Bladder Cancer Treatment (PDQ®)–Health Professional Version
General Information About Bladder Cancer
Incidence and Mortality
Bladder cancer is the sixth most common cancer in the United States after breast cancer, prostate cancer, lung cancer, colon cancer, and melanoma. It is the fourth most common cancer in men and the twelfth most common cancer in women. Of the approximately 84,000 new cases annually, about 65,000 are in men and about 19,000 are in women. Of the roughly 17,000 annual deaths, more than 12,000 are in men and fewer than 5,000 are in women. The reasons for this disparity between the sexes are not well understood.[1,2]
Estimated new cases and deaths from bladder cancer in the United States in 2025:[2]
- New cases: 84,870.
- Deaths: 17,420.
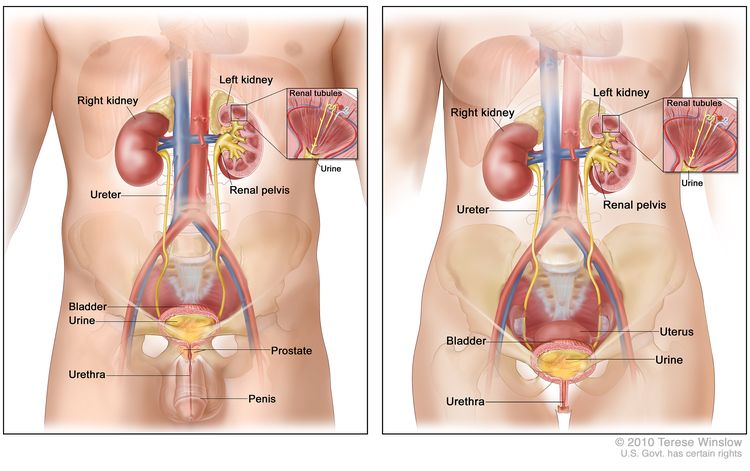
Anatomy
The urinary tract consists of the kidneys, the ureters, the bladder, and the urethra. The urinary tract is lined with transitional cell urothelium from the renal pelvis to the proximal urethra. Transitional cell carcinoma (also known as urothelial carcinoma) can develop anywhere along this pathway.
Histopathology
Under normal conditions, the bladder, the lower part of the kidneys (the renal pelvises), the ureters, and the proximal urethra are lined with a specialized mucous membrane referred to as transitional epithelium (also called urothelium). Most cancers that form in these tissues are transitional cell carcinomas (also called urothelial carcinomas) that derive from transitional epithelium. For more information, see Renal Cell Cancer Treatment and Transitional Cell Cancer of the Renal Pelvis and Ureter Treatment.
Transitional cell carcinoma of the bladder can be low-grade or high-grade:
- Low-grade bladder cancer often recurs in the bladder after treatment but rarely invades the muscular wall of the bladder or spreads to other parts of the body. Patients rarely die of low-grade bladder cancer.
- High-grade bladder cancer commonly recurs in the bladder and has a strong tendency to invade the muscular wall of the bladder and spread to other parts of the body. High-grade bladder cancer is treated more aggressively than low-grade bladder cancer and is much more likely to cause death. Almost all deaths from bladder cancer result from high-grade disease.
Bladder cancer is also divided into muscle-invasive and nonmuscle-invasive disease, based on invasion of the muscularis propria (also known as the detrusor muscle), which is the thick muscle deep in the bladder wall.
- Muscle-invasive disease is much more likely to spread to other parts of the body and is generally treated by either removing the bladder or treating the bladder with radiation and chemotherapy. As noted above, high-grade cancers are much more likely to be muscle-invasive than low-grade cancers. Thus, muscle-invasive cancers are generally treated more aggressively than nonmuscle-invasive cancers.
- Nonmuscle-invasive disease can often be treated by removing the tumor(s) via a transurethral approach. Sometimes chemotherapy or other treatments are introduced into the bladder with a catheter to help fight the cancer.
Under conditions of chronic inflammation, such as infection of the bladder with the Schistosoma haematobium parasite, squamous metaplasia may occur in the bladder. The incidence of squamous cell carcinomas of the bladder is higher under conditions of chronic inflammation than is otherwise seen. In addition to transitional cell carcinomas and squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and sarcomas can form in the bladder. In the United States, transitional cell carcinomas represent most (>90%) bladder cancers. However, a significant number of transitional cell carcinomas have areas of squamous cell or other differentiation.
