Indolent B-Cell Non-Hodgkin Lymphoma Treatment (PDQ®)–Health Professional Version
General Information About B-Cell Non-Hodgkin Lymphoma
The non-Hodgkin lymphomas (NHL) are a heterogeneous group of lymphoproliferative malignancies with differing patterns of behavior and responses to treatment.[1] This summary focuses primarily on indolent B-cell NHL. For information about B-cell and T-cell lymphomas, see Aggressive B-Cell Non-Hodgkin Lymphoma Treatment, Peripheral T-Cell Non-Hodgkin Lymphoma Treatment and Mycosis Fungoides and Other Cutaneous T-Cell Lymphomas Treatment.
Like Hodgkin lymphoma, NHL usually originates in lymphoid tissues and can spread to other organs. However, NHL is much less predictable than Hodgkin lymphoma and has a far greater tendency to spread to extranodal sites. The prognosis depends on the histological type, disease stage, and treatment.
Incidence and Mortality
Estimated new cases and deaths from all types of NHL in the United States in 2025:[2]
- New cases: 80,350.
- Deaths: 19,390.
B-cell lymphomas make up about 85% of NHL cases.[3]
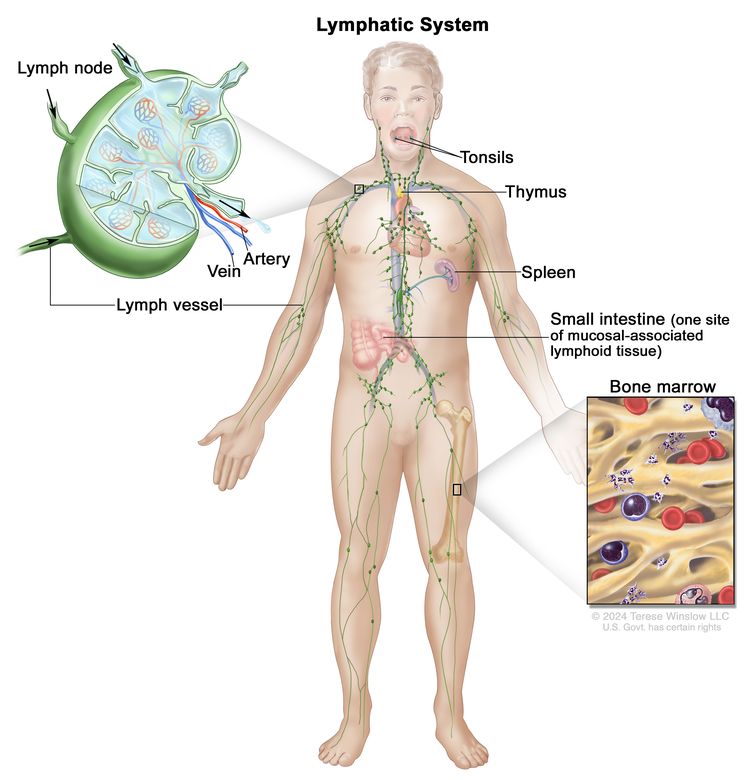
Anatomy
NHL usually originates in lymphoid tissues.
Prognosis and Survival
NHL can be divided into two prognostic groups: indolent lymphomas and aggressive lymphomas.
Indolent NHL has a relatively good prognosis, with a median survival as long as 20 years, but it is usually not curable in advanced clinical stages.[4] Early-stage (stage I and stage II) indolent NHL can be effectively treated with radiation therapy alone. Most of the indolent NHLs are nodular (or follicular) in morphology.
Aggressive NHL has a worse prognosis in the short term, but a significant number of patients can be cured with intensive combination chemotherapy regimens.
More than 70% of patients with aggressive NHL can be cured. Most relapses occur in the first 2 years after therapy. The risk of late relapse is higher in patients who manifest both indolent and aggressive histologies.[5]
Although indolent NHL responds quickly to immunotherapy, radiation therapy, and chemotherapy, a continuous rate of relapse is usually seen in advanced stages. However, patients can often be re-treated with considerable success if the disease histology remains low grade. Patients who present with, or convert to, aggressive forms of NHL may have sustained complete remissions with combination chemotherapy regimens or aggressive consolidation with marrow or stem cell support.[6,7]
Late Effects of Treatment of NHL
Late effects of treatment of non-Hodgkin lymphoma (NHL) have been observed. Impaired fertility may occur after exposure to alkylating agents.[8] For as many as three decades after diagnosis, patients are at a significantly elevated risk of developing second primary cancers, especially the following:[9-12]
- Lung cancer.
- Brain cancer.
- Kidney cancer.
- Bladder cancer.
- Melanoma.
- Hodgkin lymphoma.
- Acute nonlymphocytic leukemia.
Left ventricular dysfunction was a significant late effect in long-term survivors of high-grade NHL who received more than 200 mg/m² of doxorubicin.[8,13]
Myelodysplastic syndrome and acute myelogenous leukemia are late complications of myeloablative therapy with autologous bone marrow or peripheral blood stem cell support, as well as conventional chemotherapy-containing alkylating agents.[10,14-21] Most of these patients show clonal hematopoiesis even before the transplant, suggesting that the hematologic injury usually occurs during induction or reinduction chemotherapy.[16,22,23] A series of 605 patients who received autologous bone marrow transplant (BMT) with cyclophosphamide and total-body radiation therapy (as conditioning) were followed for a median of 10 years. The incidence of a second malignancy was 21%, and 10% of those malignancies were solid tumors.[24]
A study of young women who received autologous BMT reported successful pregnancies with children born free of congenital abnormalities.[25] Late-occurring venous thromboembolism can occur after allogeneic or autologous BMT.[26]
Some patients have osteopenia or osteoporosis at the start of therapy; bone density may worsen after therapy for lymphoma.[27]
Long-term impaired immune health was evaluated in a retrospective cohort study of 21,690 survivors of diffuse large B-cell lymphoma from the California Cancer Registry. Elevated incidence rate ratios were found up to 10 years later for pneumonia (10.8-fold), meningitis (5.3-fold), immunoglobulin deficiency (17.6-fold), and autoimmune cytopenias (12-fold).[28] Similarly, there are impaired humoral responses to COVID-19 virus vaccination in patients with lymphoma who receive B-cell–directed therapies.[29,30]
References
- Shankland KR, Armitage JO, Hancock BW: Non-Hodgkin lymphoma. Lancet 380 (9844): 848-57, 2012. [PUBMED Abstract]
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- American Cancer Society: Types of B-cell Lymphoma. American Cancer Society, 2019. Available online. Last accessed February 7, 2025.
- Tan D, Horning SJ, Hoppe RT, et al.: Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood 122 (6): 981-7, 2013. [PUBMED Abstract]
- Cabanillas F, Velasquez WS, Hagemeister FB, et al.: Clinical, biologic, and histologic features of late relapses in diffuse large cell lymphoma. Blood 79 (4): 1024-8, 1992. [PUBMED Abstract]
- Bastion Y, Sebban C, Berger F, et al.: Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J Clin Oncol 15 (4): 1587-94, 1997. [PUBMED Abstract]
- Yuen AR, Kamel OW, Halpern J, et al.: Long-term survival after histologic transformation of low-grade follicular lymphoma. J Clin Oncol 13 (7): 1726-33, 1995. [PUBMED Abstract]
- Haddy TB, Adde MA, McCalla J, et al.: Late effects in long-term survivors of high-grade non-Hodgkin's lymphomas. J Clin Oncol 16 (6): 2070-9, 1998. [PUBMED Abstract]
- Travis LB, Curtis RE, Glimelius B, et al.: Second cancers among long-term survivors of non-Hodgkin's lymphoma. J Natl Cancer Inst 85 (23): 1932-7, 1993. [PUBMED Abstract]
- Mudie NY, Swerdlow AJ, Higgins CD, et al.: Risk of second malignancy after non-Hodgkin's lymphoma: a British Cohort Study. J Clin Oncol 24 (10): 1568-74, 2006. [PUBMED Abstract]
- Hemminki K, Lenner P, Sundquist J, et al.: Risk of subsequent solid tumors after non-Hodgkin's lymphoma: effect of diagnostic age and time since diagnosis. J Clin Oncol 26 (11): 1850-7, 2008. [PUBMED Abstract]
- Major A, Smith DE, Ghosh D, et al.: Risk and subtypes of secondary primary malignancies in diffuse large B-cell lymphoma survivors change over time based on stage at diagnosis. Cancer 126 (1): 189-201, 2020. [PUBMED Abstract]
- Moser EC, Noordijk EM, van Leeuwen FE, et al.: Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood 107 (7): 2912-9, 2006. [PUBMED Abstract]
- Darrington DL, Vose JM, Anderson JR, et al.: Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol 12 (12): 2527-34, 1994. [PUBMED Abstract]
- Stone RM, Neuberg D, Soiffer R, et al.: Myelodysplastic syndrome as a late complication following autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 12 (12): 2535-42, 1994. [PUBMED Abstract]
- Armitage JO, Carbone PP, Connors JM, et al.: Treatment-related myelodysplasia and acute leukemia in non-Hodgkin's lymphoma patients. J Clin Oncol 21 (5): 897-906, 2003. [PUBMED Abstract]
- André M, Mounier N, Leleu X, et al.: Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: a GELA cohort study on 2837 patients. Blood 103 (4): 1222-8, 2004. [PUBMED Abstract]
- Oddou S, Vey N, Viens P, et al.: Second neoplasms following high-dose chemotherapy and autologous stem cell transplantation for malignant lymphomas: a report of six cases in a cohort of 171 patients from a single institution. Leuk Lymphoma 31 (1-2): 187-94, 1998. [PUBMED Abstract]
- Lenz G, Dreyling M, Schiegnitz E, et al.: Moderate increase of secondary hematologic malignancies after myeloablative radiochemotherapy and autologous stem-cell transplantation in patients with indolent lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group. J Clin Oncol 22 (24): 4926-33, 2004. [PUBMED Abstract]
- McLaughlin P, Estey E, Glassman A, et al.: Myelodysplasia and acute myeloid leukemia following therapy for indolent lymphoma with fludarabine, mitoxantrone, and dexamethasone (FND) plus rituximab and interferon alpha. Blood 105 (12): 4573-5, 2005. [PUBMED Abstract]
- Morton LM, Curtis RE, Linet MS, et al.: Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol 28 (33): 4935-44, 2010. [PUBMED Abstract]
- Mach-Pascual S, Legare RD, Lu D, et al.: Predictive value of clonality assays in patients with non-Hodgkin's lymphoma undergoing autologous bone marrow transplant: a single institution study. Blood 91 (12): 4496-503, 1998. [PUBMED Abstract]
- Lillington DM, Micallef IN, Carpenter E, et al.: Detection of chromosome abnormalities pre-high-dose treatment in patients developing therapy-related myelodysplasia and secondary acute myelogenous leukemia after treatment for non-Hodgkin's lymphoma. J Clin Oncol 19 (9): 2472-81, 2001. [PUBMED Abstract]
- Brown JR, Yeckes H, Friedberg JW, et al.: Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 23 (10): 2208-14, 2005. [PUBMED Abstract]
- Jackson GH, Wood A, Taylor PR, et al.: Early high dose chemotherapy intensification with autologous bone marrow transplantation in lymphoma associated with retention of fertility and normal pregnancies in females. Scotland and Newcastle Lymphoma Group, UK. Leuk Lymphoma 28 (1-2): 127-32, 1997. [PUBMED Abstract]
- Gangaraju R, Chen Y, Hageman L, et al.: Risk of venous thromboembolism in patients with non-Hodgkin lymphoma surviving blood or marrow transplantation. Cancer 125 (24): 4498-4508, 2019. [PUBMED Abstract]
- Westin JR, Thompson MA, Cataldo VD, et al.: Zoledronic acid for prevention of bone loss in patients receiving primary therapy for lymphomas: a prospective, randomized controlled phase III trial. Clin Lymphoma Myeloma Leuk 13 (2): 99-105, 2013. [PUBMED Abstract]
- Shree T, Li Q, Glaser SL, et al.: Impaired Immune Health in Survivors of Diffuse Large B-Cell Lymphoma. J Clin Oncol 38 (15): 1664-1675, 2020. [PUBMED Abstract]
- Ghione P, Gu JJ, Attwood K, et al.: Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood 138 (9): 811-814, 2021. [PUBMED Abstract]
- Terpos E, Trougakos IP, Gavriatopoulou M, et al.: Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood 137 (26): 3674-3676, 2021. [PUBMED Abstract]
Cellular Classification of B-Cell Non-Hodgkin Lymphoma
A pathologist should be consulted before a biopsy because some studies require special preparation of tissue (e.g., frozen tissue). Knowledge of cell surface markers and immunoglobulin and T-cell receptor gene rearrangements may help with diagnostic and therapeutic decisions. The clonal excess of light-chain immunoglobulin may differentiate malignant cells from reactive cells. Because the prognosis and the approach to treatment are influenced by histopathology, outside biopsy specimens should be carefully reviewed by a hematopathologist who is experienced in diagnosing lymphomas. Although lymph node biopsies are recommended whenever possible, sometimes immunophenotypic data are sufficient for diagnosis of lymphoma when fine-needle aspiration cytology or core needle biopsy is preferred.[1,2]
Historical Classification Systems
Historically, uniform treatment of patients with non-Hodgkin lymphoma (NHL) has been hampered by the lack of a uniform classification system. In 1982, results of a consensus study were published as the Working Formulation.[3] The Working Formulation combined results from six major classification systems into one classification. This allowed comparison of studies from different institutions and countries. The Rappaport classification, which also follows, is no longer in common use.
| Working Formulation [3] | Rappaport Classification |
|---|---|
| Low grade | |
| A. Small lymphocytic, consistent with chronic lymphocytic leukemia | Diffuse lymphocytic, well-differentiated |
| B. Follicular, predominantly small-cleaved cell | Nodular lymphocytic, poorly differentiated |
| C. Follicular, mixed small-cleaved, and large cell | Nodular mixed, lymphocytic, and histiocytic |
| Intermediate grade | |
| D. Follicular, predominantly large cell | Nodular histiocytic |
| E. Diffuse small-cleaved cell | Diffuse lymphocytic, poorly differentiated |
| F. Diffuse mixed, small and large cell | Diffuse mixed, lymphocytic, and histiocytic |
| G. Diffuse, large cell, cleaved, or noncleaved cell | Diffuse histiocytic |
| High grade | |
| H. Immunoblastic, large cell | Diffuse histiocytic |
| I. Lymphoblastic, convoluted, or nonconvoluted cell | Diffuse lymphoblastic |
| J. Small noncleaved-cell, Burkitt, or non-Burkitt | Diffuse undifferentiated Burkitt or non-Burkitt |
Current Classification Systems
As the histopathological diagnosis of NHL has become more sophisticated with the use of immunologic and genetic techniques, a number of new pathological entities have been described.[4] In addition, the understanding and treatment of many of the previously described pathological subtypes have changed. As a result, the Working Formulation has become outdated and less useful to clinicians and pathologists. European and American pathologists have proposed a new classification, the Revised European American Lymphoma (REAL) classification.[5-8] Since 1995, members of the European and American Hematopathology societies have been collaborating on a new World Health Organization (WHO) classification, which represents an updated version of the REAL system.[9,10]
Updated REAL/WHO classification
The World Health Organization (WHO) modification of the Revised European American Lymphoma (REAL) classification recognizes three major categories of lymphoid malignancies based on morphology and cell lineage: B-cell neoplasms, T-cell/natural killer (NK)-cell neoplasms, and Hodgkin lymphoma (HL). Both lymphomas and lymphoid leukemias are included in this classification because both solid and circulating phases are present in many lymphoid neoplasms and distinction between them is artificial. For example, B-cell chronic lymphocytic leukemia (CLL) and B-cell small lymphocytic lymphoma are simply different manifestations of the same neoplasm, as are lymphoblastic lymphomas and acute lymphocytic leukemias. Within the B-cell and T-cell categories, two subdivisions are recognized: precursor neoplasms, which correspond to the earliest stages of differentiation, and more mature differentiated neoplasms.[9,10]
B-cell neoplasms
- Precursor B-cell neoplasm: precursor B-acute lymphoblastic leukemia/lymphoblastic lymphoma (LBL).
- Peripheral B-cell neoplasms.
- B-cell CLL/small lymphocytic lymphoma.
- B-cell prolymphocytic leukemia.
- Lymphoplasmacytic lymphoma/immunocytoma.
- Mantle cell lymphoma.
- Follicular lymphoma.
- Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphatic tissue (MALT) type.
- Nodal marginal zone B-cell lymphoma (± monocytoid B cells).
- Splenic marginal zone lymphoma (± villous lymphocytes).
- Hairy cell leukemia.
- Plasmacytoma/plasma cell myeloma.
- Diffuse large B-cell lymphoma.
- Burkitt lymphoma.
T-cell and putative NK-cell neoplasms
- Precursor T-cell neoplasm: precursor T-acute lymphoblastic leukemia/LBL. For more information, see Acute Lymphoblastic Leukemia Treatment.
- Peripheral T-cell and NK-cell neoplasms.
- T-cell CLL/prolymphocytic leukemia.
- T-cell granular lymphocytic leukemia.
- Mycosis fungoides (including Sézary syndrome).
- Peripheral T-cell lymphoma, not otherwise specified.
- Hepatosplenic gamma/delta T-cell lymphoma.
- Subcutaneous panniculitis-like T-cell lymphoma.
- Extranodal T-/NK-cell lymphoma, nasal type.
- Nodal lymphomas of T follicular helper cell origin (including angioimmunoblastic T-cell lymphoma, follicular peripheral T-cell lymphoma, and nodal peripheral T-cell lymphoma with T follicular helper phenotype).
- Enteropathy-associated intestinal T-cell lymphoma.
- Monomorphic epitheliotropic intestinal T-cell lymphoma.
- Adult T-cell lymphoma/leukemia (human T-lymphotrophic virus [HTLV] 1+).
- Anaplastic large cell lymphoma, primary systemic type.
- Anaplastic large cell lymphoma, primary cutaneous type.
- Aggressive NK-cell leukemia.
HL
- Nodular lymphocyte-predominant HL.
- Classical HL.
- Nodular sclerosis HL.
- Lymphocyte-rich classical HL.
- Mixed-cellularity HL.
- Lymphocyte-depleted HL.
The REAL classification encompasses all lymphoproliferative neoplasms. For more information, see the following PDQ summaries:
- Acute Lymphoblastic Leukemia Treatment
- Aggressive B-Cell Non-Hodgkin Lymphoma Treatment
- AIDS-Related Lymphoma Treatment
- Chronic Lymphocytic Leukemia Treatment
- Hodgkin Lymphoma Treatment
- Hairy Cell Leukemia Treatment
- Indolent B-Cell Non-Hodgkin Lymphoma Treatment
- Mantle Cell Lymphoma Treatment
- Mycosis Fungoides and Other Cutaneous T-Cell Lymphomas Treatment
- Peripheral T-Cell Non-Hodgkin Lymphoma Treatment
- Plasma Cell Neoplasms (Including Multiple Myeloma) Treatment
- Primary Central Nervous System Lymphoma Treatment
References
- Zeppa P, Marino G, Troncone G, et al.: Fine-needle cytology and flow cytometry immunophenotyping and subclassification of non-Hodgkin lymphoma: a critical review of 307 cases with technical suggestions. Cancer 102 (1): 55-65, 2004. [PUBMED Abstract]
- Young NA, Al-Saleem T: Diagnosis of lymphoma by fine-needle aspiration cytology using the revised European-American classification of lymphoid neoplasms. Cancer 87 (6): 325-45, 1999. [PUBMED Abstract]
- National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin's Lymphoma Pathologic Classification Project. Cancer 49 (10): 2112-35, 1982. [PUBMED Abstract]
- Pugh WC: Is the working formulation adequate for the classification of the low grade lymphomas? Leuk Lymphoma 10 (Suppl 1): 1-8, 1993.
