New type of bifunctional molecules perturb K-Ras interactions with membranes
, by Johannes Morstein and Kevan M. Shokat
Johannes Morstein is an NCI K99/R00 Postdoctoral Fellow in the group of Dr. Shokat. He is working on expanding the druggability of GTPases and protein-membrane interfaces.
Kevan Shokat is a professor at the University of California at San Francisco and leader in the search for novel targeted therapeutics. He successfully developed the first covalent inhibitor of mutant KRAS (Sotorasib), which saw FDA approval in 2021 for the treatment of adults with non-small cell lung cancer (NSCLC) that carry the KRAS G12C mutation.
Most drug molecules currently are monofunctional. This means that they bind to a single biological target, which can be a receptor, transporter, or enzyme (all different types of proteins). However, in recent years several research groups have succeeded in developing bifunctional molecules, which simultaneously bind to two different biological targets. There are many clever ways this technology can be used. For example, it can bring a specific disease-causing protein close to the proteasome, which is the apparatus in cells that recycles proteins. In other words, such bifunctional molecules can result in the recycling of a protein of choice, which could be an attractive avenue in drug discovery. In particular, when a drug is not powerful enough on its own to fully inhibit (that is, to inactivate) a disease-causing protein, recycling can be a solution and provide a more effective approach leading to the removal of the disease-causing protein from cells.
So far in the field, efforts to engineer bifunctional molecules have mostly focused on designing agents that simultaneously bind to two proteins. In our study, we realized that the interaction between proteins and membranes is also very important for most cellular processes. For example, for a cell to sense clues from the environment (e.g. a hormone, growth factor, stimulant), signals have to be transmitted from the extracellular space to the intracellular space across its outer boundary, the cell membrane. Accordingly, we hypothesized that developing a bifunctional approach to modulate these protein-membrane interactions could yield another attractive avenue in drug development.
In cancer, diseased cells often grow uncontrollably through a constant overstimulation of growth signaling. This can occur when proteins typically involved in normal sensing cascades harbor mutations that lead to their constant activation even in the absence of extracellular stimulants. Most famously, mutations in the oncogene KRAS can have this consequence. Indeed, KRAS is the most frequently mutated human oncogene, which means that it is the gene associated with driving the growth of many cancers. KRAS mutations are particularly common in pancreatic cancer, lung cancer, and colorectal cancer. Unlike many other proteins, K-Ras, the protein that is the product of the KRAS oncogene, turned out to be particularly challenging to drug, and for a long time, it was deemed ‘undruggable’. Thankfully this has changed, and we are witnessing the first FDA approvals of K-Ras inhibitors and an increasing number of clinical trials.
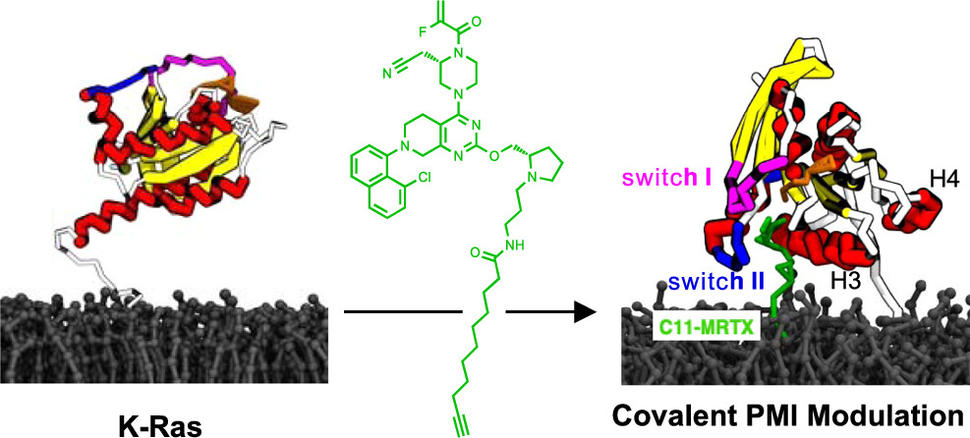
One critical feature of K-Ras is its loose association with the inner leaflet of the plasma membrane. To rephrase this in spatial terms, K-Ras sits on the inner site of the outer boundary of cells. This location and its association with the plasma membrane are necessary for its function. If K-Ras is not located there, it does not work and does not contribute to cell growth. In the current study, we therefore thought to explore a new bifunctional approach by targeting these interactions directly. To this end, we designed molecules that can bind to both K-Ras and the membrane. In a collaboration between UCSF, the Frederick National Laboratory, Los Alamos National Laboratory, and UTHealth Houston, we were able to show that these molecules indeed bind both K-Ras and the membrane and, as a result, significantly change the way the two interact.
Our bifunctional molecules glue K-Ras to the membrane at a location where K-Ras usually does not interact with the membrane. Gluing K-Ras to the membrane at a new position also leads to tighter interactions, which restrict two aspects that are critical for the function of K-Ras—its dynamic mobility on the membrane and its clustering with other K-Ras molecules. Our study was the first demonstration of a bifunctional molecule specifically engineered to target and modulate the interactions between a protein and a membrane. Our approach could be useful to study the role of protein-membrane interactions and adds a new modality in drug discovery that helps to target and ultimately stop cancer drivers.