Carcinogenesis and Risk Factors
Increasing age is the most important risk factor for most cancers. Other risk factors for bladder cancer include:
- Use of tobacco, especially cigarettes.[3]
- Family history of bladder cancer.[4]
- Genetic variants.[5-7]
- HRAS variant (Costello syndrome, facio-cutaneous-skeletal syndrome).
- RB1 variant.
- PTEN/MMAC1 variant (Cowden syndrome).
- NAT2 slow acetylator phenotype.
- GSTM1 null phenotype.
- Occupational exposure to chemicals in processed paint, dye, metal, and petroleum products that include:
- Treatment with cyclophosphamide, ifosfamide, or pelvic radiation for other malignancies.[10-12]
- Use of Chinese herbs: aristolochic acid extracted from species of Aristolochia fangchi.[13]
- Exposure to arsenic.
- Exposure to chlorinated aliphatic hydrocarbons and chlorination by-products in treated water.[16]
- Schistosoma haematobium bladder infections (bilharzial bladder cancer).[17]
- Neurogenic bladder and associated use of indwelling catheters.[18]
There is strong evidence linking exposure to carcinogens to bladder cancer. The most common risk factor for bladder cancer in the United States is cigarette smoking. It is estimated that cigarette smoking causes up to one-half of all bladder cancers and that smoking increases a person’s risk of bladder cancer two to four times above baseline risk.[19,20] Smokers with less functional polymorphisms of N-acetyltransferase-2 (known as slow acetylators) have a higher risk of bladder cancer than other smokers, presumably because of their reduced ability to detoxify carcinogens.
Certain occupational exposures have also been linked to bladder cancer, and higher rates of bladder cancer have been reported in textile dye and rubber tire industries; among painters; leather workers; shoemakers; and aluminum-, iron-, and steelworkers. Specific chemicals linked to bladder carcinogenesis include beta-naphthylamine, 4-aminobiphenyl, and benzidine. Although these chemicals are now generally banned in Western countries, many other chemicals still in use are also suspected of causing bladder cancer.[20]
Exposure to the chemotherapy drug cyclophosphamide has also been associated with an increased risk of bladder cancer.
Chronic urinary tract infections and infection with the parasite S. haematobium have also been associated with an increased risk of bladder cancer, often squamous cell carcinomas. Chronic inflammation is thought to play a key role in carcinogenesis in these settings.
Clinical Features
Bladder cancer typically presents with gross or microscopic hematuria. Less commonly, patients may complain of urinary frequency, nocturia, and dysuria, symptoms that are more common in patients with carcinoma in situ. Patients with upper urinary tract urothelial carcinomas may present with pain resulting from obstruction by the tumor.
Urothelial carcinomas are often multifocal—the entire urothelium needs to be evaluated if a tumor is found. In patients with bladder cancer, upper urinary tract imaging is essential for staging and surveillance. This can be accomplished with ureteroscopy, retrograde pyelograms during cystoscopy, intravenous pyelograms, or computed tomography (CT) urograms. Similarly, patients with an upper urinary tract transitional cell carcinoma have a high risk of developing bladder cancer; these patients need periodic cystoscopy and surveillance of the contralateral upper urinary tract.
Diagnostics
When bladder cancer is suspected, the most useful diagnostic test is cystoscopy. Radiological studies such as CT scans or ultrasound do not have sufficient sensitivity to detect bladder cancers. Cystoscopy can be performed in a urology clinic.
If cancer is seen on cystoscopy, the patient is typically scheduled for bimanual examination under anesthesia and a repeat cystoscopy in an operating room so that transurethral resection of the tumor(s) and/or biopsies can be performed. If a high-grade cancer (including carcinoma in situ) or invasive cancer is seen, the patient is staged with a CT scan of the abdomen and pelvis (or CT urogram) and either a chest x-ray or chest CT scan. Patients with a nonhepatic elevation of alkaline phosphatase or symptoms suggestive of bone metastases undergo a bone scan.
Prognostic Factors
The major prognostic factors in carcinoma of the bladder include:
- Depth of invasion into the bladder wall.
- Pathological grade of the tumor.
- Presence versus absence of carcinoma in situ.