- Harris NL, Jaffe ES, Stein H, et al.: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 84 (5): 1361-92, 1994. [PUBMED Abstract]
- Pittaluga S, Bijnens L, Teodorovic I, et al.: Clinical analysis of 670 cases in two trials of the European Organization for the Research and Treatment of Cancer Lymphoma Cooperative Group subtyped according to the Revised European-American Classification of Lymphoid Neoplasms: a comparison with the Working Formulation. Blood 87 (10): 4358-67, 1996. [PUBMED Abstract]
- Armitage JO, Weisenburger DD: New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 16 (8): 2780-95, 1998. [PUBMED Abstract]
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood 89 (11): 3909-18, 1997. [PUBMED Abstract]
- Pileri SA, Milani M, Fraternali-Orcioni G, et al.: From the R.E.A.L. Classification to the upcoming WHO scheme: a step toward universal categorization of lymphoma entities? Ann Oncol 9 (6): 607-12, 1998. [PUBMED Abstract]
- Society for Hematopathology Program: Society for Hematopathology Program. Am J Surg Pathol 21 (1): 114-121, 1997.
Stage Information for Indolent B-Cell Non-Hodgkin Lymphoma
Stage is important in selecting a treatment for patients with non-Hodgkin lymphoma (NHL). Chest and abdominal computed tomography (CT) scans are usually part of the staging evaluation for all patients with lymphoma. The staging system for NHL is similar to the staging system used for Hodgkin lymphoma (HL).
It is common for patients with NHL to have involvement of the following sites:
- Noncontiguous lymph nodes.
- Waldeyer ring.
- Epitrochlear nodes.
- Gastrointestinal tract.
- Extranodal presentations. (A single extranodal site is occasionally the only site of involvement in patients with diffuse lymphoma.)
- Bone marrow.
- Liver (especially common in patients with low-grade lymphomas).
Cytological examination of cerebrospinal fluid may be positive in patients with aggressive NHL. Involvement of hilar and mediastinal lymph nodes is less common than in HL. Mediastinal adenopathy, however, is a prominent feature of lymphoblastic lymphoma and primary mediastinal B-cell lymphoma, entities primarily found in young adults.
Most patients with NHL present with advanced (stage III or stage IV) disease often identified by CT scans or biopsies of the bone marrow and other accessible sites of involvement. In a retrospective review of over 32,000 cases of lymphoma in France, up to 40% of diagnoses were made by core needle biopsy, and 60% were made by excisional biopsy.[1] After expert review, core needle biopsy provided a definite diagnosis in 92.3% of cases; excisional biopsy provided a definite diagnosis in 98.1% of cases (P < .0001). Laparoscopic biopsy or laparotomy is not required for staging but rarely may be necessary to establish a diagnosis or histological type.[2]
Positron emission tomography (PET) with fluorine F 18-fludeoxyglucose can be used for initial staging. It can also be used for follow-up after therapy as a supplement to CT scanning.[3] Multiple studies have demonstrated that interim PET scans after two to four cycles of therapy do not provide reliable prognostic information. A large cooperative group trial (ECOG-E344 [NCT00274924]) reported problems with interobserver reproducibility. Two prospective trials and one meta-analysis showed no differences in outcomes between PET-negative and PET-positive/biopsy-negative patients.[4-7]
In a retrospective study of 130 patients with diffuse large B-cell lymphoma, PET scanning identified all clinically important marrow involvement from lymphoma, and bone marrow biopsy did not upstage any patient's lymphoma.[8] A retrospective study of 580 patients with follicular lymphoma from seven National Cancer Institute–sponsored trials showed no improvement in assessing response to therapy when bone marrow biopsy was added to radiological imaging.[9] The workup of NHL should include bone marrow biopsy when management would change (e.g., determining limited stage vs. advanced stage) or when evaluating cytopenias.
For patients with follicular lymphoma, a positive PET result after therapy has a worse prognosis; however, it is unclear whether a positive PET result is predictive when further or different therapy is implemented.[10]
Staging Subclassification System
Lugano classification
The American Joint Committee on Cancer (AJCC) has adopted the Lugano classification to evaluate and stage lymphoma.[11] The Lugano classification system replaces the Ann Arbor classification system, which was adopted in 1971 at the Ann Arbor Conference,[12] with some modifications 18 years later from the Cotswolds meeting.[13,14]
| Stage | Stage Description | Illustration |
|---|---|---|
| CSF = cerebrospinal fluid; CT = computed tomography; DLBCL = diffuse large B-cell lymphoma; NHL = non-Hodgkin lymphoma. | ||
| aHodgkin and Non-Hodgkin Lymphomas. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 937–58. | ||
| bStage II bulky may be considered either early or advanced stage based on lymphoma histology and prognostic factors. | ||
| cThe definition of disease bulk varies according to lymphoma histology. In the Lugano classification, bulk ln Hodgkin lymphoma is defined as a mass greater than one-third of the thoracic diameter on CT of the chest or a mass >10 cm. For NHL, the recommended definitions of bulk vary by lymphoma histology. In follicular lymphoma, 6 cm has been suggested based on the Follicular Lymphoma International Prognostic Index-2 and its validation. In DLBCL, cutoffs ranging from 5 cm to 10 cm have been used, although 10 cm is recommended. | ||
| Limited stage | ||
| I | Involvement of a single lymphatic site (i.e., nodal region, Waldeyer’s ring, thymus, or spleen). | 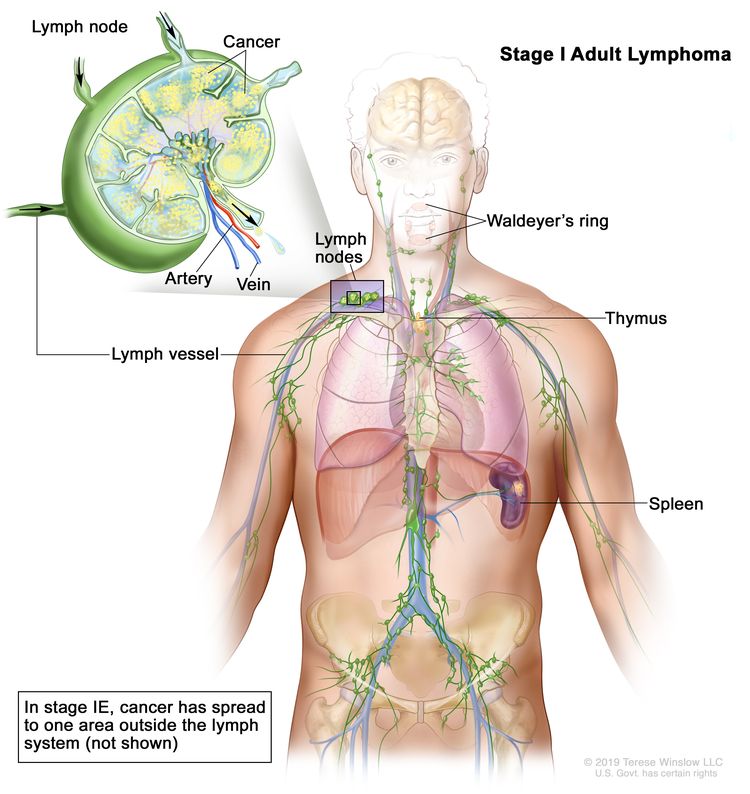 |
| IE | Single extralymphatic site in the absence of nodal involvement (rare in Hodgkin lymphoma). | |
| II | Involvement of two or more lymph node regions on the same side of the diaphragm. | 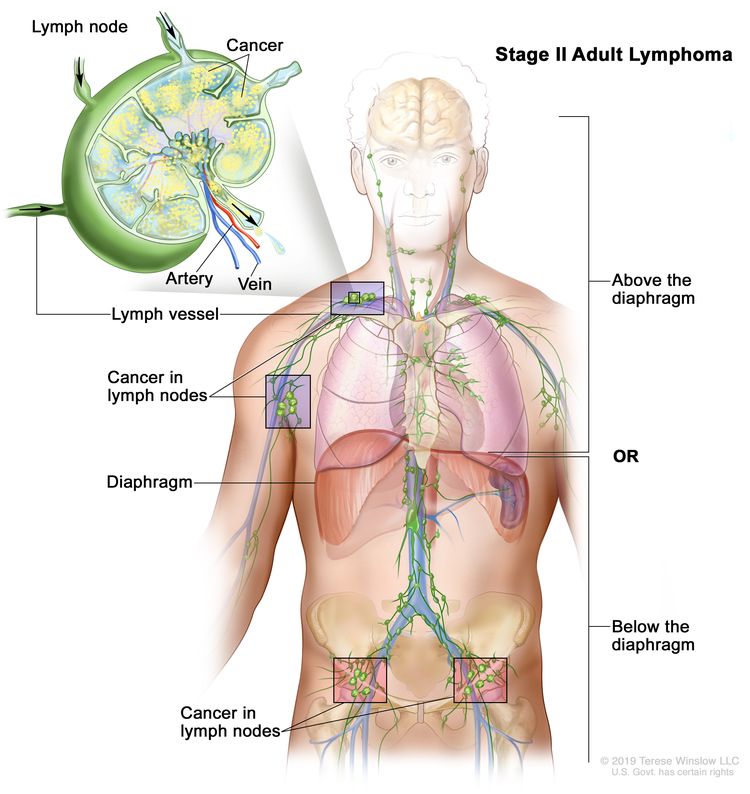 |
| IIE | Contiguous extralymphatic extension from a nodal site with or without involvement of other lymph node regions on the same side of the diaphragm. | 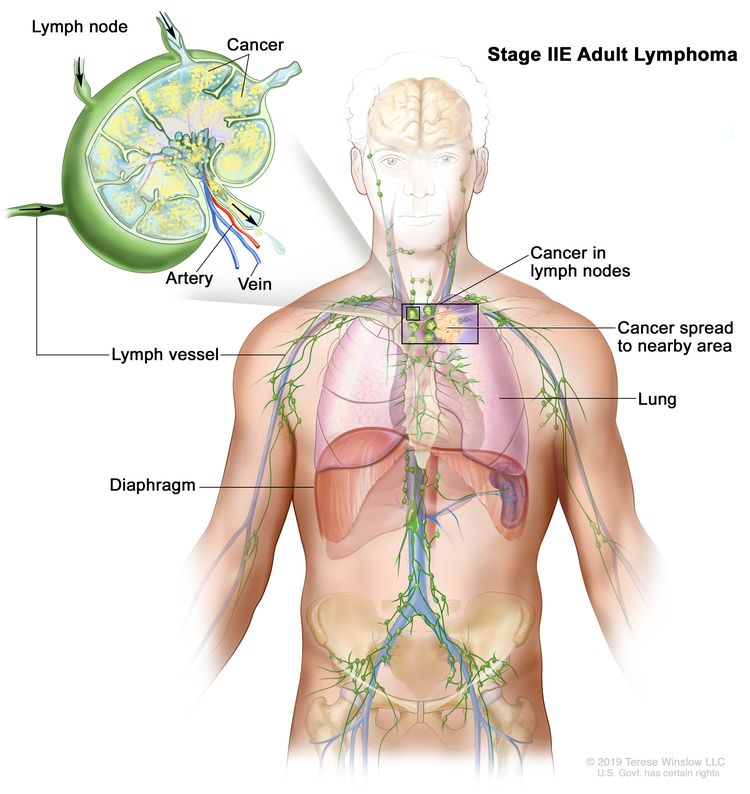 |
| II bulkyb | Stage II with disease bulk.c | |
| Advanced stage | ||
| III | Involvement of lymph node regions on both sides of the diaphragm; nodes above the diaphragm with spleen involvement. | 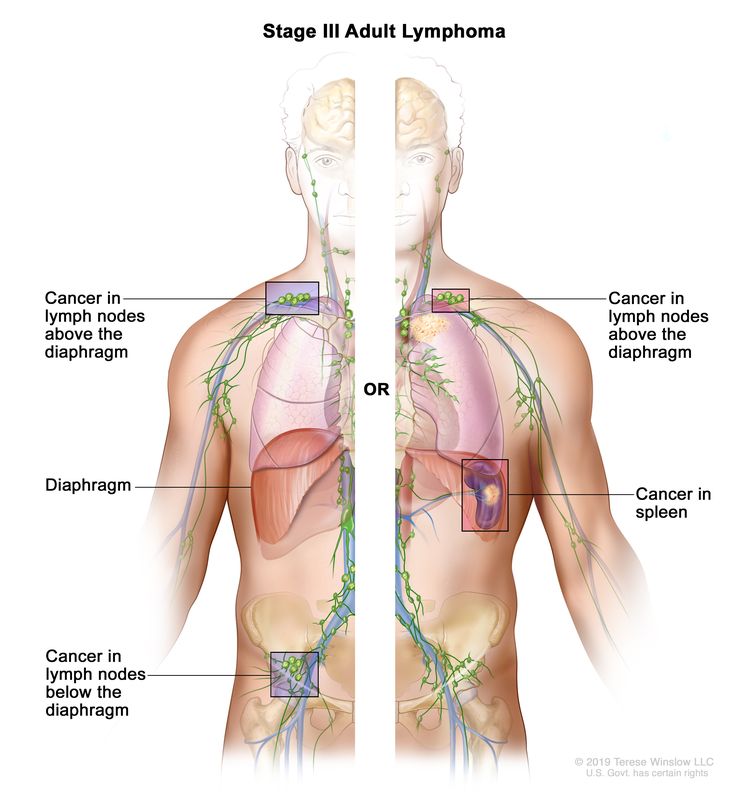 |
| IV | Diffuse or disseminated involvement of one or more extralymphatic organs, with or without associated lymph node involvement; or noncontiguous extralymphatic organ involvement in conjunction with nodal stage II disease; or any extralymphatic organ involvement in nodal stage III disease. Stage IV includes any involvement of the CSF, bone marrow, liver, or multiple lung lesions (other than by direct extension in stage IIE disease). | 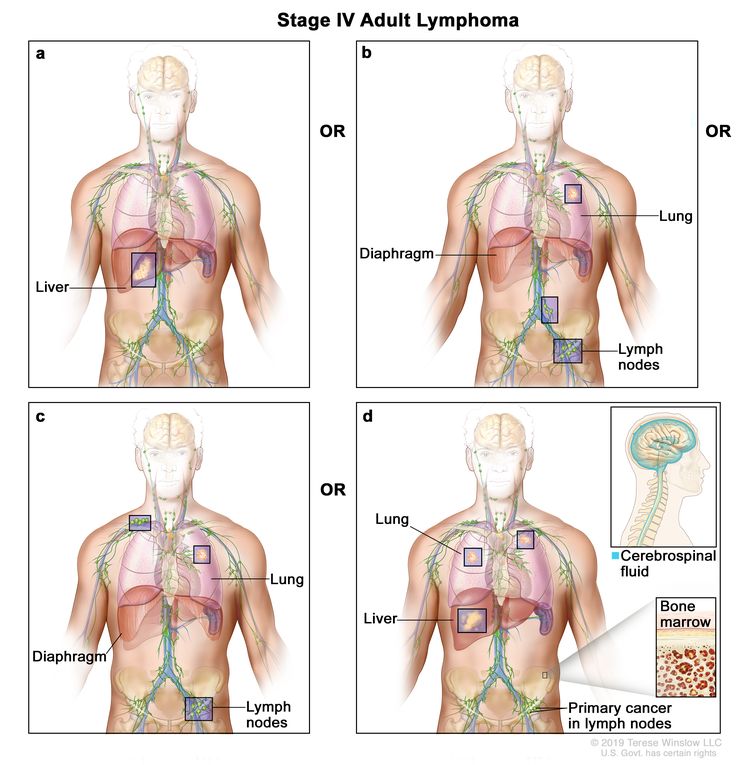 |
| Note: Hodgkin lymphoma uses A or B designation with stage group. A/B is no longer used in NHL. | ||
Occasionally, specialized staging systems are used. The physician should be aware of the system used in a specific report.
The E designation is used when extranodal lymphoid malignancies arise in tissues separate from, but near, the major lymphatic aggregates. Stage IV refers to disease that is diffusely spread throughout an extranodal site, such as the liver. If pathological proof of involvement of one or more extralymphatic sites has been documented, the symbol for the site of involvement, followed by a plus sign (+), is listed.
| N = nodes | H = liver | L = lung | M = bone marrow |
| S = spleen | P = pleura | O = bone | D = skin |
Current practice assigns a clinical stage based on the findings of the clinical evaluation and a pathological stage based on the findings from invasive procedures beyond the initial biopsy.
For example, on percutaneous biopsy, a patient with inguinal adenopathy and a positive lymphangiogram without systemic symptoms might have involvement of the liver and bone marrow. The precise stage of such a patient would be clinical stage IIA, pathological stage IVA(H+)(M+).
Several other factors that are not included in the above staging system are important for the staging and prognosis of patients with NHL. These factors include the following:
- Age.
- Performance status (PS).
- Tumor size.
- Lactate dehydrogenase (LDH) values.
- The number of extranodal sites.
References
- Syrykh C, Chaouat C, Poullot E, et al.: Lymph node excisions provide more precise lymphoma diagnoses than core biopsies: a French Lymphopath network survey. Blood 140 (24): 2573-2583, 2022. [PUBMED Abstract]
- Mann GB, Conlon KC, LaQuaglia M, et al.: Emerging role of laparoscopy in the diagnosis of lymphoma. J Clin Oncol 16 (5): 1909-15, 1998. [PUBMED Abstract]
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al.: Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32 (27): 3048-58, 2014. [PUBMED Abstract]
- Horning SJ, Juweid ME, Schöder H, et al.: Interim positron emission tomography scans in diffuse large B-cell lymphoma: an independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood 115 (4): 775-7; quiz 918, 2010. [PUBMED Abstract]
- Moskowitz CH, Schöder H, Teruya-Feldstein J, et al.: Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol 28 (11): 1896-903, 2010. [PUBMED Abstract]
- Pregno P, Chiappella A, Bellò M, et al.: Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood 119 (9): 2066-73, 2012. [PUBMED Abstract]
- Sun N, Zhao J, Qiao W, et al.: Predictive value of interim PET/CT in DLBCL treated with R-CHOP: meta-analysis. Biomed Res Int 2015: 648572, 2015. [PUBMED Abstract]
- Khan AB, Barrington SF, Mikhaeel NG, et al.: PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood 122 (1): 61-7, 2013. [PUBMED Abstract]
- Rutherford SC, Yin J, Pederson L, et al.: Relevance of Bone Marrow Biopsies for Response Assessment in US National Cancer Institute National Clinical Trials Network Follicular Lymphoma Clinical Trials. J Clin Oncol 41 (2): 336-342, 2023. [PUBMED Abstract]
- Pyo J, Won Kim K, Jacene HA, et al.: End-therapy positron emission tomography for treatment response assessment in follicular lymphoma: a systematic review and meta-analysis. Clin Cancer Res 19 (23): 6566-77, 2013. [PUBMED Abstract]
- Hodgkin and non-Hodgkin lymphoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 937–58.
- Carbone PP, Kaplan HS, Musshoff K, et al.: Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 31 (11): 1860-1, 1971. [PUBMED Abstract]
- Lister TA, Crowther D, Sutcliffe SB, et al.: Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 7 (11): 1630-6, 1989. [PUBMED Abstract]
- National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin's Lymphoma Pathologic Classification Project. Cancer 49 (10): 2112-35, 1982. [PUBMED Abstract]
Indolent B-Cell Non-Hodgkin Lymphoma
Indolent B-cell non-Hodgkin lymphoma (NHL) includes the following subtypes:
- Follicular lymphoma (grades 1–3a).
