboured KRAS codon 12 driver mutations that were confined to endometriosis epithelium (and absent from stromal cells). Subsequent studies have shown so-called somatic cancer-driver mutations to be present on all anatomic subtypes of endometriosis (including iatrogenic endometriosis seeded to the abdominal wall from previous surgery). KRAS activating mutations remain the most commonly described recurrent somatic mutation, though others have been described (e.g. affecting PIK3CA, CTNNB1, ARID1A, etc.). Furthermore, the presence of the same mutation in different endometriosis subtypes and lesions within the same patient has supported endometriosis as an oligoclonal disease with multiple epithelial clones migrating and disseminating throughout the pelvis.
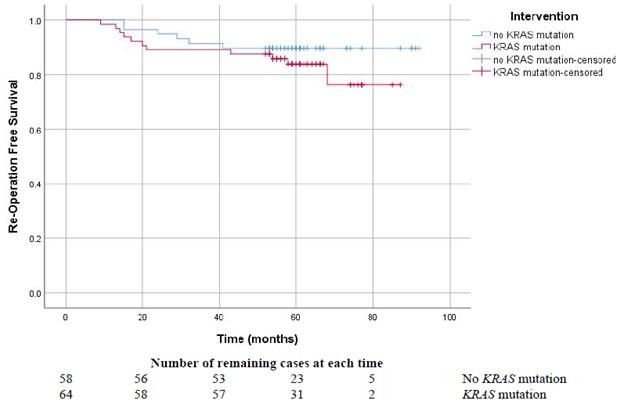
This year, we published a prospective longitudinal study of KRAS somatic driver mutations in 122 patients with endometriosis, with 5-9 year follow-ups after the index surgery. Using endometriosis tissue specimens from the index surgery, droplet digital PCR (ddPCR) was used to identify six KRAS codon 12 driver mutations: c.34G>T (p.G12C), c.35G>A (p.G12D), c.34G>C (p.G12R), c.35G>T (p.G12V), c.35G>C (p.G12A), and c.34G>A (p.G12S). Remarkably, 52.5% (64/122) of patients had at least one KRAS codon 12 mutation in at least one endometriosis lesion. Patients harbouring a mutation were significantly more likely to have the more severe subtypes of deep endometriosis and ovarian endometriomas (resulting in higher surgical stage). In the cases with a KRAS mutation, greater surgical difficulty was reported by surgeons at the time of the index surgery. Interestingly, these patients were also significantly more likely to be of non-Caucasian ethnicity (primarily East or South East Asian descent in our cohort). Intriguingly, cases with a KRAS mutation had a trend towards lower re-operation free survival (higher re-operation rate after the index surgery), although power was limited by the relatively low overall re-operation rates in the cohort. In contrast, KRAS codon 12 mutation status was not associated with pelvic pain symptoms, possibly due to the complex multifactorial nature of endometriosis-associated pain which would require a greater sample size to control for potential confounders.
Work is ongoing to further characterize the clinical phenotype of KRAS and other somatic “cancer-driver” mutations in endometriosis. Current surgical staging of endometriosis is known to have limitations, and thus there is interest in novel classification systems including the use of molecular markers. KRAS somatic mutations could form part of such a future molecular classification of endometriosis.
Please contact Dr. Paul Yong with any questions at Paul.Yong@vch.ca.