Among nonmuscle-invasive cancers, the following factors are also prognostic:[21]
- Number of tumors.
- Tumor size (e.g., >3 cm or <3 cm).
- Invasion of the lamina propria (Ta vs. T1).
- Whether the tumor is the primary tumor or a recurrence.
Most superficial tumors are well differentiated. Patients in whom superficial tumors are less differentiated, large, multiple, or associated with carcinoma in situ (Tis) in other areas of the bladder mucosa are at greatest risk of recurrence and the development of invasive cancer. These patients may be considered to have the entire urothelial surface at risk of cancer development.
Survival
Patients who die of bladder cancer almost always have disease that has metastasized from the bladder to other organs. Low-grade bladder cancers rarely grow into the muscular wall of the bladder and rarely metastasize, so patients with low-grade (grade I) bladder cancers rarely die of their cancer. Nonetheless, they may experience multiple relapses that need to be resected.
Almost all deaths from bladder cancer are among patients with high-grade disease, which has a much greater potential to invade deeply into the bladder’s muscular wall and spread to other organs.
Approximately 70% to 80% of patients with newly diagnosed bladder cancer present with superficial bladder tumors (i.e., stage Ta, Tis, or T1). The prognosis of these patients depends largely on the grade of the tumor. Patients with high-grade tumors have a significant risk of dying of their cancer, even if it is not muscle-invasive.[22] Among patients with high-grade tumors, those who present with superficial, nonmuscle-invasive bladder cancer can usually be cured, and those with muscle-invasive disease can sometimes be cured.[23-25] Studies have demonstrated that some patients with distant metastases have achieved long-term complete response after being treated with combination chemotherapy regimens, although most such patients have metastases limited to their lymph nodes and have a near-normal performance status.[26,27]
There are clinical trials suitable for patients with all stages of bladder cancer. Whenever possible, patients should consider clinical trials designed to improve upon standard therapy.
General information about clinical trials is also available from the NCI website.
Follow-Up
Bladder cancer tends to recur, even when it is noninvasive at the time of diagnosis. Therefore, standard practice is to perform surveillance of the urinary tract after a diagnosis of bladder cancer. However, no trials have been conducted to assess whether surveillance affects rates of progression, survival, or quality of life. In addition, clinical trials have not defined an optimal surveillance schedule. Urothelial carcinomas are thought to reflect a so-called field defect, whereby the cancer emerges because of genetic variants that are widely present in the patient's bladder or entire urothelium. Thus, people who have had a bladder tumor resected often subsequently have recurrent tumors in the bladder, often in different locations from the site of the initial tumor. Similarly, but less commonly, they may have tumors appear in the upper urinary tract (i.e., in the renal pelvises or ureters).
An alternative explanation for these patterns of recurrence is that cancer cells that are disrupted when a tumor is resected may reimplant elsewhere in the urothelium. Support for this second theory is that tumors are more likely to recur downstream than upstream from the initial cancer. Upper urinary tract cancers are more likely to recur in the bladder than bladder cancers are to recur in the upper urinary tract.[28-31]
References
- National Cancer Institute: SEER Cancer Stat Facts: Bladder Cancer. Bethesda, Md: National Cancer Institute. Available online. Last accessed January 31, 2025.
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Burger M, Catto JW, Dalbagni G, et al.: Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63 (2): 234-41, 2013. [PUBMED Abstract]
- Fraumeni JF Jr, Thomas LB: Malignant bladder tumors in a man and his three sons. JAMA 201 (7): 97-9, 1967.
- Marees T, Moll AC, Imhof SM, et al.: Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst 100 (24): 1771-9, 2008. [PUBMED Abstract]
- Gallagher DJ, Feifer A, Coleman JA: Genitourinary cancer predisposition syndromes. Hematol Oncol Clin North Am 24 (5): 861-83, 2010. [PUBMED Abstract]
- Lindor NM, McMaster ML, Lindor CJ, et al.: Concise handbook of familial cancer susceptibility syndromes - second edition. J Natl Cancer Inst Monogr (38): 1-93, 2008. [PUBMED Abstract]
- Stadler WM: Molecular events in the initiation and progression of bladder cancer (review). Int J Oncol 3: 549-557, 1993.