- Lymphoplasmacytic lymphoma (Waldenström macroglobulinemia).
- Marginal zone lymphoma.
Follicular Lymphoma (Grades 1–3a)
Follicular lymphoma makes up 20% of all NHL and as many as 70% of the indolent lymphomas reported in American and European clinical trials.[1-3] Most patients with follicular lymphoma are aged 50 years and older and present with widespread disease at diagnosis. Nodal involvement is most common and is often accompanied by splenic and bone marrow disease. Rearrangement of the BCL2 gene is present in more than 90% of patients with follicular lymphoma. Overexpression of the BCL2 protein is associated with the inability to eradicate the lymphoma by inhibiting apoptosis.[4]
Prognosis
Follicular lymphoma is designated as indolent because median survival ranges from 8 to 15 years, even in advanced stages.[5-7] Patients with advanced-stage follicular lymphoma are not cured with current therapeutic options. The rate of relapse is fairly consistent over time, even in patients who have achieved complete responses to treatment.[8] Watchful waiting, the deferring of treatment until the patient becomes symptomatic, is an option for patients with advanced-stage follicular lymphoma.[9,10] An international index for follicular lymphoma (the Follicular Lymphoma International Prognostic Index [FLIPI]) [11-13] identified five significant risk factors prognostic of overall survival (OS):
- Age (≤60 years vs. >60 years).
- Serum lactate dehydrogenase (LDH) (normal vs. elevated).
- Stage (stage I or stage II vs. stage III or stage IV).
- Hemoglobin level (≥120 g/L vs. <120 g/L).
- Number of nodal areas (≤4 vs. >4).
Patients with zero or one risk factor have a 10-year survival rate of 67%, and four or five risk factors confer a 10-year survival rate of 36%.[11] In a revised FLIPI-2, an elevated beta-2-microglobulin and lymph node size of more than 6 cm are proposed prognostic factors instead of serum LDH and the number of nodal areas.[14] Although the FLIPI and FLIPI-2 indices can predict progression-free survival (PFS) and OS, the scores cannot be used to establish the need for therapy or to predict response to therapy.[11,14] The primary use of FLIPI or FLIPI-2 is to assure a balance of prognostic factors or to define entry requirements in randomized clinical trials. Individuals with an adverse FLIPI score may well benefit from watchful waiting or may respond well to initial therapy. An alternative prognostic index using only beta-2-microglobulin and initial bone marrow involvement (PRIMA-PI) has the disadvantage of requiring an invasive test not usually required outside the context of a clinical trial.[15] The Follicular Lymphoma Evaluation Index (FLEX), an alternative prognostic index using only noninvasive clinical variables, outperformed FLIPI, FLIPI-2, and PRIMA-PI, using data from immunochemotherapy trials.[16] The nine clinical variables of the FLEX model include:
- Male sex.
- Histological grade 3a disease.
- More than two extranodal sites.
- Eastern Cooperative Oncology Group performance status score of 2 or higher.
- Hemoglobin level less than 120 g/L.
- Beta-2 microglobulin level above the upper limit of normal.
- LDH level above the upper limit of normal.
- Absolute blood natural killer cell count less than 100/μL.
- Bulky disease.
Three retrospective analyses, including one pooled analysis of 5,225 patients from 13 randomized clinical trials, identified a high-risk group that had a 5-year OS rate of 50% when relapses occurred within 24 months of induction chemoimmunotherapy.[17-19] A fourth retrospective analysis of 296 patients who received bendamustine and rituximab found a 2-year OS rate of 38% (95% confidence interval [CI], 20%−55%) among those with progression of disease before 24 months (POD24). Most of these patients (76%) had transformed disease (histological progression to diffuse large B-cell lymphoma [DLBCL]).[20] These patients with higher-risk POD24 disease represent a target population for clinical trials.
Therapeutic approaches
Because of the often-indolent clinical course and the lack of symptoms in some patients with follicular lymphoma, watchful waiting remains a standard of care during the initial encounter and for patients with slow asymptomatic relapsing disease. When therapy is required, numerous options may be used in varying sequences with an OS equivalence at 5 to 10 years.[9,21-23] Rituximab can be given alone or in combination with various chemotherapy options.[23-25] Rituximab can also be combined with the immunomodulating-agent lenalidomide to avoid the short- and long-term toxicities of cytotoxic agents.[26-28] Obinutuzumab is a completely humanized anti–CD20 monoclonal antibody that can be given alone or with combination chemotherapy. It may be especially useful for patients who develop severe allergic reactions to rituximab due to human antimouse antibodies.[29] CD19-directed chimeric antigen receptor T cells may be used in patients who have disease progression after two or more prior lines of therapy.[30] Mosunetuzumab, a bispecific CD20-directed CD3 T-cell engager, may also be used in this setting.[31] Consolidation therapy for relapsed disease after reinduction therapy using autologous stem cell transplant (SCT) or allogeneic SCT can be considered.[32]
Outside the context of clinical trials, the use of measurable residual disease (MRD) testing has not been shown to be predictive in directing therapy for patients with follicular lymphoma. In retrospective analyses of two randomized prospective trials, while MRD negativity was prognostic of outcome, maintenance rituximab or obinutuzumab prolonged PFS the most among patients with MRD-negative disease.[33,34][Level of evidence C2] Stopping maintenance rituximab or obinutuzumab was not indicated in patients with MRD-negative disease, negating any possible change in therapy based on that status.
Follicular lymphoma in situ and primary follicular lymphoma of the duodenum are particularly indolent variants that rarely progress or require therapy.[35,36] A so-called pediatric-type nodal follicular lymphoma has indolent behavior and rarely recurs; adult patients with this histological variant have disease characterized by a lack of BCL2 rearrangement in conjunction with a Ki-67 proliferation index greater than 30% and a localized stage I presentation.[37]
Patients with indolent lymphoma may experience a relapse with a more aggressive histology. If the clinical pattern of relapse suggests that the disease is behaving in a more aggressive manner, a biopsy can be performed, if feasible.[38] If disease conversion to a more aggressive histology is confirmed, therapy must change to a regimen applicable to that histological type.[39] Rapid growth or discordant growth between various disease sites may indicate a histological conversion.[38]
In a prospective nonrandomized study, at a median follow-up of 6.8 years, 379 of 2,652 patients (14%) subsequently transformed to a more aggressive histology after an initial diagnosis of follicular lymphoma.[40][Level of evidence C3] The median OS after subsequent transformation was 5 years. However, among 47 patients with evidence of transformation in conjunction with follicular lymphoma at the time of initial diagnosis, the OS was no worse than that of the nontransformed patients (5-year OS rate, 88%; 95% CI, 74%–95%).
Grade 3b follicular lymphoma is managed similarly to DLBCL. For more information, see Aggressive B-Cell Non-Hodgkin Lymphoma Treatment.
Lymphoplasmacytic Lymphoma (Waldenström Macroglobulinemia)
Lymphoplasmacytic lymphoma is usually associated with a monoclonal serum paraprotein of immunoglobulin M (IgM) type (Waldenström macroglobulinemia).[41] Most patients have bone marrow, lymph node, and splenic involvement, and some patients may develop hyperviscosity syndrome. Most patients with Waldenström macroglobulinemia carry the MYD88 variant, which some pathologists consider indicative for the disease.[42] Other lymphomas may also be associated with serum paraproteins. Patients with lymphoplasmacytic lymphoma should be checked for associated hepatitis C virus (HCV) infection.
Asymptomatic patients can be monitored for evidence of disease progression without immediate need for chemotherapy.[9,43,44]
Prognostic factors associated with symptoms requiring therapy include:
- Aged 70 years or older.
- Beta-2-microglobulin of 3 mg/dL or more.
- Increased serum LDH.[43]
An externally validated prognostic model uses age, albumin, and LDH levels.[45]
Therapeutic approaches
The management of lymphoplasmacytic lymphoma is similar to that of other low-grade lymphomas, especially diffuse small lymphocytic lymphoma/chronic lymphocytic leukemia.[46-48] If the serum viscosity relative to water is greater than four, the patient may have symptoms of hyperviscosity. Plasmapheresis is useful for temporary, acute symptoms such as retinopathy, congestive heart failure, and central nervous system (CNS) dysfunction. It can also be combined with chemotherapy for prolonged disease control. Symptomatic patients with a serum viscosity of four or lower are usually treated with chemoimmunotherapy or biologically directed therapies. Therapy may be required to correct hemolytic anemia in patients with chronic cold agglutinin disease; rituximab, bendamustine, and steroids are often used.[44] Occasionally, a heated room is required for patients whose cold agglutinins become activated by even minor chilling. Sutimlimab, an immunoglobulin G4 monoclonal antibody that selectively inhibits the complement pathway at C15, can reduce hemolysis when therapies directed at the lymphoplasmacytic lymphoma are ineffective.[49]
First-line regimens include zanubrutinib (a Bruton tyrosine kinase [BTK] inhibitor), rituximab, and ibrutinib (another BTK inhibitor), rituximab alone, the nucleoside analogues, and alkylating agents, either as single agents or as part of combination chemotherapy.[50-53] In a randomized prospective trial, 150 symptomatic patients (including patients with previously untreated and relapsing disease) received either ibrutinib and rituximab or rituximab and a placebo. With a median follow-up of 50 months, the 4.5-year PFS rate was 68% in the ibrutinib-and-rituximab arm (95% CI, 55%–78%) and 25% in the rituximab-and-placebo arm (95% CI, 15%–37%) (hazard ratio [HR], 0.25; 95% CI, 0.15–0.42; P < .0001). The OS rate at 30 months was no different in the two arms (92%–94%).[52][Level of evidence B1] Zanubrutinib was compared with ibrutinib in a randomized prospective clinical trial of 164 patients with relapsed disease and 38 with previously untreated disease.[53] With a median follow-up of 44.4 months, the PFS rate was similar in both groups at 70% to 78% (HR, 0.63; 95% CI, 0.36–1.12), and the OS rate was similar in both groups at 85% to 87% (HR, 0.75; 95% CI, 0.36–1.59).[54] The zanubrutinib group had fewer cases of atrial fibrillation (11 vs. 1) and 50% fewer cases of hypertension (statistics not provided).[53][Level of evidence C3] BTK inhibition with ibrutinib allowed all 13 patients with cold-antibody–mediated autoimmune hemolytic anemia and acrocyanosis to attain clinical remission regardless of underlying pathology or MYD88 variant status.[55][Level of evidence C3]
Previously untreated patients who received rituximab had response rates of 60% to 80%, but close monitoring of the serum IgM is required because of a sudden rise in this paraprotein at the start of therapy.[56-58][Level of evidence C3] The rise of IgM after rituximab can be avoided with the concomitant use of an alkylating agent, such as cyclophosphamide, or the proteosome inhibitors bortezomib or ixazomib.[44,59-61] A combination of bortezomib, dexamethasone, and rituximab has been used without causing IgM rebound.[62-64] Previously untreated patients with lymphoplasmacytic lymphoma who received the nucleoside analogues cladribine and fludarabine showed similar response rates.[51,65,66][Level of evidence C3] Patients who received single-agent alkylators, bendamustine, bortezomib, venetoclax, and combination chemotherapy with or without rituximab also showed similar response rates.[51,59,61,67-71][Level of evidence C3] In the rare case of lymphoplasmacytic lymphoma involving the CNS (Bing-Neel syndrome), ibrutinib resulted in an 85% response rate in an anecdotal series of 28 patients.[72][Level of evidence C3]
Myeloablative therapy with autologous or allogeneic hematopoietic stem cell support is under clinical evaluation.[73-76] Candidates for this approach should avoid long-term use of alkylating agents or purine nucleoside analogues, which can deplete hematopoietic stem cells or predispose patients to myelodysplasia or acute leukemia.[56,77] After relapse from alkylating-agent therapy, 92 patients with lymphoplasmacytic lymphoma were randomly assigned to receive either fludarabine or cyclophosphamide, doxorubicin, and prednisone. Although relapse-free survival favored fludarabine (median duration of 19 months vs. 3 months; P < .01), no difference was observed in OS.[78][Level of evidence B1]
Marginal Zone Lymphoma
When marginal zone lymphomas involve the nodes, they are called monocytoid B-cell lymphomas or nodal marginal zone B-cell lymphomas. When they involve extranodal sites (e.g., gastrointestinal tract, thyroid, lung, breast, orbit, and skin), they are called mucosa-associated lymphatic tissue (MALT) lymphomas.[79,80] Splenic marginal zone lymphoma is a distinct clinical entity, which usually presents with massive splenomegaly. A variant form of MALT lymphoma is known as immunoproliferative small intestinal disease (IPSID).[80] A prognostic index for all of the marginal zone lymphomas has three adverse prognostic factors: aged 70 years or older, stage III or stage IV disease, and high LDH level.[81] Fewer than 10% of patients transform to a higher-grade lymphoma. In one retrospective review, risk factors for transformation included elevated LDH, more than four nodal sites at the time of initial diagnosis of marginal zone lymphoma, and failure to achieve complete response after initial treatment.[82]
Gastric MALT
Many patients have a history of autoimmune disease, such as Hashimoto thyroiditis or Sjögren syndrome, or of Helicobacter gastritis. Most patients present with stage I or stage II extranodal disease, which is most often in the stomach. Treatment of H. pylori infection may resolve most cases of localized gastric involvement.[83,84] After standard antibiotic regimens, 50% of patients show resolution of gastric MALT by endoscopy after 3 months. Other patients may show resolution after 12 to 18 months of observation. Of the patients who attain complete remission, 30% demonstrate monoclonality by immunoglobulin heavy chain rearrangement on stomach biopsies with a 5-year median follow-up.[85] The clinical implication of this finding is unknown. Translocation t(11;18) in patients with gastric MALT predicts poor response to both antibiotic therapy and oral alkylator therapy, and predicts negative H. pylori testing results.[86-88] Patients with stable asymptomatic disease and persistently positive biopsies have been successfully followed with a watchful waiting approach until disease progression.[84] Patients with disease progression are treated with radiation therapy,[89-94] rituximab,[95] surgery (total gastrectomy or partial gastrectomy plus radiation therapy),[96] chemotherapy,[97] or combined-modality therapy.[98] A single-arm prospective trial enrolled 24 patients with newly diagnosed or relapsed H. pylori–negative gastric MALT.[94] Most patients had a complete response to radiation therapy at 4 Gy, and 20 Gy was applied as salvage therapy. The 3-year local control rate was 96% (95% CI, 88%–100%).[94][Level of evidence C3] Endoscopic ultrasonography may help clinicians monitor responses in these patients.[99] Four case series encompassing more than 100 patients with stage IE or IIE DLBCL with or without associated MALT (but H. pylori-positive) reported durable complete remissions in more than 50% of the patients after treatment of H. pylori.[100-103]
Extragastric MALT
Localized involvement of other sites can be treated with radiation therapy or surgery.[90-92,104-107] Patients with extragastric MALT lymphoma have a higher relapse rate than patients with gastric MALT lymphoma in some series, and relapses can happen many years and even decades later.[108] Many of these recurrences involve different MALT sites than the original location.[109] When disseminated to lymph nodes, bone marrow, or blood, this entity behaves like other low-grade lymphomas.[110,111] A prospective randomized trial of 401 patients with nongastric extranodal MALT compared chlorambucil alone versus rituximab plus chlorambucil versus rituximab alone.[112] With a median follow-up of 7.4 years, the event-free survival was 68% in the rituximab-plus-chlorambucil arm, 51% in the rituximab-alone arm, and 50% in the chlorambucil-alone arm (P = .0009). However, the 5-year OS rate was 90% in all arms.[112] For patients with ocular adnexal MALT, antibiotic therapy using doxycycline that targeted Chlamydia psittaci resulted in durable remissions for almost one-half of the patients in a review of the literature that included 131 patients.[113][Level of evidence C3] These responses to doxycycline are mainly seen in Italian trials and less often in trials conducted in other geographic sites.[114] Large B-cell lymphomas of MALT sites are classified and treated as diffuse large cell lymphomas.[115] A large, retrospective review of primary ocular adnexal MALT found that after 10 years of follow-up, 4% of stage I patients treated with radiation therapy had disease transformation to DLBCL, and 3% developed CNS involvement.[116]
Nodal marginal zone lymphoma
Patients with nodal marginal zone lymphoma (monocytoid B-cell lymphoma) are treated with watchful waiting or therapies as described for lymphoplasmacytic lymphoma. Rituximab alone, obinutuzumab alone, or combinations with cytotoxic agents (such as bendamustine, CVP [cyclophosphamide, vincristine, and prednisone] or CHOP [cyclophosphamide, doxorubicin, vincristine, and prednisone]) can be used.[117-119][Level of evidence C3] Zanubrutinib is approved for patients with disease relapse after a rituximab-containing regimen. This approval was based on a single-arm phase II study.[120] With a median follow-up of 15.7 months, the overall response rate was 68.2%, and the complete response rate was 25.8%. The median duration of response was 93% at 12 months.[120][Level of evidence C3] Ibrutinib also showed similar efficacy in patients with relapsed marginal zone lymphoma.[121][Level of evidence C3] Patients with marginal zone lymphoma comprised a small percentage (about 15%) of the population for a trial that established the benefit of rituximab with lenalidomide in patients with relapsed or refractory indolent lymphoma (AUGMENT trial).[122][Level of evidence C3] Similar to follicular lymphoma, patients with POD24 who required initiation of therapy had a worse prognosis than did the patients without POD24 (3-year OS rate, 53% vs. 95%).[123] Among patients with concomitant HCV infection, 40% to 60% attained a complete or partial remission after loss of detectable HCV RNA with antiviral treatment.[124,125][Level of evidence C3]
Mediterranean abdominal lymphoma
The disease variously known as Mediterranean abdominal lymphoma, heavy-chain disease, or IPSID, which occurs in young adults in eastern Mediterranean countries, is another version of MALT lymphoma. This disease responds to antibiotics in its early stages.[126] Campylobacter jejuni has been identified as one of the bacterial species associated with IPSID, and antibiotic therapy may result in disease remission.[127]
Splenic marginal zone lymphoma
Splenic marginal zone lymphoma is an indolent lymphoma that is marked by massive splenomegaly and peripheral blood and bone marrow involvement, usually without adenopathy.[128,129] This type of lymphoma is also known as splenic lymphoma with villous lymphocytes. Splenectomy may result in prolonged remission.[130,131]
Management is similar to that of other low-grade lymphomas and usually involves rituximab alone or rituximab in combination with purine analogues or alkylating agent chemotherapy.[132] Splenic marginal zone lymphoma responds less well to chemotherapy, which would ordinarily be effective for chronic lymphocytic leukemia.[128,132,133] Among small numbers of patients with splenic marginal zone lymphoma (splenic lymphoma with villous lymphocytes) and HCV infection, most attained a complete or partial remission after loss of detectable HCV RNA with treatment using interferon alfa with or without ribavirin.[124,134]; [135][Level of evidence C3] In contrast, no responses to interferon were seen in six HCV-negative patients.
References
- Armitage JO, Weisenburger DD: New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 16 (8): 2780-95, 1998. [PUBMED Abstract]
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood 89 (11): 3909-18, 1997. [PUBMED Abstract]
- Society for Hematopathology Program: Society for Hematopathology Program. Am J Surg Pathol 21 (1): 114-121, 1997.
