Senescent macrophages promote oncogenic KRAS-driven lung cancer and suppress anti-tumour immunity
, by Dr Scott Haston, Daniel Muñoz-Espin, and Juan Pedro Martinez-Barbera
Dr Scott Haston obtained a BSc in Anatomical Sciences (University of Dundee, Scotland) in 2013 and a PhD in Genetics and Cell Biology (University College London) in 2019. Scott is currently a senior Postdoctoral Research Fellow in the laboratory of Professor Juan Pedro-Martinez-Barbera at University College London's Institute of Child Health. With 10 years of experience in genetics, cell biology and genetically engineered mouse models, Scott's research delves into the connections between cellular senescence, tumorigenesis, and biological aging. Specifically, Scott investigates whether cellular senescence and its associated pro-inflammatory signalling dynamics promote tumour formation and whether this is therapeutically targetable to prevent tumourigenesis or restrict tumour growth, improving ageing outcomes. His PhD research unveiled an important role for cellular senescence in driving paediatric pituitary tumourigenesis non-cell autonomously. Following this, in his postdoctoral career, he continues to make use of novel genetically engineered mouse models, revealing new insights into the influence of cellular senescence on lung ageing and cancer biology. Through collaborations, he extends this research into other tumourigenic and age-associated pathological contexts.
Daniel Muñoz-Espin is group leader in the Early Cancer Institute (Department of Onclogy, University of Cambridge) and Co-director of the CRUK Cambridge Centre Thoracic Cancer Programme. His laboratory works on the fundamental processes and mechanisms triggering cancer initiation and progression, with a particular focus on the lung tumour microenvironment. Also, Daniel´s group uses this knowledge to design, develop and validate novel tools for cancer early detection and therapeutic interventions, including imaging probes, activatable pro-drugs and nanotechnologies. Daniel did his PhD (2002-2006) in the laboratory of Prof Margarita Salas (Autonomous University of Madrid, Spain) and in the group of Prof Jeff Errington (University of Oxford, UK). After a first postdoctoral stage at the Centre of Molecular Biology Severo Ochoa in Madrid (2007-2010), he moved to the group of Prof Manuel Serrano at the Spanish National Cancer Research Centre – CNIO, where he was trained in cellular senescence and mouse models of cancer and ageing (2011-2015). His main work, published in Cell and Nature Reviews Molecular Cell Biology, culminated with two awarded grants: a “Ramon y Cajal Programme Senior Grant” and a “National Programme Grant for Researched Aimed at the H2020 Societal Changes”. In 2016, Dr Muñoz-Espin joined the University of Cambridge as a principal investigator to establish his own group. Among other sources, Daniel was funded with an MRC New Investigator Research Grant (2017-2021), the CRUK Early Detection Project (2018-2021), and by a CRUK Programme Foundations Award (2020-2026).
Juan Pedro Martinez-Barbera is a Professor in Developmental Biology and Cancer at the University College London Great Ormond Street Institute of Child Health. His research program aims to understand the role of cellular senescence in paediatric and adult cancers by combining developmental, molecular and cellular approaches and revealing novel therapies including senotherapies. His laboratory has developed mouse models that allow the study of cellular senescence in vivo. For example, his research has revealed that cellular senescence through its secretome drives cell transformation and tumour initiation in the context of craniopharyngioma, a clinically relevant paediatric brain tumour, and that the senescent cells are sensitive to senolytics. These studies have identified targetable pathways in humans, which are being tested in ongoing clinical trials. More recently, his laboratory’s research has expanded into other tumours, including paediatric low and high -grade glioma, as well as KRAS-driven lung adenocarcinoma where they have revealed a key role of senescent macrophages in tumour development.
Introduction:
Early investigations in cancer biology predominantly focused on the cell-autonomous role of oncogenes like RAS in driving cellular transformation and tumour development. However, in recent years, a growing appreciation has emerged in the non-cell autonomous effects of these oncogenes and the interactions between early tumour cells and their surrounding tissue environment. Non-cell autonomy in cancer development extends beyond the intrinsic genetic alterations in cancer cells. Oncogenes like RAS have effects that ripple through the tumour microenvironment (TME), impacting the immune response and influencing the body's ability to combat the tumour. The interplay between the tumour and the immune landscape is of particular significance as immunotherapy has shown great promise in cancer treatment, although its effectiveness is often limited by the complex dynamics within the TME.