- Brown T, Slack R, Rushton L, et al.: Occupational cancer in Britain. Urinary tract cancers: bladder and kidney. Br J Cancer 107 (Suppl 1): S76-84, 2012. [PUBMED Abstract]
- Nieder AM, Porter MP, Soloway MS: Radiation therapy for prostate cancer increases subsequent risk of bladder and rectal cancer: a population based cohort study. J Urol 180 (5): 2005-9; discussion 2009-10, 2008. [PUBMED Abstract]
- Abern MR, Dude AM, Tsivian M, et al.: The characteristics of bladder cancer after radiotherapy for prostate cancer. Urol Oncol 31 (8): 1628-34, 2013. [PUBMED Abstract]
- Monach PA, Arnold LM, Merkel PA: Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: a data-driven review. Arthritis Rheum 62 (1): 9-21, 2010. [PUBMED Abstract]
- Cosyns JP: Aristolochic acid and 'Chinese herbs nephropathy': a review of the evidence to date. Drug Saf 26 (1): 33-48, 2003. [PUBMED Abstract]
- Letašiová S, Medve'ová A, Šovčíková A, et al.: Bladder cancer, a review of the environmental risk factors. Environ Health 11 (Suppl 1): S11, 2012. [PUBMED Abstract]
- Fernández MI, López JF, Vivaldi B, et al.: Long-term impact of arsenic in drinking water on bladder cancer health care and mortality rates 20 years after end of exposure. J Urol 187 (3): 856-61, 2012. [PUBMED Abstract]
- Villanueva CM, Cantor KP, Grimalt JO, et al.: Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 165 (2): 148-56, 2007. [PUBMED Abstract]
- Kantor AF, Hartge P, Hoover RN, et al.: Urinary tract infection and risk of bladder cancer. Am J Epidemiol 119 (4): 510-5, 1984. [PUBMED Abstract]
- Locke JR, Hill DE, Walzer Y: Incidence of squamous cell carcinoma in patients with long-term catheter drainage. J Urol 133 (6): 1034-5, 1985. [PUBMED Abstract]
- Brennan P, Bogillot O, Greiser E, et al.: The contribution of cigarette smoking to bladder cancer in women (pooled European data). Cancer Causes Control 12 (5): 411-7, 2001. [PUBMED Abstract]
- Kirkali Z, Chan T, Manoharan M, et al.: Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 66 (6 Suppl 1): 4-34, 2005. [PUBMED Abstract]
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al.: Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49 (3): 466-5; discussion 475-7, 2006. [PUBMED Abstract]
- Herr HW: Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol 163 (1): 60-1; discussion 61-2, 2000. [PUBMED Abstract]
- Stein JP, Lieskovsky G, Cote R, et al.: Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 19 (3): 666-75, 2001. [PUBMED Abstract]
- Madersbacher S, Hochreiter W, Burkhard F, et al.: Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol 21 (4): 690-6, 2003. [PUBMED Abstract]
- Manoharan M, Ayyathurai R, Soloway MS: Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 104 (9): 1227-32, 2009. [PUBMED Abstract]
- Loehrer PJ, Einhorn LH, Elson PJ, et al.: A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 10 (7): 1066-73, 1992. [PUBMED Abstract]
- von der Maase H, Sengelov L, Roberts JT, et al.: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23 (21): 4602-8, 2005. [PUBMED Abstract]
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al.: Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 164 (3 Pt 1): 680-4, 2000. [PUBMED Abstract]
- Nieder AM, Brausi M, Lamm D, et al.: Management of stage T1 tumors of the bladder: International Consensus Panel. Urology 66 (6 Suppl 1): 108-25, 2005. [PUBMED Abstract]
- Babjuk M, Oosterlinck W, Sylvester R, et al.: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 54 (2): 303-14, 2008. [PUBMED Abstract]
- Babjuk M, Oosterlinck W, Sylvester R, et al.: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 59 (6): 997-1008, 2011. [PUBMED Abstract]
Cellular Classification of Bladder Cancer
More than 90% of bladder cancers are transitional cell carcinomas derived from the uroepithelium. About 2% to 7% are squamous cell carcinomas, and 2% are adenocarcinomas.[1] Adenocarcinomas may be of urachal origin or nonurachal origin; the latter type is generally thought to arise from metaplasia of chronically irritated transitional epithelium. Small cell carcinomas also may develop in the bladder.[2,3] Sarcomas of the bladder are very rare.