- López-Guillermo A, Cabanillas F, McDonnell TI, et al.: Correlation of bcl-2 rearrangement with clinical characteristics and outcome in indolent follicular lymphoma. Blood 93 (9): 3081-7, 1999. [PUBMED Abstract]
- Peterson BA, Petroni GR, Frizzera G, et al.: Prolonged single-agent versus combination chemotherapy in indolent follicular lymphomas: a study of the cancer and leukemia group B. J Clin Oncol 21 (1): 5-15, 2003. [PUBMED Abstract]
- Swenson WT, Wooldridge JE, Lynch CF, et al.: Improved survival of follicular lymphoma patients in the United States. J Clin Oncol 23 (22): 5019-26, 2005. [PUBMED Abstract]
- Liu Q, Fayad L, Cabanillas F, et al.: Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas M.D. Anderson Cancer Center. J Clin Oncol 24 (10): 1582-9, 2006. [PUBMED Abstract]
- Kahl BS, Yang DT: Follicular lymphoma: evolving therapeutic strategies. Blood 127 (17): 2055-63, 2016. [PUBMED Abstract]
- Ardeshna KM, Smith P, Norton A, et al.: Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet 362 (9383): 516-22, 2003. [PUBMED Abstract]
- Armitage JO, Longo DL: Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood 127 (23): 2804-8, 2016. [PUBMED Abstract]
- Solal-Céligny P, Roy P, Colombat P, et al.: Follicular lymphoma international prognostic index. Blood 104 (5): 1258-65, 2004. [PUBMED Abstract]
- Perea G, Altés A, Montoto S, et al.: Prognostic indexes in follicular lymphoma: a comparison of different prognostic systems. Ann Oncol 16 (9): 1508-13, 2005. [PUBMED Abstract]
- Buske C, Hoster E, Dreyling M, et al.: The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood 108 (5): 1504-8, 2006. [PUBMED Abstract]
- Federico M, Bellei M, Marcheselli L, et al.: Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 27 (27): 4555-62, 2009. [PUBMED Abstract]
- Bachy E, Maurer MJ, Habermann TM, et al.: A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood 132 (1): 49-58, 2018. [PUBMED Abstract]
- Mir F, Mattiello F, Grigg A, et al.: Follicular Lymphoma Evaluation Index (FLEX): A new clinical prognostic model that is superior to existing risk scores for predicting progression-free survival and early treatment failure after frontline immunochemotherapy. Am J Hematol 95 (12): 1503-1510, 2020. [PUBMED Abstract]
- Casulo C, Byrtek M, Dawson KL, et al.: Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol 33 (23): 2516-22, 2015. [PUBMED Abstract]
- Shi Q, Flowers CR, Hiddemann W, et al.: Thirty-Month Complete Response as a Surrogate End Point in First-Line Follicular Lymphoma Therapy: An Individual Patient-Level Analysis of Multiple Randomized Trials. J Clin Oncol 35 (5): 552-560, 2017. [PUBMED Abstract]
- Casulo C, Dixon JG, Le-Rademacher J, et al.: Validation of POD24 as a robust early clinical end point of poor survival in FL from 5225 patients on 13 clinical trials. Blood 139 (11): 1684-1693, 2022. [PUBMED Abstract]
- Freeman CL, Kridel R, Moccia AA, et al.: Early progression after bendamustine-rituximab is associated with high risk of transformation in advanced stage follicular lymphoma. Blood 134 (9): 761-764, 2019. [PUBMED Abstract]
- Brice P, Bastion Y, Lepage E, et al.: Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 15 (3): 1110-7, 1997. [PUBMED Abstract]
- Young RC, Longo DL, Glatstein E, et al.: The treatment of indolent lymphomas: watchful waiting v aggressive combined modality treatment. Semin Hematol 25 (2 Suppl 2): 11-6, 1988. [PUBMED Abstract]
- Luminari S, Ferrari A, Manni M, et al.: Long-Term Results of the FOLL05 Trial Comparing R-CVP Versus R-CHOP Versus R-FM for the Initial Treatment of Patients With Advanced-Stage Symptomatic Follicular Lymphoma. J Clin Oncol 36 (7): 689-696, 2018. [PUBMED Abstract]
- Lockmer S, Østenstad B, Hagberg H, et al.: Chemotherapy-Free Initial Treatment of Advanced Indolent Lymphoma Has Durable Effect With Low Toxicity: Results From Two Nordic Lymphoma Group Trials With More Than 10 Years of Follow-Up. J Clin Oncol : JCO1800262, 2018. [PUBMED Abstract]
- Cartron G, Bachy E, Tilly H, et al.: Randomized Phase III Trial Evaluating Subcutaneous Rituximab for the First-Line Treatment of Low-Tumor Burden Follicular Lymphoma: Results of a LYSA Study. J Clin Oncol 41 (19): 3523-3533, 2023. [PUBMED Abstract]
- Morschhauser F, Fowler NH, Feugier P, et al.: Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N Engl J Med 379 (10): 934-947, 2018. [PUBMED Abstract]
- Leonard JP, Trnený M, Izutsu K, et al.: Augment: a phase III randomized study of lenalidomide Plus rituximab (R2) vs rituximab/placebo in patients with relapsed/refractory indolent non-Hodgkin lymphoma. [Abstract] Blood 132 (Suppl 1): A-445, 2018.
- Zucca E, Rondeau S, Vanazzi A, et al.: Short regimen of rituximab plus lenalidomide in follicular lymphoma patients in need of first-line therapy. Blood 134 (4): 353-362, 2019. [PUBMED Abstract]
- Marcus R, Davies A, Ando K, et al.: Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med 377 (14): 1331-1344, 2017. [PUBMED Abstract]
- Jacobson CA, Chavez JC, Sehgal AR, et al.: Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol 23 (1): 91-103, 2022. [PUBMED Abstract]
- Bartlett NL, Sehn LH, Matasar MJ, et al.: Mosunetuzumab monotherapy demonstrates durable efficacy with a manageable safety profile in patients with relapsed/refractory follicular lymphoma who received ≥2 prior therapies: updated results from a pivotal phase II study. [Abstract] Blood 140 (Suppl 1): A-610, 1467-70, 2022.
- Schaaf M, Reiser M, Borchmann P, et al.: High-dose therapy with autologous stem cell transplantation versus chemotherapy or immuno-chemotherapy for follicular lymphoma in adults. Cochrane Database Syst Rev 1: CD007678, 2012. [PUBMED Abstract]
- Luminari S, Manni M, Galimberti S, et al.: Response-Adapted Postinduction Strategy in Patients With Advanced-Stage Follicular Lymphoma: The FOLL12 Study. J Clin Oncol 40 (7): 729-739, 2022. [PUBMED Abstract]
- Pott C, Jurinovic V, Trotman J, et al.: Minimal Residual Disease Status Predicts Outcome in Patients With Previously Untreated Follicular Lymphoma: A Prospective Analysis of the Phase III GALLIUM Study. J Clin Oncol 42 (5): 550-561, 2024. [PUBMED Abstract]
- Schmatz AI, Streubel B, Kretschmer-Chott E, et al.: Primary follicular lymphoma of the duodenum is a distinct mucosal/submucosal variant of follicular lymphoma: a retrospective study of 63 cases. J Clin Oncol 29 (11): 1445-51, 2011. [PUBMED Abstract]
- Jegalian AG, Eberle FC, Pack SD, et al.: Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood 118 (11): 2976-84, 2011. [PUBMED Abstract]
- Louissaint A, Ackerman AM, Dias-Santagata D, et al.: Pediatric-type nodal follicular lymphoma: an indolent clonal proliferation in children and adults with high proliferation index and no BCL2 rearrangement. Blood 120 (12): 2395-404, 2012. [PUBMED Abstract]
- Sarkozy C, Trneny M, Xerri L, et al.: Risk Factors and Outcomes for Patients With Follicular Lymphoma Who Had Histologic Transformation After Response to First-Line Immunochemotherapy in the PRIMA Trial. J Clin Oncol 34 (22): 2575-82, 2016. [PUBMED Abstract]
- Tsimberidou AM, O'Brien S, Khouri I, et al.: Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol 24 (15): 2343-51, 2006. [PUBMED Abstract]
- Wagner-Johnston ND, Link BK, Byrtek M, et al.: Outcomes of transformed follicular lymphoma in the modern era: a report from the National LymphoCare Study (NLCS). Blood 126 (7): 851-7, 2015. [PUBMED Abstract]
- Leblond V, Kastritis E, Advani R, et al.: Treatment recommendations from the Eighth International Workshop on Waldenström's Macroglobulinemia. Blood 128 (10): 1321-8, 2016. [PUBMED Abstract]
- Treon SP, Xu L, Yang G, et al.: MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N Engl J Med 367 (9): 826-33, 2012. [PUBMED Abstract]
- Dhodapkar MV, Hoering A, Gertz MA, et al.: Long-term survival in Waldenstrom macroglobulinemia: 10-year follow-up of Southwest Oncology Group-directed intergroup trial S9003. Blood 113 (4): 793-6, 2009. [PUBMED Abstract]
- Ansell SM, Kyle RA, Reeder CB, et al.: Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines. Mayo Clin Proc 85 (9): 824-33, 2010. [PUBMED Abstract]
- Zanwar S, Le-Rademacher J, Durot E, et al.: Simplified Risk Stratification Model for Patients With Waldenström Macroglobulinemia. J Clin Oncol 42 (21): 2527-2536, 2024. [PUBMED Abstract]
- Kapoor P, Ansell SM, Fonseca R, et al.: Diagnosis and Management of Waldenström Macroglobulinemia: Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy (mSMART) Guidelines 2016. JAMA Oncol 3 (9): 1257-1265, 2017. [PUBMED Abstract]
- Dimopoulos MA, Kastritis E: How I treat Waldenström macroglobulinemia. Blood 134 (23): 2022-2035, 2019. [PUBMED Abstract]
- Gertz MA: Waldenstrom Macroglobulinemia: Tailoring Therapy for the Individual. J Clin Oncol 40 (23): 2600-2608, 2022. [PUBMED Abstract]
- Röth A, Berentsen S, Barcellini W, et al.: Sutimlimab in patients with cold agglutinin disease: results of the randomized placebo-controlled phase 3 CADENZA trial. Blood 140 (9): 980-991, 2022. [PUBMED Abstract]
- Buske C, Dimopoulos MA, Grunenberg A, et al.: Bortezomib-Dexamethasone, Rituximab, and Cyclophosphamide as First-Line Treatment for Waldenström's Macroglobulinemia: A Prospectively Randomized Trial of the European Consortium for Waldenström's Macroglobulinemia. J Clin Oncol 41 (14): 2607-2616, 2023. [PUBMED Abstract]
- Leblond V, Johnson S, Chevret S, et al.: Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenström macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J Clin Oncol 31 (3): 301-7, 2013. [PUBMED Abstract]
- Buske C, Tedeschi A, Trotman J, et al.: Ibrutinib Plus Rituximab Versus Placebo Plus Rituximab for Waldenström's Macroglobulinemia: Final Analysis From the Randomized Phase III iNNOVATE Study. J Clin Oncol 40 (1): 52-62, 2022. [PUBMED Abstract]
- Tam CS, Opat S, D'Sa S, et al.: A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 136 (18): 2038-2050, 2020. [PUBMED Abstract]
- Dimopoulos MA, Opat S, D'Sa S, et al.: Zanubrutinib Versus Ibrutinib in Symptomatic Waldenström Macroglobulinemia: Final Analysis From the Randomized Phase III ASPEN Study. J Clin Oncol 41 (33): 5099-5106, 2023. [PUBMED Abstract]
- Jalink M, Berentsen S, Castillo JJ, et al.: Effect of ibrutinib treatment on hemolytic anemia and acrocyanosis in cold agglutinin disease/cold agglutinin syndrome. Blood 138 (20): 2002-2005, 2021. [PUBMED Abstract]
- Gertz MA, Anagnostopoulos A, Anderson K, et al.: Treatment recommendations in Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol 30 (2): 121-6, 2003. [PUBMED Abstract]
- Dimopoulos MA, Zervas C, Zomas A, et al.: Treatment of Waldenström's macroglobulinemia with rituximab. J Clin Oncol 20 (9): 2327-33, 2002. [PUBMED Abstract]
- Treon SP, Branagan AR, Hunter Z, et al.: Paradoxical increases in serum IgM and viscosity levels following rituximab in Waldenstrom's macroglobulinemia. Ann Oncol 15 (10): 1481-3, 2004. [PUBMED Abstract]
- Dimopoulos MA, Chen C, Kastritis E, et al.: Bortezomib as a treatment option in patients with Waldenström macroglobulinemia. Clin Lymphoma Myeloma Leuk 10 (2): 110-7, 2010. [PUBMED Abstract]
- Gavriatopoulou M, García-Sanz R, Kastritis E, et al.: BDR in newly diagnosed patients with WM: final analysis of a phase 2 study after a minimum follow-up of 6 years. Blood 129 (4): 456-459, 2017. [PUBMED Abstract]
- Kersten MJ, Amaador K, Minnema MC, et al.: Combining Ixazomib With Subcutaneous Rituximab and Dexamethasone in Relapsed or Refractory Waldenström's Macroglobulinemia: Final Analysis of the Phase I/II HOVON124/ECWM-R2 Study. J Clin Oncol 40 (1): 40-51, 2022. [PUBMED Abstract]
- Treon SP, Ioakimidis L, Soumerai JD, et al.: Primary therapy of Waldenström macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J Clin Oncol 27 (23): 3830-5, 2009. [PUBMED Abstract]
- Dimopoulos MA, García-Sanz R, Gavriatopoulou M, et al.: Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood 122 (19): 3276-82, 2013. [PUBMED Abstract]
- Treon SP, Tripsas CK, Meid K, et al.: Carfilzomib, rituximab, and dexamethasone (CaRD) treatment offers a neuropathy-sparing approach for treating Waldenström's macroglobulinemia. Blood 124 (4): 503-10, 2014. [PUBMED Abstract]
- Dimopoulos MA, Alexanian R: Waldenstrom's macroglobulinemia. Blood 83 (6): 1452-9, 1994. [PUBMED Abstract]
- Laszlo D, Andreola G, Rigacci L, et al.: Rituximab and subcutaneous 2-chloro-2'-deoxyadenosine combination treatment for patients with Waldenstrom macroglobulinemia: clinical and biologic results of a phase II multicenter study. J Clin Oncol 28 (13): 2233-8, 2010. [PUBMED Abstract]
- García-Sanz R, Montoto S, Torrequebrada A, et al.: Waldenström macroglobulinaemia: presenting features and outcome in a series with 217 cases. Br J Haematol 115 (3): 575-82, 2001. [PUBMED Abstract]
- Buske C, Hoster E, Dreyling M, et al.: The addition of rituximab to front-line therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG). Leukemia 23 (1): 153-61, 2009. [PUBMED Abstract]
- Ghobrial IM, Hong F, Padmanabhan S, et al.: Phase II trial of weekly bortezomib in combination with rituximab in relapsed or relapsed and refractory Waldenstrom macroglobulinemia. J Clin Oncol 28 (8): 1422-8, 2010. [PUBMED Abstract]
- Rummel MJ, Niederle N, Maschmeyer G, et al.: Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381 (9873): 1203-10, 2013. [PUBMED Abstract]
- Castillo JJ, Allan JN, Siddiqi T, et al.: Venetoclax in Previously Treated Waldenström Macroglobulinemia. J Clin Oncol 40 (1): 63-71, 2022. [PUBMED Abstract]
- Castillo JJ, Itchaki G, Paludo J, et al.: Ibrutinib for the treatment of Bing-Neel syndrome: a multicenter study. Blood 133 (4): 299-305, 2019. [PUBMED Abstract]
- Dreger P, Glass B, Kuse R, et al.: Myeloablative radiochemotherapy followed by reinfusion of purged autologous stem cells for Waldenström's macroglobulinaemia. Br J Haematol 106 (1): 115-8, 1999. [PUBMED Abstract]
- Desikan R, Dhodapkar M, Siegel D, et al.: High-dose therapy with autologous haemopoietic stem cell support for Waldenström's macroglobulinaemia. Br J Haematol 105 (4): 993-6, 1999. [PUBMED Abstract]
- Martin P, Chadburn A, Christos P, et al.: Intensive treatment strategies may not provide superior outcomes in mantle cell lymphoma: overall survival exceeding 7 years with standard therapies. Ann Oncol 19 (7): 1327-30, 2008. [PUBMED Abstract]
- Kyriakou C, Canals C, Cornelissen JJ, et al.: Allogeneic stem-cell transplantation in patients with Waldenström macroglobulinemia: report from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 28 (33): 4926-34, 2010. [PUBMED Abstract]
- Leleu X, Soumerai J, Roccaro A, et al.: Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenström macroglobulinemia treated with nucleoside analogs. J Clin Oncol 27 (2): 250-5, 2009. [PUBMED Abstract]
- Leblond V, Lévy V, Maloisel F, et al.: Multicenter, randomized comparative trial of fludarabine and the combination of cyclophosphamide-doxorubicin-prednisone in 92 patients with Waldenström macroglobulinemia in first relapse or with primary refractory disease. Blood 98 (9): 2640-4, 2001. [PUBMED Abstract]
- Zucca E, Bertoni F: The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood 127 (17): 2082-92, 2016. [PUBMED Abstract]
- Rossi D, Bertoni F, Zucca E: Marginal-Zone Lymphomas. N Engl J Med 386 (6): 568-581, 2022. [PUBMED Abstract]
- Thieblemont C, Cascione L, Conconi A, et al.: A MALT lymphoma prognostic index. Blood 130 (12): 1409-1417, 2017. [PUBMED Abstract]
- Alderuccio JP, Zhao W, Desai A, et al.: Risk Factors for Transformation to Higher-Grade Lymphoma and Its Impact on Survival in a Large Cohort of Patients With Marginal Zone Lymphoma From a Single Institution. J Clin Oncol : JCO1800138, 2018. [PUBMED Abstract]
- Zullo A, Hassan C, Andriani A, et al.: Eradication therapy for Helicobacter pylori in patients with gastric MALT lymphoma: a pooled data analysis. Am J Gastroenterol 104 (8): 1932-7; quiz 1938, 2009. [PUBMED Abstract]
- Nakamura S, Sugiyama T, Matsumoto T, et al.: Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut 61 (4): 507-13, 2012. [PUBMED Abstract]
- Wündisch T, Thiede C, Morgner A, et al.: Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 23 (31): 8018-24, 2005. [PUBMED Abstract]
- Ye H, Liu H, Raderer M, et al.: High incidence of t(11;18)(q21;q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood 101 (7): 2547-50, 2003. [PUBMED Abstract]
- Lévy M, Copie-Bergman C, Gameiro C, et al.: Prognostic value of translocation t(11;18) in tumoral response of low-grade gastric lymphoma of mucosa-associated lymphoid tissue type to oral chemotherapy. J Clin Oncol 23 (22): 5061-6, 2005. [PUBMED Abstract]
- Nakamura S, Ye H, Bacon CM, et al.: Clinical impact of genetic aberrations in gastric MALT lymphoma: a comprehensive analysis using interphase fluorescence in situ hybridisation. Gut 56 (10): 1358-63, 2007. [PUBMED Abstract]
- Schechter NR, Yahalom J: Low-grade MALT lymphoma of the stomach: a review of treatment options. Int J Radiat Oncol Biol Phys 46 (5): 1093-103, 2000. [PUBMED Abstract]
- Tsang RW, Gospodarowicz MK, Pintilie M, et al.: Stage I and II MALT lymphoma: results of treatment with radiotherapy. Int J Radiat Oncol Biol Phys 50 (5): 1258-64, 2001. [PUBMED Abstract]
- Tsang RW, Gospodarowicz MK, Pintilie M, et al.: Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 21 (22): 4157-64, 2003. [PUBMED Abstract]
- Tsai HK, Li S, Ng AK, et al.: Role of radiation therapy in the treatment of stage I/II mucosa-associated lymphoid tissue lymphoma. Ann Oncol 18 (4): 672-8, 2007. [PUBMED Abstract]