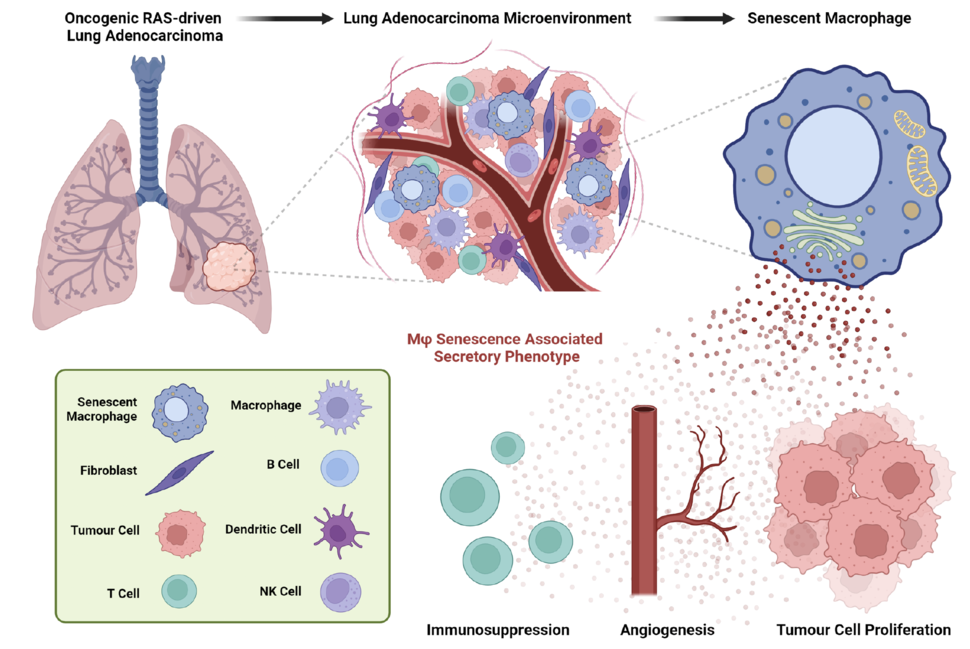
This is where the concept of cellular senescence becomes crucial. Cellular senescence is an evolutionarily conserved chronic damage and stress response, in which cells lose their ability to divide and alter their metabolic and transcriptomic profiles, accompanied by the acquisition of a senescence-associated secretory phenotype (SASP). The SASP implies a complex and highly dynamic cellular secretion pattern comprised of pro-inflammatory chemokine and cytokines, growth factors and extracellular matrix remodelling factors. In a context-dependent manner, the SASP can influence the behaviour of neighbouring normal and cancer cells, generating either tumour-promoting or tumour-suppressive effects. Senescent cells are known to passively accumulate in tissues as organisms age, but are also induced in response to persistent cellular stress, such as tissue damage or oncogene activation 1,2. However, identifying and characterizing the role of senescent cells within the TME has proven to be a challenging task, but it has the potential to unlock new therapeutic modalities for the treatment of cancer 3.
Research Approach and Findings:
Our study was designed with the aim of unravelling the potential role of senescent cell populations within the complex landscape of KRAS-driven lung tumours 4. To achieve this, we generated a novel genetically engineered mouse model, termed p16-FDR. This model enables the visualisation, fate mapping and selective ablation of p16INK4a (CDKN2A)-expressing cell populations in vivo, which is one of the best singular markers of senescent cell populations 5.
By utilising the p16-FDR mouse model and single-cell transcriptomic approaches, we were able to discern the prevalent presence of macrophages and, to a lesser extent, endothelial cells with senescent characteristics in murine KRAS-driven lung tumours. Deeper molecular characterisation unveiled a unique repertoire of pro-tumorigenic SASP factors and distinctive surface proteins expressed by the senescent tumour-associated macrophages. We leveraged the ability of the p16-FDR model or the senolytic compound (drug capable of selectively killing senescent cell populations while sparing their non-senescent counterparts) ABT-737, a BCL-2/BCL-xL inhibitor, to ablate senescent cell populations during lung tumourigenesis.