Pathological grade of transitional cell carcinomas, which is based on cellular atypia, nuclear abnormalities, and the number of mitotic figures, is of great prognostic importance.
References
- Al-Ahmadie H, Lin O, Reuter VE: Pathology and cytology of tumors of the urinary tract. In: Scardino PT, Linehan WM, Zelefsky MJ, et al., eds.: Comprehensive Textbook of Genitourinary Oncology. 4th ed. Lippincott Williams & Wilkins, 2011, pp 295-316.
- Koay EJ, Teh BS, Paulino AC, et al.: A Surveillance, Epidemiology, and End Results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trends. Cancer 117 (23): 5325-33, 2011. [PUBMED Abstract]
- Fahed E, Hansel DE, Raghavan D, et al.: Small cell bladder cancer: biology and management. Semin Oncol 39 (5): 615-8, 2012. [PUBMED Abstract]
Stage Information for Bladder Cancer
The clinical staging of carcinoma of the bladder is determined by the depth of invasion of the bladder wall by the tumor. This determination requires a cystoscopic examination that includes a biopsy and examination under anesthesia to assess the following:
- Size and mobility of palpable masses.
- Degree of induration of the bladder wall.
- Presence of extravesical extension or invasion of adjacent organs.
Clinical staging, even when computed tomography (CT) and/or magnetic resonance imaging (MRI) scans and other imaging modalities are used, often underestimates the extent of tumor, particularly in cancers that are less differentiated and more deeply invasive. CT imaging is the standard staging modality. A clinical benefit from obtaining MRI or positron emission tomography scans instead of CT imaging has not been demonstrated.[1,2]
AJCC Stage Groupings and TNM Definitions
The American Joint Committee on Cancer (AJCC) has designated staging by TNM (tumor, node, metastasis) classification to define bladder cancer.[3]
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Urinary bladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 757–65. | |||
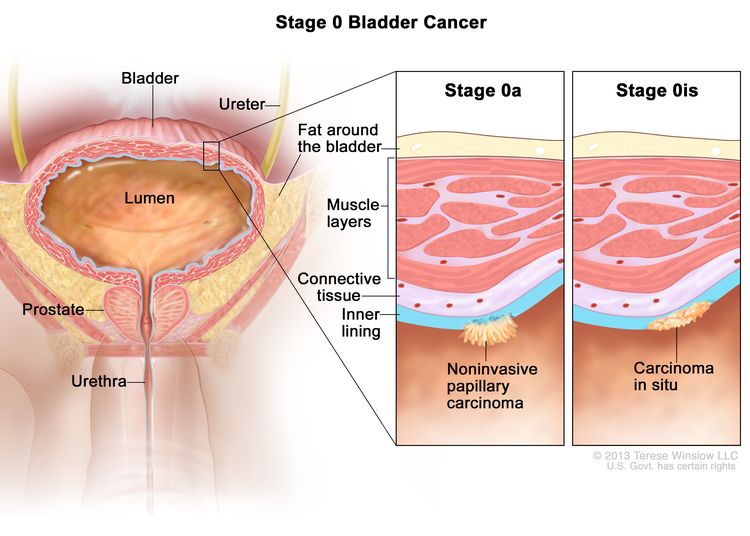
| 0a | Ta, N0, M0 | Ta = Noninvasive papillary carcinoma. |
 |