- De Leo AN, Bates JE, Lockney NA, et al.: Radiotherapy in Early-stage Gastric MALT: Improved Survival Without Increased Cardiac Death. Am J Clin Oncol 43 (11): 770-775, 2020. [PUBMED Abstract]
- Gunther JR, Xu J, Bhutani MS, et al.: Response-adapted ultra-low-dose 4 Gy radiation as definitive therapy of gastric MALT lymphoma: a single-centre, pilot trial. Lancet Haematol 11 (7): e521-e529, 2024. [PUBMED Abstract]
- Martinelli G, Laszlo D, Ferreri AJ, et al.: Clinical activity of rituximab in gastric marginal zone non-Hodgkin's lymphoma resistant to or not eligible for anti-Helicobacter pylori therapy. J Clin Oncol 23 (9): 1979-83, 2005. [PUBMED Abstract]
- Cogliatti SB, Schmid U, Schumacher U, et al.: Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 101 (5): 1159-70, 1991. [PUBMED Abstract]
- Zinzani PL, Magagnoli M, Galieni P, et al.: Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol 17 (4): 1254, 1999. [PUBMED Abstract]
- Thieblemont C, Bastion Y, Berger F, et al.: Mucosa-associated lymphoid tissue gastrointestinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol 15 (4): 1624-30, 1997. [PUBMED Abstract]
- Pavlick AC, Gerdes H, Portlock CS: Endoscopic ultrasound in the evaluation of gastric small lymphocytic mucosa-associated lymphoid tumors. J Clin Oncol 15 (5): 1761-6, 1997. [PUBMED Abstract]
- Morgner A, Miehlke S, Fischbach W, et al.: Complete remission of primary high-grade B-cell gastric lymphoma after cure of Helicobacter pylori infection. J Clin Oncol 19 (7): 2041-8, 2001. [PUBMED Abstract]
- Chen LT, Lin JT, Shyu RY, et al.: Prospective study of Helicobacter pylori eradication therapy in stage I(E) high-grade mucosa-associated lymphoid tissue lymphoma of the stomach. J Clin Oncol 19 (22): 4245-51, 2001. [PUBMED Abstract]
- Chen LT, Lin JT, Tai JJ, et al.: Long-term results of anti-Helicobacter pylori therapy in early-stage gastric high-grade transformed MALT lymphoma. J Natl Cancer Inst 97 (18): 1345-53, 2005. [PUBMED Abstract]
- Kuo SH, Yeh KH, Wu MS, et al.: Helicobacter pylori eradication therapy is effective in the treatment of early-stage H pylori-positive gastric diffuse large B-cell lymphomas. Blood 119 (21): 4838-44; quiz 5057, 2012. [PUBMED Abstract]
- Uno T, Isobe K, Shikama N, et al.: Radiotherapy for extranodal, marginal zone, B-cell lymphoma of mucosa-associated lymphoid tissue originating in the ocular adnexa: a multiinstitutional, retrospective review of 50 patients. Cancer 98 (4): 865-71, 2003. [PUBMED Abstract]
- Bayraktar S, Bayraktar UD, Stefanovic A, et al.: Primary ocular adnexal mucosa-associated lymphoid tissue lymphoma (MALT): single institution experience in a large cohort of patients. Br J Haematol 152 (1): 72-80, 2011. [PUBMED Abstract]
- Stefanovic A, Lossos IS: Extranodal marginal zone lymphoma of the ocular adnexa. Blood 114 (3): 501-10, 2009. [PUBMED Abstract]
- Vazquez A, Khan MN, Sanghvi S, et al.: Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue of the salivary glands: a population-based study from 1994 to 2009. Head Neck 37 (1): 18-22, 2015. [PUBMED Abstract]
- Raderer M, Streubel B, Woehrer S, et al.: High relapse rate in patients with MALT lymphoma warrants lifelong follow-up. Clin Cancer Res 11 (9): 3349-52, 2005. [PUBMED Abstract]
- Sretenovic M, Colovic M, Jankovic G, et al.: More than a third of non-gastric malt lymphomas are disseminated at diagnosis: a single center survey. Eur J Haematol 82 (5): 373-80, 2009. [PUBMED Abstract]
- Nathwani BN, Drachenberg MR, Hernandez AM, et al.: Nodal monocytoid B-cell lymphoma (nodal marginal-zone B-cell lymphoma). Semin Hematol 36 (2): 128-38, 1999. [PUBMED Abstract]
- Raderer M, Wöhrer S, Streubel B, et al.: Assessment of disease dissemination in gastric compared with extragastric mucosa-associated lymphoid tissue lymphoma using extensive staging: a single-center experience. J Clin Oncol 24 (19): 3136-41, 2006. [PUBMED Abstract]
- Zucca E, Conconi A, Martinelli G, et al.: Final Results of the IELSG-19 Randomized Trial of Mucosa-Associated Lymphoid Tissue Lymphoma: Improved Event-Free and Progression-Free Survival With Rituximab Plus Chlorambucil Versus Either Chlorambucil or Rituximab Monotherapy. J Clin Oncol 35 (17): 1905-1912, 2017. [PUBMED Abstract]
- Kiesewetter B, Raderer M: Antibiotic therapy in nongastrointestinal MALT lymphoma: a review of the literature. Blood 122 (8): 1350-7, 2013. [PUBMED Abstract]
- Grünberger B, Hauff W, Lukas J, et al.: 'Blind' antibiotic treatment targeting Chlamydia is not effective in patients with MALT lymphoma of the ocular adnexa. Ann Oncol 17 (3): 484-7, 2006. [PUBMED Abstract]
- Kuo SH, Chen LT, Yeh KH, et al.: Nuclear expression of BCL10 or nuclear factor kappa B predicts Helicobacter pylori-independent status of early-stage, high-grade gastric mucosa-associated lymphoid tissue lymphomas. J Clin Oncol 22 (17): 3491-7, 2004. [PUBMED Abstract]
- Desai A, Joag MG, Lekakis L, et al.: Long-term course of patients with primary ocular adnexal MALT lymphoma: a large single-institution cohort study. Blood 129 (3): 324-332, 2017. [PUBMED Abstract]
- Herold M, Hoster E, Janssens A, et al.: Immunochemotherapy and Maintenance With Obinutuzumab or Rituximab in Patients With Previously Untreated Marginal Zone Lymphoma in the Randomized GALLIUM Trial. Hemasphere 6 (3): e699, 2022. [PUBMED Abstract]
- Salar A, Domingo-Domenech E, Panizo C, et al.: Long-term results of a phase 2 study of rituximab and bendamustine for mucosa-associated lymphoid tissue lymphoma. Blood 130 (15): 1772-1774, 2017. [PUBMED Abstract]
- Alderuccio JP, Arcaini L, Watkins MP, et al.: An international analysis evaluating frontline bendamustine with rituximab in extranodal marginal zone lymphoma. Blood Adv 6 (7): 2035-2044, 2022. [PUBMED Abstract]
- Opat S, Tedeschi A, Linton K, et al.: The MAGNOLIA Trial: Zanubrutinib, a Next-Generation Bruton Tyrosine Kinase Inhibitor, Demonstrates Safety and Efficacy in Relapsed/Refractory Marginal Zone Lymphoma. Clin Cancer Res 27 (23): 6323-6332, 2021. [PUBMED Abstract]
- Noy A, de Vos S, Coleman M, et al.: Durable ibrutinib responses in relapsed/refractory marginal zone lymphoma: long-term follow-up and biomarker analysis. Blood Adv 4 (22): 5773-5784, 2020. [PUBMED Abstract]
- Leonard JP, Trneny M, Izutsu K, et al.: AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol 37 (14): 1188-1199, 2019. [PUBMED Abstract]
- Luminari S, Merli M, Rattotti S, et al.: Early progression as a predictor of survival in marginal zone lymphomas: an analysis from the FIL-NF10 study. Blood 134 (10): 798-801, 2019. [PUBMED Abstract]
- Vallisa D, Bernuzzi P, Arcaini L, et al.: Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkin's lymphoma: a multicenter Italian experience. J Clin Oncol 23 (3): 468-73, 2005. [PUBMED Abstract]
- Merli M, Rattotti S, Spina M, et al.: Direct-Acting Antivirals as Primary Treatment for Hepatitis C Virus-Associated Indolent Non-Hodgkin Lymphomas: The BArT Study of the Fondazione Italiana Linfomi. J Clin Oncol 40 (35): 4060-4070, 2022. [PUBMED Abstract]
- Isaacson PG: Gastrointestinal lymphoma. Hum Pathol 25 (10): 1020-9, 1994. [PUBMED Abstract]
- Lecuit M, Abachin E, Martin A, et al.: Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 350 (3): 239-48, 2004. [PUBMED Abstract]
- Arcaini L, Paulli M, Boveri E, et al.: Splenic and nodal marginal zone lymphomas are indolent disorders at high hepatitis C virus seroprevalence with distinct presenting features but similar morphologic and phenotypic profiles. Cancer 100 (1): 107-15, 2004. [PUBMED Abstract]
- Arcaini L, Rossi D, Paulli M: Splenic marginal zone lymphoma: from genetics to management. Blood 127 (17): 2072-81, 2016. [PUBMED Abstract]
- Bertoni F, Zucca E: State-of-the-art therapeutics: marginal-zone lymphoma. J Clin Oncol 23 (26): 6415-20, 2005. [PUBMED Abstract]
- Parry-Jones N, Matutes E, Gruszka-Westwood AM, et al.: Prognostic features of splenic lymphoma with villous lymphocytes: a report on 129 patients. Br J Haematol 120 (5): 759-64, 2003. [PUBMED Abstract]
- Arcaini L, Lazzarino M, Colombo N, et al.: Splenic marginal zone lymphoma: a prognostic model for clinical use. Blood 107 (12): 4643-9, 2006. [PUBMED Abstract]
- Iannitto E, Ambrosetti A, Ammatuna E, et al.: Splenic marginal zone lymphoma with or without villous lymphocytes. Hematologic findings and outcomes in a series of 57 patients. Cancer 101 (9): 2050-7, 2004. [PUBMED Abstract]
- Hermine O, Lefrère F, Bronowicki JP, et al.: Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med 347 (2): 89-94, 2002. [PUBMED Abstract]
- Kelaidi C, Rollot F, Park S, et al.: Response to antiviral treatment in hepatitis C virus-associated marginal zone lymphomas. Leukemia 18 (10): 1711-6, 2004. [PUBMED Abstract]
Treatment Option Overview for Indolent B-Cell Non-Hodgkin Lymphoma
Treatment of indolent non-Hodgkin lymphoma (NHL) depends on the histological type and stage. Many of the improvements in survival have been made because of clinical trials that have attempted to improve on conventional or standard therapy.
In asymptomatic patients with indolent forms of advanced NHL, treatment may be deferred until the patient becomes symptomatic as the disease progresses. When treatment is deferred, the clinical course of patients with indolent NHL varies; frequent and careful observation is required so that effective treatment can be initiated when the clinical course of the disease accelerates. Some patients have a prolonged indolent course, but others have disease that rapidly evolves into more aggressive types of NHL that require immediate treatment.
Radiation techniques differ somewhat from those used in the treatment of Hodgkin lymphoma. The dose of radiation therapy usually varies from 25 Gy to 50 Gy and is dependent on factors that include the histological type of lymphoma, the patient’s stage and overall condition, the goal of treatment (curative or palliative), the proximity of sensitive surrounding organs, and whether the patient is being treated with radiation therapy alone or in combination with chemotherapy. Given the patterns of disease presentations and relapse, treatment may need to include unusual sites such as Waldeyer ring, epitrochlear nodes, or mesenteric nodes. The associated morbidity of the treatment must be considered carefully. Most patients who receive radiation are treated on only one side of the diaphragm. Localized presentations of extranodal NHL may be treated with involved-field techniques with significant (>50%) success.
In situations where mediastinal radiation therapy would encompass the left side of the heart or would increase breast cancer risk in young female patients, proton therapy may be considered to reduce radiation dose to organs at risk.[1] For more information, see the Superior Vena Cava Syndrome section in Cardiopulmonary Syndromes.
Even though existing treatments cure a significant fraction of patients with lymphoma, numerous clinical trials that explore treatment improvements are in progress. If possible, patients can be included in these studies. Standardized guidelines for response assessment have been suggested for use in clinical trials.[2]
Several retrospective reviews suggest that routine surveillance scans offer little to no value in patients with diffuse-large B-cell lymphoma (DLBCL) who have attained a clinical complete remission after induction therapy. Prognostic value is also difficult to identify for an interim positron emission tomography–computed tomography scan during induction therapy for DLBCL.[3-6]
Active hepatitis B or hepatitis C can be assessed before treatment with rituximab and/or chemotherapy.[7,8] Patients with detectable hepatitis B virus (HBV) benefit from prophylaxis with entecavir in the context of rituximab therapy.[9,10] Patients with a resolved HBV infection (defined as hepatitis B surface antigen-negative but hepatitis B core antibody-positive) are at risk of reactivation of HBV and require monitoring of HBV DNA. In a retrospective study of 326 patients, prophylactic nucleoside analogue therapy lowered HBV reactivation from 10.8% to 2.1%.[11] Similarly, prophylaxis for herpes zoster with acyclovir or valacyclovir and prophylaxis for Pneumocystis with trimethoprim/sulfamethoxazole or dapsone are usually given to patients receiving rituximab with or without combination chemotherapy. Long-term impaired immune health was evaluated in a retrospective cohort study of 21,690 survivors of DLBCL from the California Cancer Registry. Elevated incidence rate ratios were found up to 10 years later for pneumonia (10.8-fold), meningitis (5.3-fold), immunoglobulin deficiency (17.6-fold), and autoimmune cytopenias (12-fold).[12]
Among 2,508 patients in a Danish registry, the incidence of doxorubicin-induced congestive heart failure increased for 115 NHL survivors with a history of cardiac disease (hazard ratio [HR], 2.71; 95% confidence interval [CI], 1.15−6.36) and/or multiple cardiovascular risk factors (HR, 2.86; 95% CI, 1.56−5.23).[13]
References
- Dabaja BS, Hoppe BS, Plastaras JP, et al.: Proton therapy for adults with mediastinal lymphomas: the International Lymphoma Radiation Oncology Group guidelines. Blood 132 (16): 1635-1646, 2018. [PUBMED Abstract]
- Cheson BD, Horning SJ, Coiffier B, et al.: Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17 (4): 1244, 1999. [PUBMED Abstract]
- Mamot C, Klingbiel D, Hitz F, et al.: Final Results of a Prospective Evaluation of the Predictive Value of Interim Positron Emission Tomography in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP-14 (SAKK 38/07). J Clin Oncol 33 (23): 2523-9, 2015. [PUBMED Abstract]
- Thompson CA, Ghesquieres H, Maurer MJ, et al.: Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol 32 (31): 3506-12, 2014. [PUBMED Abstract]
- El-Galaly TC, Jakobsen LH, Hutchings M, et al.: Routine Imaging for Diffuse Large B-Cell Lymphoma in First Complete Remission Does Not Improve Post-Treatment Survival: A Danish-Swedish Population-Based Study. J Clin Oncol 33 (34): 3993-8, 2015. [PUBMED Abstract]
- Huntington SF, Svoboda J, Doshi JA: Cost-effectiveness analysis of routine surveillance imaging of patients with diffuse large B-cell lymphoma in first remission. J Clin Oncol 33 (13): 1467-74, 2015. [PUBMED Abstract]
- Niitsu N, Hagiwara Y, Tanae K, et al.: Prospective analysis of hepatitis B virus reactivation in patients with diffuse large B-cell lymphoma after rituximab combination chemotherapy. J Clin Oncol 28 (34): 5097-100, 2010. [PUBMED Abstract]
- Dong HJ, Ni LN, Sheng GF, et al.: Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol 57 (3): 209-14, 2013. [PUBMED Abstract]
- Huang YH, Hsiao LT, Hong YC, et al.: Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 31 (22): 2765-72, 2013. [PUBMED Abstract]
- Li H, Zhang HM, Chen LF, et al.: Prophylactic lamivudine to improve the outcome of HBsAg-positive lymphoma patients during chemotherapy: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 39 (1): 80-92, 2015. [PUBMED Abstract]
- Kusumoto S, Arcaini L, Hong X, et al.: Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood 133 (2): 137-146, 2019. [PUBMED Abstract]
- Shree T, Li Q, Glaser SL, et al.: Impaired Immune Health in Survivors of Diffuse Large B-Cell Lymphoma. J Clin Oncol 38 (15): 1664-1675, 2020. [PUBMED Abstract]
- Salz T, Zabor EC, de Nully Brown P, et al.: Preexisting Cardiovascular Risk and Subsequent Heart Failure Among Non-Hodgkin Lymphoma Survivors. J Clin Oncol 35 (34): 3837-3843, 2017. [PUBMED Abstract]
Treatment of Indolent Stage I and Indolent, Contiguous Stage II B-Cell Non-Hodgkin Lymphoma
Although localized presentations are uncommon in B-cell non-Hodgkin lymphoma (NHL), the goal of treatment is to cure the disease in patients with confirmed localized occurrence after undergoing appropriate staging.
Treatment Options for Indolent Stage I and Indolent, Contiguous Stage II B-Cell NHL
Treatment options for indolent stage I and indolent, contiguous stage II B-cell NHL include:
In a prospective randomized trial, 150 patients with stage I or stage II follicular lymphoma were randomly assigned to 30 Gy of involved-field radiation therapy alone or radiation therapy plus six cycles of R-CVP (rituximab, cyclophosphamide, vincristine, prednisolone). With a median follow-up of 9.6 years, the 10-year progression-free survival (PFS) rate favored combined-modality therapy, at 59% (95% confidence interval [CI], 46%–74%) versus 41% for radiation therapy alone (95% CI, 30%–57%) (P = .033). There was no difference in overall survival (OS) (87% and 95%; P = .40).[1][Level of evidence B1]
The National Lymphocare Study identified 471 patients with stage I follicular lymphoma. Of those patients, 206 were rigorously staged with a bone marrow aspirate and biopsy, and computed tomography (CT) scans or positron emission tomography (PET)-CT scans.[2] Nonrandomized treatments included radiation therapy (27%), rituximab-chemotherapy (R-chemotherapy) (28%), watchful waiting (17%), R-chemotherapy plus radiation therapy (13%), and rituximab alone (12%), although more than one-third of the patients started with watchful waiting. With a median follow-up of 57 months, PFS favored R-chemotherapy or R-chemotherapy plus radiation therapy, but OS was nearly identical, all over 90%.[2][Level of evidence C2] Clinical trials are required to answer the following questions:[3]
- If the PET-CT scan is clear after excisional biopsy, is watchful waiting or radiation therapy preferred?
- Should rituximab be added to radiation therapy for stage I follicular lymphoma?
- Is there any role for R-chemotherapy plus radiation therapy?