Remarkably, we discovered that these senescent tumour-associated macrophages have potent pro-tumourigenic potential. Their removal from the lung tumour microenvironment resulted in decreased lung tumour burden, reduced tumour cell proliferation and increased engagement of anti-tumour immune responses, collectively resulting in significantly increased mouse survival. Of translational importance, our findings highlighted the conserved existence of senescent macrophages in human lung pre-malignant lesions, but not in advanced adenocarcinomas, indicating their potential involvement in the early stages of human lung tumorigenesis.
Unexpectedly, we also uncovered a significant population of senescent macrophages that exist in naturally-aged mouse lungs. Remarkably, these age-associated senescent macrophages share the unique transcriptomic signature, SASP composition and surface markers with the senescent macrophages found during lung tumourigenesis. This observation provides further translational significance of our findings to the broader physiological context of ageing and the development of age-associated pathologies. Notably, our study aligns with the complementary research conducted by Dr Darren Baker’s group 6, which further reinforces the critical role of senescent macrophages in shaping the tumour/immune microenvironment and negatively influencing tumour behaviour.
In light of ongoing debates surrounding macrophage senescence 7, it is imperative to recognize the evidence of the detrimental effects of populations of tumour-associated macrophages 8, regardless of the semantic intricacies of qualifying a cell as senescent. Crucially, our findings suggest that these deleterious macrophage-mediated effects can be effectively attenuated through the application of senolytic compounds, offering a promising novel avenue for targeted therapeutic interventions in the context of cancer management 9,10.
Implications for Lung Cancer Treatment and Prevention:
Our results show that the removal of senescent macrophages results in a significantly decreased tumour burden and extended mouse survival. Of note, about one third of the mice developing multiple lung adenomas never developed adenocarcinomas. Therefore, this result opens up the possibility of using senolytic therapies (e.g. Bcl-2 inhibitors) as a novel strategy to intercept KRAS-driven lung cancer at early tumour stages and/or a preventative modality for patients at high risk of developing lung cancer, for instance in patients bearing multifocal premalignant primary lesions. The scRNAseq data has revealed potential immunosuppressive SASP factors, suggesting that senomorphic strategies aiming to inhibit these factors might be relevant to preventing cancer onset. Finally, the potential use of anti-CSF1R antibodies, or other identified possible targets (such as CD163 or FOLR2), to deplete tumour-associated macrophages and boost immunotherapeutic responses warrants further investigations.
Potential areas for future research and preclinical validation may include (i) the use of senolysis in combination with current, standard of care, senescence-inducing cancer treatment regimens (also known as “one-two punch” approach), (ii) senomorphic strategies to turn a tumour-promoting and/or immunosuppressive SASP into a tumour-suppressive and immunoregulatory SASP and (iii) the development of immunotherapy-combination anti-cancer strategies facilitating the clearance of protumorigenic senescent cells; for example, immune checkpoint inhibitors (e.g. anti-PD-L1) 11, monoclonal antibodies 4, treatment with senescent vaccines 12, immune system modulators 13 or senolytic CAR-T cells 14.
Successful implementation of the above strategies relies heavily on a detailed understanding of the specific senescent ecosystems that contribute to early tumorigenesis and their underlying mechanisms of tumour promotion and progression. In particular, in the case of senescent macrophages accumulating in the early tumour microenvironment, it will be crucial to dissect how oncogene-induced senescent lung epithelial cells lead to macrophage senescence, how senescent macrophages promote tumorigenesis and the specific SASP factors responsible, and to what extent age-induced senescent macrophages can contribute to lung cancer onset.
Conclusions:
Conceptually, our work underscores two major discoveries: At the basic cancer research level, it demonstrates the crucial contribution of specific senescent populations of the precancerous niche, in particular macrophages and their associated cell extrinsic mechanisms (SASP), in promoting lung cancer initiation and progression. At the translational level, this work is a proof-of-concept of how targeting a (senescent) tumour microenvironment by distinct therapeutic modalities can impair (or even prevent) lung carcinogenesis.
This strategy has the potential to be implemented to treat or intercept lung cancer at early stages, or as a preventative therapeutic modality for patients at high risk of developing lung cancer, such as long-term heavy smokers bearing primary lesions. Further research is however needed to identify and validate senescence specific biomarkers and targets, allowing us to ultimately translate our findings to clinical settings.