| N0 = No lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| 0is | Tis, N0, M0 | Tis = Urothelial carcinoma in situ: flat tumor. | |
| N0 = No lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Urinary bladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 757–65. | |||
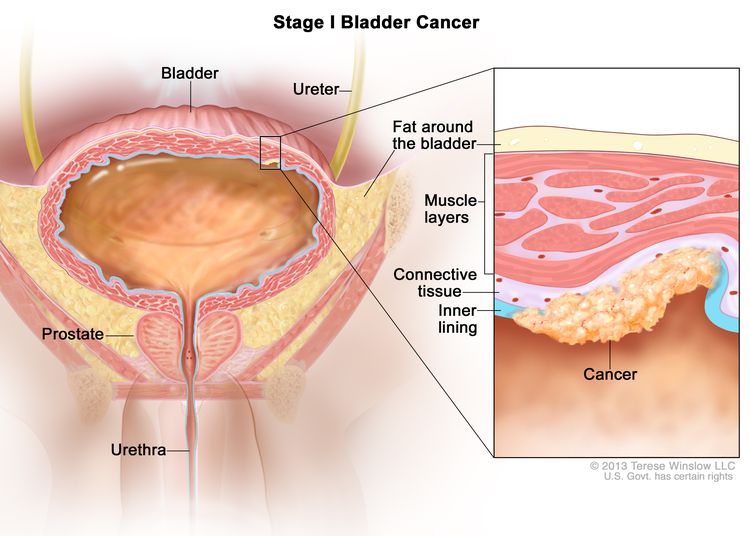
| I | T1, N0, M0 | T1 = Tumor invades lamina propria (subepithelial connective tissue). |
 |
| N0 = No lymph node metastasis | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration | |
|---|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; p = pathological. | ||||
| aReprinted with permission from AJCC: Urinary bladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 757–65. | ||||
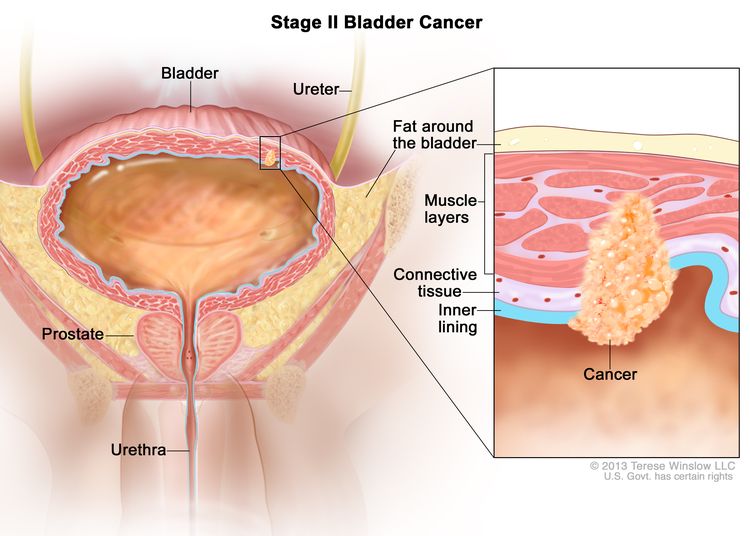
| II | T2a, N0, M0 | pT2a = Tumor invades superficial muscularis propria (inner half). |
 |
|
| N0 = No lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| T2b, N0, M0 | pT2b = Tumor invades deep muscularis propria (outer half). | |||
| N0 = No lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; p = pathological. | |||
| aReprinted with permission from AJCC: Urinary bladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 757–65. | |||
| IIIA | T3a, T3b, T4a, N0, M0 | –pT3a = Microscopically. |
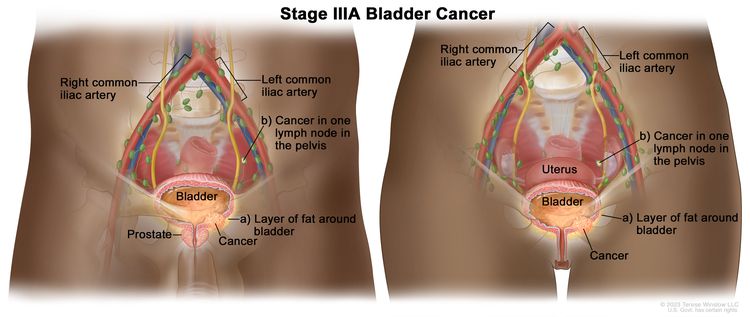 |
| –pT3b = Macroscopically (extravesical mass). | |||
| –T4a = Extravesical tumor invades directly into prostatic stroma, uterus, vagina. | |||
| N0 = No lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| T1–T4a, N1, M0 | T1 = Tumor invades lamina propria (subepithelial connective tissue). | ||
| T2 = Tumor invades muscularis propria. | |||
| –pT2a = Tumor invades superficial muscularis propria (inner half). | |||
| –pT2b = Tumor invades deep muscularis propria (outer half). | |||
| T3 = Tumor invades perivesical soft tissue. | |||
| –pT3a = Microscopically. | |||
| –pT3b = Macroscopically (extravesical mass). | |||
| T4 = Extravesical tumor directly invades any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, abdominal wall. | |||