Radiation therapy
Long-term disease control within radiation fields can be achieved in a significant number of patients with indolent stage I or stage II NHL by using radiation therapy. This requires dosages of radiation that usually range from 25 Gy to 40 Gy to involved sites or to extended fields that cover adjacent nodal sites.[1,4-6] Almost one-half of all patients treated with radiation therapy alone will have out-of-field relapse within 10 years.[1,6,7]
A retrospective review of 512 patients from an international consortium evaluated patients with early-stage follicular lymphoma who received at least 24 Gy of localized radiation therapy at initial presentation. With a median follow-up of 52 months, 29.1% of patients developed recurrent lymphoma at a median of 23 months (range, 1−143 months).[8] With a median follow-up of 33 months after relapse, the 3-year OS rate was 91.4% after patients received subsequent systemic chemotherapy that involved rituximab.[8]
Very low-dose radiation therapy with 4 Gy (2 Gy × 2 fractions) can result in 50% remission rates for patients who cannot tolerate higher doses.[9] In a multicenter, randomized, prospective trial, 548 patients with follicular or marginal zone lymphoma received radiation therapy, either 4 Gy in 2 fractions or 24 Gy in 12 fractions.[10]
- At a median follow-up of 73.8 months, the 5-year local complete response rate was 89.9% (85.5%–93.1%) after 24 Gy and 70.4% (64.7%–75.4%) after 4 Gy (hazard ratio, 3.46; 95% CI, 2.25–5.33; P < .0001).[10]
- Although durable local control was superior for patients who received 24 Gy, the 4 Gy regimen was nearly comparable with reductions in radiation exposure, time undergoing therapy, and cost.
Rituximab with or without chemotherapy
When radiation therapy is contraindicated, or when an alternative treatment is preferred, patients with symptomatic disease who require therapy may receive rituximab with or without chemotherapy (as outlined below for more advanced-stage patients).[11] The value of adjuvant treatment with radiation to decrease relapse, plus rituximab (an anti–CD20 monoclonal antibody) either alone or in combination with chemotherapy, has been extrapolated from trials of patients with advanced-stage disease and has not been confirmed.[12,13]
Watchful waiting
Watchful waiting can be considered for asymptomatic patients.[14] Watchful waiting has never been compared with up-front radiation therapy in a prospective randomized trial. A retrospective analysis of the Surveillance, Epidemiology and End Results (SEER) Program database in patients diagnosed over a span of 30 years showed improved outcomes for up-front radiation therapy.[15]
Other therapies as designated for patients with advanced-stage disease
Patients with disease unable to be encompassed by radiation therapy are treated as outlined for patients with stage III or stage IV low-grade lymphoma.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- MacManus M, Fisher R, Roos D, et al.: Randomized Trial of Systemic Therapy After Involved-Field Radiotherapy in Patients With Early-Stage Follicular Lymphoma: TROG 99.03. J Clin Oncol 36 (29): 2918-2925, 2018. [PUBMED Abstract]
- Friedberg JW, Byrtek M, Link BK, et al.: Effectiveness of first-line management strategies for stage I follicular lymphoma: analysis of the National LymphoCare Study. J Clin Oncol 30 (27): 3368-75, 2012. [PUBMED Abstract]
- Montoto S: Management of localized-stage follicular lymphoma: changing the paradigm? J Clin Oncol 30 (27): 3328-9, 2012. [PUBMED Abstract]
- Haas RL, Poortmans P, de Jong D, et al.: High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J Clin Oncol 21 (13): 2474-80, 2003. [PUBMED Abstract]
- Guckenberger M, Alexandrow N, Flentje M: Radiotherapy alone for stage I-III low grade follicular lymphoma: long-term outcome and comparison of extended field and total nodal irradiation. Radiat Oncol 7: 103, 2012. [PUBMED Abstract]
- Brady JL, Binkley MS, Hajj C, et al.: Definitive radiotherapy for localized follicular lymphoma staged by 18F-FDG PET-CT: a collaborative study by ILROG. Blood 133 (3): 237-245, 2019. [PUBMED Abstract]
- Guadagnolo BA, Li S, Neuberg D, et al.: Long-term outcome and mortality trends in early-stage, Grade 1-2 follicular lymphoma treated with radiation therapy. Int J Radiat Oncol Biol Phys 64 (3): 928-34, 2006. [PUBMED Abstract]
- Binkley MS, Brady JL, Hajj C, et al.: Salvage Treatment and Survival for Relapsed Follicular Lymphoma Following Primary Radiation Therapy: A Collaborative Study on Behalf of ILROG. Int J Radiat Oncol Biol Phys 104 (3): 522-529, 2019. [PUBMED Abstract]
- Hoskin PJ, Kirkwood AA, Popova B, et al.: 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol 15 (4): 457-63, 2014. [PUBMED Abstract]
- Hoskin P, Popova B, Schofield O, et al.: 4 Gy versus 24 Gy radiotherapy for follicular and marginal zone lymphoma (FoRT): long-term follow-up of a multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol 22 (3): 332-340, 2021. [PUBMED Abstract]
- Cartron G, Bachy E, Tilly H, et al.: Randomized Phase III Trial Evaluating Subcutaneous Rituximab for the First-Line Treatment of Low-Tumor Burden Follicular Lymphoma: Results of a LYSA Study. J Clin Oncol 41 (19): 3523-3533, 2023. [PUBMED Abstract]
- Kelsey SM, Newland AC, Hudson GV, et al.: A British National Lymphoma Investigation randomised trial of single agent chlorambucil plus radiotherapy versus radiotherapy alone in low grade, localised non-Hodgkins lymphoma. Med Oncol 11 (1): 19-25, 1994. [PUBMED Abstract]
- Seymour JF, Pro B, Fuller LM, et al.: Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin's lymphoma. J Clin Oncol 21 (11): 2115-22, 2003. [PUBMED Abstract]
- Advani R, Rosenberg SA, Horning SJ: Stage I and II follicular non-Hodgkin's lymphoma: long-term follow-up of no initial therapy. J Clin Oncol 22 (8): 1454-9, 2004. [PUBMED Abstract]
- Pugh TJ, Ballonoff A, Newman F, et al.: Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation: a Surveillance, Epidemiology, and End Results database analysis. Cancer 116 (16): 3843-51, 2010. [PUBMED Abstract]
Treatment of Indolent, Noncontiguous Stage II/III/IV B-Cell Non-Hodgkin Lymphoma
Optimal treatment of advanced stages of low-grade non-Hodgkin lymphoma (NHL) is controversial because current therapeutic options result in low cure rates. Numerous clinical trials are in progress to evaluate treatment issues, and patients are urged to participate. The rate of relapse is fairly constant over time, even in patients who have achieved complete response to treatment. Relapse may occur many years after treatment. Currently, no randomized trials provide guidance to clinicians about the initial choice of watchful waiting, rituximab, nucleoside analogues, alkylating agents, combination chemotherapy, radiolabeled monoclonal antibodies, or combinations of these options.[1]; [2][Level of evidence B1]
For patients with indolent, noncontiguous stage II and stage III NHL, central lymphatic radiation therapy has been proposed but is not usually recommended as a form of treatment.[3,4]
Patients with a resolved hepatitis B virus (HBV) infection (defined as hepatitis B surface antigen-negative but hepatitis B core antibody-positive) are at risk of reactivation of HBV and require monitoring of HBV DNA. In a retrospective study of 326 patients, prophylactic nucleoside analogue therapy lowered HBV reactivation from 10.8% to 2.1%.[5]
Treatment Options for Indolent, Noncontiguous Stage II/III/IV B-Cell NHL
Treatment options for indolent, noncontiguous stage II/III/IV B-cell NHL include:
Because none of the therapies listed above are curative for advanced-stage disease, innovative approaches are under clinical evaluation.
Watchful waiting for asymptomatic patients
Because relapse may occur many years after treatment, even in patients who have achieved complete responses, deferred treatment (i.e., watchful waiting until the patient becomes symptomatic before initiating treatment) can be considered.[2,6-8] The Follicular Lymphoma International Prognostic Index (FLIPI) and the revised FLIPI-2 can predict progression-free survival (PFS) and overall survival (OS), but the scores cannot be used to establish the need for therapy in asymptomatic patients.[9,10]
Evidence (watchful waiting):
- Three randomized trials compared watchful waiting with immediate chemotherapy.[7,11]; [12][Level of evidence A1]
- All three trials showed no difference in cause-specific survival or OS.
- For patients randomly assigned to watchful waiting, the median time to require therapy was 2 to 3 years. One-third of patients undergoing watchful waiting never required treatment (one-half died of other causes and the other half remained progression free after 10 years).
- A selected group of 107 patients with advanced-stage follicular lymphoma were managed with initial watchful waiting; subsequent therapy was delayed for a median of 55 months. These patients achieved equivalent freedom from treatment failure and OS compared with a similar cohort treated immediately with rituximab.[13][Level of evidence C2] This implies that watchful waiting remains a relevant approach even in the rituximab era.
Rituximab alone or in combination with cytotoxic agents used in front-line therapy
Standard therapy includes rituximab, an anti–CD20 monoclonal antibody, either alone, as was shown in the ECOG-E4402 trial (NCT00075946),[14-19] or in combination with purine nucleoside analogues, such as fludarabine or cladribine, alkylating agents (with or without steroids), or combination chemotherapy. Rituximab may be considered as first-line therapy, either alone or in combination with other agents. Rituximab may be given intravenously (IV) or subcutaneously (SQ), and biosimilar versions, such as CT-P10 and GP2013, have shown equivalent efficacy and safety.[20-22] Combinations include the following:
- R-bendamustine: rituximab + bendamustine.[23-25]
- R-F: rituximab + fludarabine.[26]
- R-CVP: rituximab + cyclophosphamide + vincristine + prednisone.[27-30]
- R-CHOP: rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone.[29-33] A Cochrane meta-analysis could not identify any OS benefit of adding doxorubicin to chemotherapy regimens with rituximab or to chemotherapy regimens without rituximab.[34][Level of evidence A1]
- R-FM: rituximab + fludarabine + mitoxantrone.[29,30,35]
- R-FCM: rituximab + fludarabine + cyclophosphamide + mitoxantrone.[36]
Evidence (rituximab with or without chemotherapy):
- A prospective randomized trial of 534 patients with previously untreated, advanced-stage, follicular lymphoma compared R-CHOP, R-FM, and R-CVP.[29]
- With a median follow-up of 84 months, there was no difference in OS (8-year OS rate, 83%; 95% confidence interval [CI], 79%–87%), but the 8-year PFS rates favored R-CHOP (52%) and R-FM (49%) over R-CVP (42%) (P for the three regimens = .037).[29][Level of evidence B1]
- Four randomized prospective studies of previously untreated patients (involving more than 1,300 patients) and one Cochrane meta-analysis that included both untreated and previously treated patients (involving almost 1,000 patients) have compared rituximab plus combination chemotherapy with chemotherapy alone.[28,33,37]; [38][Level of evidence A1]
- Rituximab plus chemotherapy was superior in terms of event-free survival (EFS) or PFS (ranging from 2–3 years) in all of the studies and in terms of OS in all but one study (absolute benefit ranging from 6%–13% at 4 years, P < .04; hazard ratio [HR], 0.63 [0.51–0.79] for the meta-analysis).
- All of these trials were performed in symptomatic patients who required therapy. These results do not negate watchful waiting when appropriate.
- Fluorine F 18-fludeoxyglucose positron emission tomography–computed tomography (18F-FDG PET-CT) scan status at the completion of rituximab plus chemotherapy induction therapy is strongly predictive of outcome. It is not yet known whether acting on the results of the scans translates into better outcomes.[39,40]
- In a prospective randomized trial (NCT00991211), 527 patients with indolent and mantle cell lymphoma were randomly assigned to receive either bendamustine and rituximab or R-CHOP.[24][Level of evidence B1]
- With a median follow-up of 45 months, the median PFS favored the bendamustine arm (69 months vs. 31 months [HR, 0.58; 95% CI, 0.44–0.74; P < .0001]) but with no difference in OS.
- Compared with the R-CHOP arm, the bendamustine arm was associated with significantly lower rates of alopecia, hematologic toxicity, stomatitis, peripheral neuropathy, and infections.
- In a similar prospective randomized trial, 447 patients with indolent and mantle cell lymphoma were assigned to bendamustine and rituximab versus R-CHOP or R-CVP.[25][Level of evidence B1]
- With a median follow-up of 65 months, the 5-year PFS rate favored bendamustine and rituximab, 65.5% versus 55.8% (HR, 0.61; 95% CI, 0.45–0.85; P = .0025), but with no difference in OS.
- Increased deaths in the bendamustine-and-rituximab arm from cardiovascular causes (seven vs. one) and from secondary malignancies other than lymphoma (five vs. three) may have contributed to the lack of OS advantage.
Lenalidomide and rituximab
The combination of the immunomodulating agent lenalidomide with rituximab (the so-called R2 regimen) has been proposed as an alternative regimen to combinations involving cytotoxic agents and their subsequent short- and long-term toxicities.
Evidence (lenalidomide and rituximab):
- In a randomized prospective trial (RELEVANCE [NCT01650701]) of 1,030 patients with previously untreated follicular lymphoma, rituximab plus lenalidomide for 18 months was compared with rituximab plus chemotherapy (usually R-CHOP).[41,42] All patients received rituximab maintenance for up to 2 years.
- With a median follow-up of 72 months, the 6-year PFS rates (60% and 59%) and 3-year OS rates (89%) were identical (HR for PFS, 1.03; 95% CI, 0.84–1.27; P = .78) (HR for OS was not reported).[41,42][Level of evidence A1]
- This trial established that the R2 regimen is as effective as rituximab plus cytotoxic chemotherapy options. The transformation rate to aggressive lymphoma per year was 0.68% in the R2 group and 0.45% in the R-chemotherapy group. With a median follow-up of 72 months, there were no new safety signals.[42]
- In a randomized prospective trial of 358 patients with resistant/refractory indolent lymphoma (usually follicular lymphoma), the R2 regimen was compared with rituximab alone.[43]
- With a median follow-up of 28 months, the median PFS was 39.4 months for R2 and 14.1 months for rituximab alone (P < .0001), with no difference in OS.[43][Level of evidence B1]
Maintenance rituximab
After induction therapy with rituximab only or with rituximab plus chemotherapy, rituximab can be used once every 2 to 3 months as maintenance therapy. Several studies have evaluated this approach.
Evidence (maintenance rituximab for previously untreated patients):
- In the PRIMA study (NCT00140582), 1,018 patients with high-risk, previously untreated, symptomatic disease achieved complete response or partial response after induction therapy with immunochemotherapy (usually R-CHOP). Patients were then randomly assigned to 2 years of maintenance rituximab versus no maintenance.[44][Level of evidence B1]
- With a median follow-up of 9.0 years, median PFS favored rituximab maintenance (10.5 years) compared with observation (4.1 years) (HR, 0.61; 95% CI, 0.52−0.73; P < .001), but with no difference in OS.
- In the United Kingdom/International Study (NCT00112931), 379 previously untreated patients with asymptomatic, low-burden disease were randomly assigned to watchful waiting versus rituximab induction only versus rituximab induction followed by 2 years of rituximab maintenance.[45][Level of evidence A3]
- Although OS and histological transformation rates were not different at 3 years, maintenance rituximab was favored based on quality-of-life studies (Mental Adjustment to Cancer Scale P = .0004 at 7 months; Illness Coping Score P = .0012 at 7 months) and time-to-initiation of new treatment by 3 years (54% for watchful waiting vs. 12% for rituximab maintenance [HR, 0.21; 95% CI, 0.14–0.31; P < .0001]).[45][Level of evidence A3]
- This study suggested that for some patients, watch and wait resulted in watch and worry.[46] However, from the perspective of OS and histological transformation rates, no benefit could be seen with rituximab maintenance.
- In the RESORT study (NCT00075946), 289 previously untreated patients with asymptomatic, low-burden disease were randomly assigned to receive rituximab induction alone, with a re-treatment strategy that used rituximab at relapse versus rituximab induction plus maintenance rituximab every 13 weeks until treatment failure.[47][Level of evidence B3]
- With a median follow-up of 8.7 years, the 7-year rate for freedom from cytotoxic chemotherapy or radiation therapy was 83% for patients who received maintenance rituximab and 63% for patients who received induction alone (HR, 2.37; 95% CI, 1.5–3.8; P = .0001). There was no difference in the 10-year OS rate (83% vs. 84%; nonsignificant P = .5972). A re-treatment strategy achieved comparable disease control using significantly fewer doses of rituximab.
- Maintenance rituximab induces prolonged B-cell depletion, but morbidity from infections was not evaluated after 2015, when the full protocol data stopped (12 years after the study began).
These three randomized trials in previously untreated patients showed no advantage for the use of rituximab maintenance versus observation and reinduction of therapy at the time of relapse. The trials suggest a benefit for maintenance rituximab after reinduction for relapsed disease. Many questions remain about rituximab maintenance, particularly about truncating therapy at 2 years and long-term safety and efficacy. A trial extending rituximab maintenance to 5 years showed similar EFS or OS versus 1 year of maintenance after induction therapy with rituximab in previously untreated patients.[48][Level of evidence A1]
- The FOLL12 study (NCT02063685) included 807 patients with previously untreated high-tumor burden follicular lymphoma. Patients received rituximab plus chemotherapy induction and were randomly assigned to either standard rituximab maintenance (every 8 weeks for 2 years) or to postinduction treatment (monitoring, rituximab maintenance, or radioimmunotherapy) based on their complete metabolic response and measurable residual disease (MRD)–negative status.[49]
- With a median follow-up of 53 months, the 3-year PFS rate was 86% for patients who received standard maintenance and 72% for patients who received response-based treatment (P < .001). The 3-year OS rate was the same in both groups (98% vs. 97%; P = .238).[49][Level of evidence B1]
- This trial does not support the use of an end-of-treatment PET-CT scan to guide the use of maintenance rituximab.
- A prospective trial included 202 patients with previously untreated low-tumor burden follicular lymphoma. Patients were randomly assigned to receive either four weekly doses of IV rituximab (standard dose, 375 mg/m2) or one dose of IV rituximab followed by three weekly doses of SQ rituximab (1,400 mg) and maintenance doses in months 3, 5, 7, and 9.[19]
- With a median follow-up of 50.2 months, the 4-year PFS rate was 58.1% (95% CI, 47.5%–67.4%) for patients in the SQ arm who received maintenance therapy, and 41.2% (95% CI, 30.6%–51.6%) (HR, 0.585; 0.939–0.871; P = .0076) for the patients in the IV arm who did not receive maintenance therapy.[19][Level of evidence B1]
- There was no difference in OS or time-to-next treatment.
- Outside the context of clinical trials, the use of MRD testing has not been shown to be predictive in directing therapy for patients with follicular lymphoma. In retrospective analyses of two randomized prospective trials, while MRD negativity was prognostic of outcome, maintenance rituximab or obinutuzumab prolonged PFS the most among patients with MRD-negative disease.[49,50][Level of evidence C2] Stopping maintenance rituximab or obinutuzumab was not indicated in patients with MRD-negative disease, negating any possible change in therapy based on that status.
In summary, for previously untreated patients, all of the studies showed improved PFS, with no change in OS.
Evidence (maintenance rituximab for previously treated patients):
- In a prospective randomized trial of 465 patients with relapsed follicular lymphoma, responders to R-CHOP or CHOP were further randomly assigned to receive rituximab maintenance (1 dose every 3 months for 2 years) or no maintenance.[51][Level of evidence B1]
- At a median follow-up of 6 years, rituximab maintenance was better for median PFS (44 months vs. 16 months, P < .001) and borderline for 5-year OS (74% vs. 64%, P = .07).
- This benefit for maintenance was evident even for patients who received rituximab during induction therapy. Most patients in both arms received extensive rituximab during postprotocol salvage treatment.
- In a prospective randomized trial of 280 patients with relapsed follicular lymphoma, responders to chemotherapy and autologous stem cell transplant consolidation were randomly assigned to receive four doses of rituximab maintenance or no maintenance.[52][Level of evidence B1]
- With an 8.3-year median follow-up, the 10-year PFS rates favored maintenance (54% vs. 37% [HR, 0.66; 95% CI, 0.47–0.91; P = .012]), but there was no difference in OS.
- A meta-analysis of nine randomized clinical trials with a total of 2,586 patients with follicular lymphoma, most of whom had relapsed disease, compared rituximab maintenance with no maintenance and showed improved OS for rituximab maintenance in previously treated patients (HRdeath, 0.72; 95% CI, 0.57–0.91).[53][Level of evidence A1]
For previously treated patients, there is more evidence to suggest an OS advantage with the use of rituximab maintenance.
Obinutuzumab alone or in combination with cytotoxic agents used in front-line therapy
Obinutuzumab is a glycoengineered type II anti–CD20 monoclonal antibody with greater antibody-dependent cellular cytotoxicity than rituximab.
Evidence (obinutuzumab):
- A prospective randomized trial (NCT01332968) of 1,202 patients with previously untreated follicular lymphoma compared obinutuzumab combined with bendamustine (50%), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) (33%), or CVP (cyclophosphamide, vincristine, and prednisone) (10%) with rituximab combined with the same chemotherapy regimens (based on investigator choice).[54] After six cycles of combination therapy, patients had 2 years of maintenance therapy, receiving the same antibody every 2 months.
- With a median follow-up of 34.5 months, the 3-year PFS rate was 80% in the obinutuzumab group and 73.3% in the rituximab group (HR, 0.66; 95% CI, 0.51–0.85; P = .001).[54][Level of evidence B1]
- There was no difference in OS.
- Compared with what has been seen historically, there was a high rate of toxic deaths among patients using bendamustine in the obinutuzumab arm (5.6%) and in the rituximab arm (4.4%). For patients with indolent low-grade lymphoma, with median survivals exceeding 15 years, the number of toxic deaths during first-line therapy seems excessive. By comparison, the toxic death rate was 1% to 2% when either antibody was combined with CHOP or CVP.
Several issues have been raised about this study:
- The side effects were significantly higher with obinutuzumab in terms of infusion reactions and subsequent adverse events.
- Obinutuzumab costs significantly more than rituximab.
In summary, in the absence of any change in OS, switching from rituximab to obinutuzumab in combination with chemotherapy for previously untreated follicular lymphoma is a difficult choice. The PFS differences may be attributable to the imbalance in monoclonal antibody dosing, and the increased side effects and costs are mitigating factors. In this trial, bendamustine combined with either antibody led to unacceptable rates of toxic death.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hagenbeek A, Eghbali H, Monfardini S, et al.: Phase III intergroup study of fludarabine phosphate compared with cyclophosphamide, vincristine, and prednisone chemotherapy in newly diagnosed patients with stage III and IV low-grade malignant Non-Hodgkin's lymphoma. J Clin Oncol 24 (10): 1590-6, 2006. [PUBMED Abstract]
- Gribben JG: How I treat indolent lymphoma. Blood 109 (11): 4617-26, 2007. [PUBMED Abstract]
- Jacobs JP, Murray KJ, Schultz CJ, et al.: Central lymphatic irradiation for stage III nodular malignant lymphoma: long-term results. J Clin Oncol 11 (2): 233-8, 1993. [PUBMED Abstract]
- Mendenhall NP, Million RR: Comprehensive lymphatic irradiation for stage II-III non-Hodgkin's lymphoma. Am J Clin Oncol 12 (3): 190-4, 1989. [PUBMED Abstract]
- Kusumoto S, Arcaini L, Hong X, et al.: Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood 133 (2): 137-146, 2019. [PUBMED Abstract]
- Eek R, Falkson G: The low-grade lymphoproliferative disorders. Oncology 54 (6): 441-58, 1997 Nov-Dec. [PUBMED Abstract]
- Ardeshna KM, Smith P, Norton A, et al.: Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet 362 (9383): 516-22, 2003. [PUBMED Abstract]
- Portlock CS, Rosenberg SA: No initial therapy for stage III and IV non-Hodgkin's lymphomas of favorable histologic types. Ann Intern Med 90 (1): 10-13, 1979.
- Solal-Céligny P, Roy P, Colombat P, et al.: Follicular lymphoma international prognostic index. Blood 104 (5): 1258-65, 2004. [PUBMED Abstract]
- Federico M, Bellei M, Marcheselli L, et al.: Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 27 (27): 4555-62, 2009. [PUBMED Abstract]
- Brice P, Bastion Y, Lepage E, et al.: Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 15 (3): 1110-7, 1997. [PUBMED Abstract]
- Young RC, Longo DL, Glatstein E, et al.: The treatment of indolent lymphomas: watchful waiting v aggressive combined modality treatment. Semin Hematol 25 (2 Suppl 2): 11-6, 1988. [PUBMED Abstract]
- Solal-Céligny P, Bellei M, Marcheselli L, et al.: Watchful waiting in low-tumor burden follicular lymphoma in the rituximab era: results of an F2-study database. J Clin Oncol 30 (31): 3848-53, 2012. [PUBMED Abstract]
- Ghielmini M, Schmitz SF, Cogliatti SB, et al.: Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 103 (12): 4416-23, 2004. [PUBMED Abstract]
- Witzig TE, Vukov AM, Habermann TM, et al.: Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade I non-Hodgkin's lymphoma: a phase II trial in the North Central Cancer Treatment Group. J Clin Oncol 23 (6): 1103-8, 2005. [PUBMED Abstract]
- Hainsworth JD, Litchy S, Shaffer DW, et al.: Maximizing therapeutic benefit of rituximab: maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma--a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 23 (6): 1088-95, 2005. [PUBMED Abstract]
- Kahl BS, Hong F, Williams ME, et al.: Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. J Clin Oncol 32 (28): 3096-102, 2014. [PUBMED Abstract]
- Buske C, Hiddemann W: Rituximab maintenance therapy in indolent NHL: a clinical review. Leuk Res 30 (Suppl 1): S11-5, 2006. [PUBMED Abstract]
- Cartron G, Bachy E, Tilly H, et al.: Randomized Phase III Trial Evaluating Subcutaneous Rituximab for the First-Line Treatment of Low-Tumor Burden Follicular Lymphoma: Results of a LYSA Study. J Clin Oncol 41 (19): 3523-3533, 2023. [PUBMED Abstract]
- Kim WS, Buske C, Ogura M, et al.: Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 compared with rituximab in patients with previously untreated advanced-stage follicular lymphoma: a randomised, double-blind, parallel-group, non-inferiority phase 3 trial. Lancet Haematol 4 (8): e362-e373, 2017. [PUBMED Abstract]
- Davies A, Merli F, Mihaljević B, et al.: Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial. Lancet Haematol 4 (6): e272-e282, 2017. [PUBMED Abstract]
- Jurczak W, Moreira I, Kanakasetty GB, et al.: Rituximab biosimilar and reference rituximab in patients with previously untreated advanced follicular lymphoma (ASSIST-FL): primary results from a confirmatory phase 3, double-blind, randomised, controlled study. Lancet Haematol 4 (8): e350-e361, 2017. [PUBMED Abstract]
- Robinson KS, Williams ME, van der Jagt RH, et al.: Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol 26 (27): 4473-9, 2008. [PUBMED Abstract]
- Rummel MJ, Niederle N, Maschmeyer G, et al.: Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381 (9873): 1203-10, 2013. [PUBMED Abstract]
- Flinn IW, van der Jagt R, Kahl B, et al.: First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J Clin Oncol 37 (12): 984-991, 2019. [PUBMED Abstract]
- Czuczman MS, Koryzna A, Mohr A, et al.: Rituximab in combination with fludarabine chemotherapy in low-grade or follicular lymphoma. J Clin Oncol 23 (4): 694-704, 2005. [PUBMED Abstract]
- Marcus R, Imrie K, Belch A, et al.: CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 105 (4): 1417-23, 2005. [PUBMED Abstract]
- Marcus R, Imrie K, Solal-Celigny P, et al.: Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 26 (28): 4579-86, 2008. [PUBMED Abstract]
- Federico M, Luminari S, Dondi A, et al.: R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol 31 (12): 1506-13, 2013. [PUBMED Abstract]
- Luminari S, Ferrari A, Manni M, et al.: Long-Term Results of the FOLL05 Trial Comparing R-CVP Versus R-CHOP Versus R-FM for the Initial Treatment of Patients With Advanced-Stage Symptomatic Follicular Lymphoma. J Clin Oncol 36 (7): 689-696, 2018. [PUBMED Abstract]
- Czuczman MS, Weaver R, Alkuzweny B, et al.: Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol 22 (23): 4711-6, 2004. [PUBMED Abstract]
- Hainsworth JD, Litchy S, Morrissey LH, et al.: Rituximab plus short-duration chemotherapy as first-line treatment for follicular non-Hodgkin's lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 23 (7): 1500-6, 2005. [PUBMED Abstract]
- Hiddemann W, Kneba M, Dreyling M, et al.: Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 106 (12): 3725-32, 2005. [PUBMED Abstract]
- Itchaki G, Gafter-Gvili A, Lahav M, et al.: Anthracycline-containing regimens for treatment of follicular lymphoma in adults. Cochrane Database Syst Rev 7: CD008909, 2013. [PUBMED Abstract]
- Zinzani PL, Pulsoni A, Perrotti A, et al.: Fludarabine plus mitoxantrone with and without rituximab versus CHOP with and without rituximab as front-line treatment for patients with follicular lymphoma. J Clin Oncol 22 (13): 2654-61, 2004. [PUBMED Abstract]
- Forstpointner R, Dreyling M, Repp R, et al.: The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 104 (10): 3064-71, 2004. [PUBMED Abstract]
- Herold M, Haas A, Srock S, et al.: Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol 25 (15): 1986-92, 2007. [PUBMED Abstract]
- Salles GA, Mounier N, de Guibert S, et al.: Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: final analysis of the GELA-GOELAMS FL2000 study with a 5-year follow-up. [Abstract] Blood 110 (11): A-792, 2007.
- Dupuis J, Berriolo-Riedinger A, Julian A, et al.: Impact of [(18)F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d'Etudes des Lymphomes de l'Adulte and GOELAMS. J Clin Oncol 30 (35): 4317-22, 2012. [PUBMED Abstract]
- Trotman J, Fournier M, Lamy T, et al.: Positron emission tomography-computed tomography (PET-CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET-CT in a subset of PRIMA trial participants. J Clin Oncol 29 (23): 3194-200, 2011. [PUBMED Abstract]
- Morschhauser F, Fowler NH, Feugier P, et al.: Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N Engl J Med 379 (10): 934-947, 2018. [PUBMED Abstract]
- Morschhauser F, Nastoupil L, Feugier P, et al.: Six-Year Results From RELEVANCE: Lenalidomide Plus Rituximab (R2) Versus Rituximab-Chemotherapy Followed by Rituximab Maintenance in Untreated Advanced Follicular Lymphoma. J Clin Oncol 40 (28): 3239-3245, 2022. [PUBMED Abstract]
- Leonard JP, Trneny M, Izutsu K, et al.: AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol 37 (14): 1188-1199, 2019. [PUBMED Abstract]
- Bachy E, Seymour JF, Feugier P, et al.: Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients With Follicular Lymphoma: Long-Term Results of the PRIMA Study. J Clin Oncol 37 (31): 2815-2824, 2019. [PUBMED Abstract]
- Ardeshna KM, Qian W, Smith P, et al.: Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol 15 (4): 424-35, 2014. [PUBMED Abstract]
- Ansell SM: Follicular lymphoma: watch and wait is watch and worry. Lancet Oncol 15 (4): 368-9, 2014. [PUBMED Abstract]
- Kahl BS, Jegede OA, Peterson C, et al.: Long-Term Follow-Up of the RESORT Study (E4402): A Randomized Phase III Comparison of Two Different Rituximab Dosing Strategies for Low-Tumor Burden Follicular Lymphoma. J Clin Oncol 42 (7): 774-778, 2024. [PUBMED Abstract]
- Taverna C, Martinelli G, Hitz F, et al.: Rituximab Maintenance for a Maximum of 5 Years After Single-Agent Rituximab Induction in Follicular Lymphoma: Results of the Randomized Controlled Phase III Trial SAKK 35/03. J Clin Oncol 34 (5): 495-500, 2016. [PUBMED Abstract]
- Luminari S, Manni M, Galimberti S, et al.: Response-Adapted Postinduction Strategy in Patients With Advanced-Stage Follicular Lymphoma: The FOLL12 Study. J Clin Oncol 40 (7): 729-739, 2022. [PUBMED Abstract]
- Pott C, Jurinovic V, Trotman J, et al.: Minimal Residual Disease Status Predicts Outcome in Patients With Previously Untreated Follicular Lymphoma: A Prospective Analysis of the Phase III GALLIUM Study. J Clin Oncol 42 (5): 550-561, 2024. [PUBMED Abstract]
- van Oers MH, Tönnissen E, Van Glabbeke M, et al.: BCL-2/IgH polymerase chain reaction status at the end of induction treatment is not predictive for progression-free survival in relapsed/resistant follicular lymphoma: results of a prospective randomized EORTC 20981 phase III intergroup study. J Clin Oncol 28 (13): 2246-52, 2010. [PUBMED Abstract]
- Pettengell R, Schmitz N, Gisselbrecht C, et al.: Rituximab purging and/or maintenance in patients undergoing autologous transplantation for relapsed follicular lymphoma: a prospective randomized trial from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 31 (13): 1624-30, 2013. [PUBMED Abstract]
- Vidal L, Gafter-Gvili A, Salles G, et al.: Rituximab maintenance for the treatment of patients with follicular lymphoma: an updated systematic review and meta-analysis of randomized trials. J Natl Cancer Inst 103 (23): 1799-806, 2011. [PUBMED Abstract]
- Marcus R, Davies A, Ando K, et al.: Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med 377 (14): 1331-1344, 2017. [PUBMED Abstract]
Treatment of Indolent, Recurrent B-Cell Non-Hodgkin Lymphoma
In general, treatment with standard agents rarely produces a cure in patients with relapsed B-cell non-Hodgkin Lymphoma (NHL). Sustained remissions after relapse can often be obtained in patients with indolent lymphomas, but relapse will usually ensue. Favorable survival after relapse has been associated with an age younger than 60 years, complete remission rather than partial remission, and duration of response longer than 2 years.[1] Even patients in the most favorable subset, however, have a tenfold greater mortality compared with age-adjusted U.S. population rates.[2]
Patients who experience a relapse of indolent lymphoma can often achieve disease control with single-agent or combination chemotherapy, rituximab (an anti–CD20 monoclonal antibody), lenalidomide, radiolabeled anti–CD20 monoclonal antibodies, or palliative radiation therapy.[3,4] However, long-term freedom from second relapse is uncommon and multiple relapses usually occur. Patients with indolent lymphoma may experience a relapse with a more aggressive histology. If the clinical pattern of relapse suggests that the disease is behaving in a more aggressive manner, a biopsy can be performed. If a more aggressive histology is confirmed, therapy must change to a regimen applicable to that histological type.[5] Rapid growth or discordant growth between various disease sites may indicate a histological conversion.
In a retrospective review of 325 patients diagnosed between 1972 and 1999, the 10-year risk of histological transformation was 30%.[6] In this series, high-risk factors for subsequent histological transformation were advanced stage, high-risk Follicular Lymphoma International Prognostic Index, and watchful waiting (as opposed to treatment being initiated at diagnosis). The median survival after transformation was 1 to 2 years, with 25% of patients alive at 5 years and with approximately 10% to 20% of patients alive 10 years after re-treatment.[7]
A prospective trial of 631 patients with follicular lymphoma and with a median follow-up of 60 months in the rituximab era (2002–2009) found a 5-year transformation rate to a higher-grade histology of 11%.[8] The median overall survival (OS) after transformation was 50 months, and the 5-year OS rate was 66%, if the transformation occurred more than 18 months after a diagnosis of follicular lymphoma. This series described a better prognosis for patients with transformation than in the era before rituximab.
For descriptions of the regimens used to treat histological conversions, see the Treatment of Aggressive, Recurrent B-Cell Non-Hodgkin Lymphoma section in Aggressive B-Cell Non-Hodgkin Lymphoma Treatment. The durability of the second remission may be short, and clinical trials can be considered.
Treatment Options for Indolent, Recurrent B-Cell NHL
Treatment options for indolent, recurrent B-cell NHL include:
- Rituximab alone or in combination with cytotoxic agents used in front-line therapy.
- Obinutuzumab alone or in combination with cytotoxic agents used in front-line therapy.
- Lenalidomide and rituximab.
- Zanubrutinib and obinutuzumab.
- EZH2 inhibitor.
- Bispecific T-cell engagers.
- Chimeric antigen receptor (CAR) T-cell therapy.
- Stem cell transplant (SCT).
- Palliative radiation therapy.
Rituximab alone or in combination with cytotoxic agents used in front-line therapy
Rituximab results in a 40% to 50% response rate in patients with relapsed indolent B-cell lymphomas.[9-13] Rituximab can also be combined with combination chemotherapy.[14,15]
Evidence (rituximab):
Obinutuzumab alone or in combination with cytotoxic agents used in front-line therapy
Obinutuzumab is a CD20-binding monoclonal antibody with alternative epitope binding.
Evidence (obinutuzumab):
- A randomized prospective trial (NCT01059630) included 396 patients with rituximab-refractory indolent lymphoma (mostly follicular lymphoma). Patients received obinutuzumab plus bendamustine, followed by obinutuzumab maintenance therapy, for 2 years versus bendamustine alone with no maintenance therapy.[19,20][Level of evidence A1]
- With a median follow-up of 31.8 months, the 2-year OS rate favored the obinutuzumab combination (74.5% vs. 65.1%) (hazard ratio [HR], 0.67; 95% confidence interval [CI], 0.47–0.96; P = .027). The median PFS also favored the obinutuzumab combination (25.8 months [95% CI, 19.5–41.1] vs. 14.1 months [95% CI, 12.6–16.0]) (HR, 0.57; 95% CI, 0.44–0.73; P < .001).[20][Level of evidence A1]
- The contribution of maintenance therapy to the outcome could not be assessed in this design.
Lenalidomide and rituximab
Responses of 20% to 56% have been reported for lenalidomide, especially in patients with follicular lymphoma and small lymphocytic lymphoma, with even higher responses noted for the combination of lenalidomide and rituximab.[21,22][Level of evidence C3]
Zanubrutinib and obinutuzumab
Evidence (zanubrutinib and obinutuzumab):
- A randomized, multicenter, phase II study (ROSEWOOD [NCT03332017]) included 217 patients with relapsed or refractory follicular lymphoma after receiving two or more prior lines of therapy. Patients received either the oral Bruton tyrosine kinase inhibitor zanubrutinib plus obinutuzumab or obinutuzumab alone until disease progression.[23][Level of evidence B1]
- With a median follow-up of 19.0 months, the median PFS was 28.0 months for patients who received zanubrutinib plus obinutuzumab and 10.4 months for patients who received obinutuzumab alone (HR, 0.50; 95% CI, 0.33–0.75; P < .001).
- The overall response rate was 69% (95% CI, 61%–76%) in the zanubrutinib-plus-obinutuzumab arm and 46% (95% CI, 34%–58%) in the obinutuzumab-alone arm (P = .0012).
The U.S. Food and Drug Administration approved zanubrutinib and obinutuzumab for patients with relapsed or refractory follicular lymphoma after two or more prior lines of therapy.
EZH2 inhibitor
Tazemetostat
Tazemetostat is an inhibitor of EZH2, a histone methyltransferase essential to the formation of lymph node germinal centers, especially with activating variants of EZH2.
Evidence (tazemetostat):
- A phase II study included 99 patients with relapsed or refractory follicular lymphoma, 45 of whom had activating EZH2 variants, and 54 of whom had wild-type EZH2.[24]
- Treatment with tazemetostat resulted in an objective response rate of 69% (95% CI, 53%–82%) for patients with activating EZH2 variants versus 35% (95% CI, 23%–49%) for patients with wild-type EZH2.[24]
- With a median follow-up of 22 months, the median PFS was 13.8 months (95% CI, 10.7–22.0) for patients with activating EZH2 variants and 11.1 months (95% CI, 3.7–14.6) for patients with wild-type EZH2.[24][Level of evidence C3]
- Grade 3 or 4 treatment-related adverse events occurred in 4% of patients.
Bispecific T-cell engagers
Bispecific T-cell engagers bind to CD20 (or CD19) and to CD3 to direct T cells to eliminate malignant B cells.[25,26] Similar to CAR T-cell therapy, almost one-half of patients who receive this therapy experience cytokine release syndrome.
Mosunetuzumab
Mosunetuzumab is a bispecific T-cell engager that binds to CD20 and CD3.[25]
Evidence (mosunetuzumab):
- A single-arm, multicenter, phase II study included 90 patients with relapsed or refractory follicular lymphoma who had received two or more prior lines of therapy (including an anti-CD20 therapy and an alkylating agent). Patients received mosunetuzumab.[27]
- With a median follow-up of 37.4 months, the objective response rate was 77.8% (95% CI, 67.8%–85.9%), and the complete response rate was 60.0% (95% CI, 49.1%–70.2%), per investigator assessment. The median PFS (per investigator assessment) was 24.0 months. The 3-year OS rate was 82.4% (95% CI, 73.8%–91.0%).[27][Level of evidence C1]
- Cytokine release syndrome occurred in 44.4% of patients; 97.2% of cases were grade 1 or 2 in severity.
Epcoritamab
Epcoritamab is a bispecific T-cell engager that binds to CD20 and CD3.
Evidence (epcoritamab):
- A single-arm multicenter study (NCT03625037), included 128 patients with relapsed or refractory follicular lymphoma who had received two or more lines of therapy. Patients received epcoritamab.[28]
- With a median follow-up of 17.4 months, the overall response rate was 82.0 % (95% CI, 74.3%–88.3%) and the complete response rate was 62.5% (95% CI, 53.5%–70.9%).[28][Level of evidence C3]
- Only 2% of patients had grade 3 or higher cytokine release syndrome events.
Chimeric antigen receptor (CAR) T-cell therapy
CAR T-cell therapy, with the autologous anti-CD19 therapeutics axicabtagene ciloleucel (axi-cel), lisocabtagene maraleucel (liso-cel), or tisagenlecleucel (tisa-cel), has been approved for patients with relapsed follicular lymphoma after two or more lines of prior therapy.
Evidence (CAR T-cell therapy):
- In a phase II trial, 159 patients with relapsed or refractory follicular lymphoma (n = 127), marginal zone lymphoma (n = 31), or diffuse large B-cell lymphoma (n = 1) received axi-cel.[29,30]
- With a median follow-up of 41.7 months, the overall response rate was 92% (95% CI, 85%–97%), and the complete response rate was 74%.
- The 18-month PFS rate was 64.8% (95% CI, 54.2%–73.5%).[29][Level of evidence C2]
- The median PFS was 40.2 months for patients with follicular lymphoma and was not reached for patients with marginal zone lymphoma.[30][Level of evidence C2]
- Cytokine release syndrome occurred in 78% of patients and was grade 3 or 4 in 6% of patients.
- Tocilizumab was required in 50% of all patients, and 5% required vasopressors. Grade 3 or 4 neurological events occurred in 15% of patients.
- In a phase II trial, 98 patients with relapsed or refractory follicular lymphoma after two or more lines of prior therapy received anti-CD19 CAR T-cell therapy with tisa-cel.[31]
- With a median follow-up of 16.6 months, the complete response rate was 69.1% (95% CI, 58.8%–78.3%), and the overall response rate was 86.2% (95% CI, 77.5%–92.4%).[31][Level of evidence C3]
- Grade 3 or 4 cytokine release syndrome occurred in 48.5% of patients, and 37.1% had grade 3 or 4 neurotoxicity.
- A phase II trial (NCT04245839) included 130 patients with relapsed or refractory follicular lymphoma who had received two or more prior lines of therapy. Patients had high-risk features (progression of disease within 24 months after first-line chemoimmunotherapy or disease refractory to rituximab and alkylators). Patients received liso-cel.[32]
- With a median follow-up of 18.9 months, the overall response rate was 97% (95% CI, 91.6%–99.4%), and the complete response rate was 94% (95% CI, 87.5%–97.8%).[32][Level of evidence C3]
- Cytokine release syndrome occurred in 56% of patients and was grade 3 or higher in 1% of patients. Neurological side effects occurred in 15% of patients and was grade 3 or higher in 2% of patients.
CAR T cells are being used for high-risk patients whose disease has relapsed rapidly after chemoimmunotherapy. Such an approach is considered in the context of numerous other available agents.
Stem cell transplant
In many institutions, autologous or allogeneic SCTs are being used for high-risk patients whose disease has relapsed rapidly after chemoimmunotherapy. Such an approach is considered in the context of numerous other available agents.[33-37]
Evidence (SCT):
- The German Low-Grade Lymphoma Study Group treated 307 patients with follicular lymphoma with two cycles of CHOP (cyclophosphamide,
doxorubicin, vincristine, and prednisone)-like induction chemotherapy and then randomly assigned them to receive autologous SCT or interferon maintenance.[38]
- With a median follow-up of 4.2 years, the 5-year PFS rate was 65% for patients who received a transplant versus 33% for patients who received interferon (P < .001). There was no difference in OS.[38][Level of evidence B1]
Palliative radiation therapy
Palliation may be achieved with very low-dose (4 Gy) involved-field radiation therapy in two fractions for patients with indolent and aggressive relapsed disease.[39] In a prospective randomized trial, treatment with 4 Gy was inferior to treatment with 24 Gy in 12 fractions in PFS (77% vs. 92%, P < .0001).[40][Level of evidence B1]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Casulo C, Byrtek M, Dawson KL, et al.: Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol 33 (23): 2516-22, 2015. [PUBMED Abstract]
- Weisdorf DJ, Andersen JW, Glick JH, et al.: Survival after relapse of low-grade non-Hodgkin's lymphoma: implications for marrow transplantation. J Clin Oncol 10 (6): 942-7, 1992. [PUBMED Abstract]
- Peterson BA: Current treatment of follicular low-grade lymphomas. Semin Oncol 26 (5 Suppl 14): 2-11, 1999. [PUBMED Abstract]
- Haas RL, Poortmans P, de Jong D, et al.: High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J Clin Oncol 21 (13): 2474-80, 2003. [PUBMED Abstract]
- Tsimberidou AM, O'Brien S, Khouri I, et al.: Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol 24 (15): 2343-51, 2006. [PUBMED Abstract]
- Montoto S, Davies AJ, Matthews J, et al.: Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol 25 (17): 2426-33, 2007. [PUBMED Abstract]
- Yuen AR, Kamel OW, Halpern J, et al.: Long-term survival after histologic transformation of low-grade follicular lymphoma. J Clin Oncol 13 (7): 1726-33, 1995. [PUBMED Abstract]
- Link BK, Maurer MJ, Nowakowski GS, et al.: Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/MayoClinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol 31 (26): 3272-8, 2013. [PUBMED Abstract]
- Davis TA, White CA, Grillo-López AJ, et al.: Single-agent monoclonal antibody efficacy in bulky non-Hodgkin's lymphoma: results of a phase II trial of rituximab. J Clin Oncol 17 (6): 1851-7, 1999. [PUBMED Abstract]
- Piro LD, White CA, Grillo-López AJ, et al.: Extended Rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol 10 (6): 655-61, 1999. [PUBMED Abstract]
- Davis TA, Grillo-López AJ, White CA, et al.: Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: safety and efficacy of re-treatment. J Clin Oncol 18 (17): 3135-43, 2000. [PUBMED Abstract]
- Hainsworth JD, Litchy S, Shaffer DW, et al.: Maximizing therapeutic benefit of rituximab: maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma--a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 23 (6): 1088-95, 2005. [PUBMED Abstract]
- Lockmer S, Østenstad B, Hagberg H, et al.: Chemotherapy-Free Initial Treatment of Advanced Indolent Lymphoma Has Durable Effect With Low Toxicity: Results From Two Nordic Lymphoma Group Trials With More Than 10 Years of Follow-Up. J Clin Oncol : JCO1800262, 2018. [PUBMED Abstract]
- Forstpointner R, Dreyling M, Repp R, et al.: The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 104 (10): 3064-71, 2004. [PUBMED Abstract]
- Canellos GP: CHOP may have been part of the beginning but certainly not the end: issues in risk-related therapy of large-cell lymphoma. J Clin Oncol 15 (5): 1713-6, 1997. [PUBMED Abstract]
- van Oers MH, Van Glabbeke M, Giurgea L, et al.: Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol 28 (17): 2853-8, 2010. [PUBMED Abstract]
- van Oers MH, Klasa R, Marcus RE, et al.: Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood 108 (10): 3295-301, 2006. [PUBMED Abstract]
- Martinelli G, Schmitz SF, Utiger U, et al.: Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol 28 (29): 4480-4, 2010. [PUBMED Abstract]
- Sehn LH, Chua N, Mayer J, et al.: Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol 17 (8): 1081-93, 2016. [PUBMED Abstract]
- Cheson BD, Chua N, Mayer J, et al.: Overall Survival Benefit in Patients With Rituximab-Refractory Indolent Non-Hodgkin Lymphoma Who Received Obinutuzumab Plus Bendamustine Induction and Obinutuzumab Maintenance in the GADOLIN Study. J Clin Oncol 36 (22): 2259-2266, 2018. [PUBMED Abstract]
- Witzig TE, Wiernik PH, Moore T, et al.: Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. J Clin Oncol 27 (32): 5404-9, 2009. [PUBMED Abstract]
- Leonard JP, Jung SH, Johnson J, et al.: Randomized Trial of Lenalidomide Alone Versus Lenalidomide Plus Rituximab in Patients With Recurrent Follicular Lymphoma: CALGB 50401 (Alliance). J Clin Oncol 33 (31): 3635-40, 2015. [PUBMED Abstract]
- Zinzani PL, Mayer J, Flowers CR, et al.: ROSEWOOD: A Phase II Randomized Study of Zanubrutinib Plus Obinutuzumab Versus Obinutuzumab Monotherapy in Patients With Relapsed or Refractory Follicular Lymphoma. J Clin Oncol 41 (33): 5107-5117, 2023. [PUBMED Abstract]
- Morschhauser F, Tilly H, Chaidos A, et al.: Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol 21 (11): 1433-1442, 2020. [PUBMED Abstract]
- Budde LE, Sehn LH, Matasar M, et al.: Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol 23 (8): 1055-1065, 2022. [PUBMED Abstract]
- Hutchings M, Mous R, Clausen MR, et al.: Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 398 (10306): 1157-1169, 2021. [PUBMED Abstract]
- Sehn LH, Bartlett NL, Matasar MJ, et al.: Long-term 3-year follow-up of mosunetuzumab in relapsed or refractory follicular lymphoma after ≥2 prior therapies. Blood 145 (7): 708-719, 2025. [PUBMED Abstract]
- Linton KM, Vitolo U, Jurczak W, et al.: Epcoritamab monotherapy in patients with relapsed or refractory follicular lymphoma (EPCORE NHL-1): a phase 2 cohort of a single-arm, multicentre study. Lancet Haematol 11 (8): e593-e605, 2024. [PUBMED Abstract]
- Jacobson CA, Chavez JC, Sehgal AR, et al.: Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol 23 (1): 91-103, 2022. [PUBMED Abstract]
- Neelapu SS, Chavez JC, Sehgal AR, et al.: Three-year follow-up analysis of axicabtagene ciloleucel in relapsed/refractory indolent non-Hodgkin lymphoma (ZUMA-5). Blood 143 (6): 496-506, 2024. [PUBMED Abstract]
- Fowler NH, Dickinson M, Dreyling M, et al.: Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med 28 (2): 325-332, 2022. [PUBMED Abstract]
- Morschhauser F, Dahiya S, Palomba ML, et al.: Lisocabtagene maraleucel in follicular lymphoma: the phase 2 TRANSCEND FL study. Nat Med 30 (8): 2199-2207, 2024. [PUBMED Abstract]
- Freedman A, Friedberg JW, Gribben J: High-dose therapy for follicular lymphoma. Oncology (Huntingt) 14 (3): 321-6, 329; discussion 330-2, 338, 2000. [PUBMED Abstract]
- Brice P, Simon D, Bouabdallah R, et al.: High-dose therapy with autologous stem-cell transplantation (ASCT) after first progression prolonged survival of follicular lymphoma patients included in the prospective GELF 86 protocol. Ann Oncol 11 (12): 1585-90, 2000. [PUBMED Abstract]
- Khouri IF, McLaughlin P, Saliba RM, et al.: Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood 111 (12): 5530-6, 2008. [PUBMED Abstract]
- Sebban C, Brice P, Delarue R, et al.: Impact of rituximab and/or high-dose therapy with autotransplant at time of relapse in patients with follicular lymphoma: a GELA study. J Clin Oncol 26 (21): 3614-20, 2008. [PUBMED Abstract]
- Thomson KJ, Morris EC, Milligan D, et al.: T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol 28 (23): 3695-700, 2010. [PUBMED Abstract]
- Lenz G, Dreyling M, Schiegnitz E, et al.: Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood 104 (9): 2667-74, 2004. [PUBMED Abstract]
- Haas RL, Poortmans P, de Jong D, et al.: Effective palliation by low dose local radiotherapy for recurrent and/or chemotherapy refractory non-follicular lymphoma patients. Eur J Cancer 41 (12): 1724-30, 2005. [PUBMED Abstract]
- Hoskin PJ, Kirkwood AA, Popova B, et al.: 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol 15 (4): 457-63, 2014. [PUBMED Abstract]
Other Lymphoproliferative and Related Disorders
Castleman Disease
A biopsy of localized or multifocal collections of lymph nodes may lead to a diagnosis of Castleman disease (CD). Strictly speaking, this uncommon diagnosis is not a lymphoma or even a malignancy. Yet, many patients with CD may be seen and treated by hematologists or oncologists.
Localized or unicentric CD is usually asymptomatic and occurs in the mediastinum, which is the most common presentation for CD.[1] Watchful waiting, surgery, or radiation therapy can be used to treat this indolent form. Multicentric CD (30% of CD patients) presents with lymphadenopathy in multiple sites; symptoms such as fever, night sweats, weight loss, and fatigue; and laboratory abnormalities such as anemia, low albumin level, elevated C-reactive protein level, and high fibrinogen level.[1] Multicentric CD (MCD) is subdivided into human herpes virus-8–associated MCD (usually with HIV or severe immunocompromise) or idiopathic MCD. Cytopenias and cytokine storm are attributed to interleukin-6 (IL-6) overproduction. MCD is a feature seen in POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin abnormalities) syndrome [2] and TAFRO (thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly) syndrome.[3,4] Therapy with siltuximab (an anti–IL-6 monoclonal antibody), rituximab (an anti-CD20 monoclonal antibody), or chemotherapeutic agents has been presented in anecdotal nonrandomized series.[5-8]
True Histiocytic Lymphoma
True histiocytic lymphomas are very rare tumors that show histiocytic differentiation and express histiocytic markers in the absence of B-cell or T-cell lineage-specific immunologic markers.[9,10] Care must be taken with immunophenotypic tests to exclude anaplastic large cell lymphoma or hemophagocytic syndromes caused by viral infections, especially Epstein-Barr virus.
Therapeutic options
Therapy is modeled after the treatment of comparably staged diffuse large cell lymphomas, but the optimal approach remains to be defined.
References
- van Rhee F, Voorhees P, Dispenzieri A, et al.: International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood 132 (20): 2115-2124, 2018. [PUBMED Abstract]
- Dispenzieri A: POEMS Syndrome: 2019 Update on diagnosis, risk-stratification, and management. Am J Hematol 94 (7): 812-827, 2019. [PUBMED Abstract]
- Zhang Y, Suo SS, Yang HJ, et al.: Clinical features and treatment of 7 Chinese TAFRO syndromes from 96 de novo Castleman diseases: a 10-year retrospective study. J Cancer Res Clin Oncol 146 (2): 357-365, 2020. [PUBMED Abstract]
- Fujimoto S, Sakai T, Kawabata H, et al.: Is TAFRO syndrome a subtype of idiopathic multicentric Castleman disease? Am J Hematol 94 (9): 975-983, 2019. [PUBMED Abstract]
- Tonialini L, Bonfichi M, Ferrero S, et al.: Siltuximab in relapsed/refractory multicentric Castleman disease: Experience of the Italian NPP program. Hematol Oncol 36 (4): 689-692, 2018. [PUBMED Abstract]
- Dong Y, Zhang L, Nong L, et al.: Effectiveness of rituximab-containing treatment regimens in idiopathic multicentric Castleman disease. Ann Hematol 97 (9): 1641-1647, 2018. [PUBMED Abstract]
- Zhang L, Zhao AL, Duan MH, et al.: Phase 2 study using oral thalidomide-cyclophosphamide-prednisone for idiopathic multicentric Castleman disease. Blood 133 (16): 1720-1728, 2019. [PUBMED Abstract]
- van Rhee F, Wong RS, Munshi N, et al.: Siltuximab for multicentric Castleman's disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol 15 (9): 966-74, 2014. [PUBMED Abstract]
- Soslow RA, Davis RE, Warnke RA, et al.: True histiocytic lymphoma following therapy for lymphoblastic neoplasms. Blood 87 (12): 5207-12, 1996. [PUBMED Abstract]
- Kamel OW, Gocke CD, Kell DL, et al.: True histiocytic lymphoma: a study of 12 cases based on current definition. Leuk Lymphoma 18 (1-2): 81-6, 1995. [PUBMED Abstract]
Latest Updates to This Summary (05/14/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About B-Cell Non-Hodgkin Lymphoma
Revised text to state that aggressive non-Hodgkin lymphoma (NHL) has a worse prognosis in the short term, but a significant number of patients can be cured with intensive combination chemotherapy regimens. More than 70% of patients with aggressive NHL can be cured.
Indolent B-Cell Non-Hodgkin Lymphoma
Revised text about the Follicular Lymphoma International Prognostic Index to state that patients with zero or one risk factor have a 10-year survival rate of 67%, and four or five risk factors confer a 10-year survival rate of 36%. Also added text about the nine prognostic variables identified by the Follicular Lymphoma Evaluation Index model.
Treatment of Indolent, Recurrent B-Cell Non-Hodgkin Lymphoma
Revised text about the results of a single-arm, multicenter, phase II study of 90 patients with relapsed or refractory follicular lymphoma who received mosunetuzumab (cited Sehn et al. as reference 27 and level of evidence C1).
Revised text about the results of a phase II trial of 159 patients with relapsed or refractory follicular lymphoma, marginal zone lymphoma, or diffuse large B-cell lymphoma who received axicabtagene ciloleucel (cited Neelapu et al. as reference 30 and level of evidence C2).
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of indolent adult B-cell non-Hodgkin lymphoma. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Indolent B-Cell Non-Hodgkin Lymphoma Treatment are:
- Eric J. Seifter, MD (Johns Hopkins University)
- Cole H. Sterling, MD (Johns Hopkins University)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Indolent B-Cell Non-Hodgkin Lymphoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/lymphoma/hp/indolent-b-cell-lymphoma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 37437080]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.