| –T4a = Extravesical tumor invades directly into prostatic stroma, uterus, vagina. | |||
| N1 = Single regional lymph node metastasis in the true pelvis (perivesical, obturator, internal and external iliac, or sacral lymph node). | |||
| M0 = No distant metastasis. | |||
| IIIB | T1–4a, N2, N3, M0 | T1 = Tumor invades lamina propria (subepithelial connective tissue). |
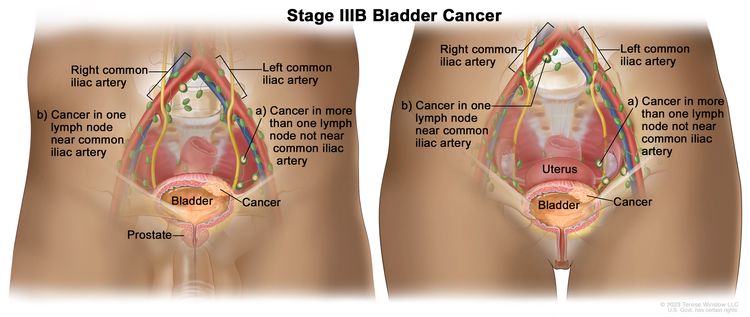 |
| T2 = Tumor invades muscularis propria. | |||
| –pT2a = Tumor invades superficial muscularis propria (inner half). | |||
| –pT2b = Tumor invades deep muscularis propria (outer half). | |||
| T3 = Tumor invades perivesical soft tissue. | |||
| –pT3a = Microscopically. | |||
| pT3b = Macroscopically (extravesical mass). | |||
| T4 = Extravesical tumor directly invades any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, abdominal wall. | |||
| –T4a = Extravesical tumor invades directly into prostatic stroma, uterus, vagina. | |||
| N2 = Multiple regional lymph node metastasis in the true pelvis (perivesical, obturator, internal and external iliac, or sacral lymph node metastasis). | |||
| N3 = Lymph node metastasis to the common iliac lymph nodes. | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; p = pathological. | |||
| aReprinted with permission from AJCC: Urinary bladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 757–65. | |||
| IVA | T4b, N0, M0 | –T4b = Extravesical tumor invades pelvic wall, abdominal wall. |
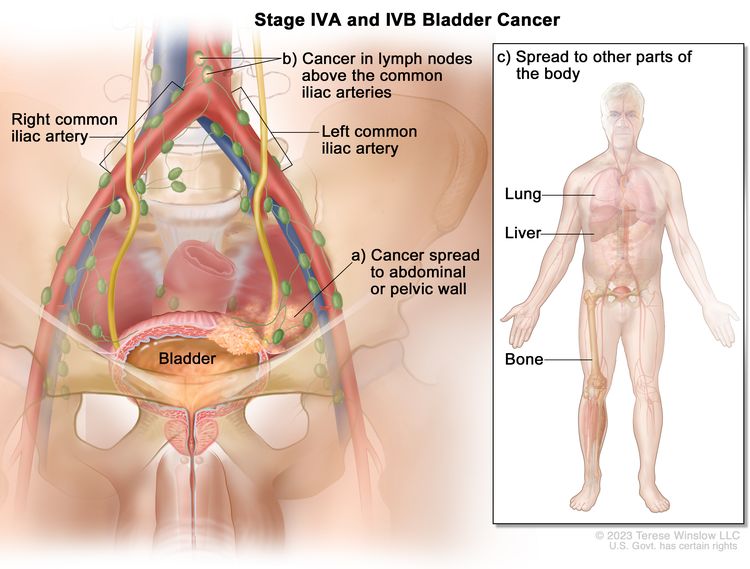 |
| N0 = No lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| Any T, Any N, M1a | TX = Primary tumor cannot be assessed. | ||
| T0 = No evidence of primary tumor. | |||
| –Ta = Noninvasive papillary carcinoma. | |||
| Tis = Urothelial carcinoma in situ: flat tumor. | |||
| T1 = Tumor invades lamina propria (subepithelial connective tissue). | |||
| T2 = Tumor invades muscularis propria. | |||
| –pT2a = Tumor invades superficial muscularis propria (inner half). | |||
| –pT2b = Tumor invades deep muscularis propria (outer half). | |||
| T3 = Tumor invades perivesical soft tissue. | |||
| –pT3a = Microscopically. | |||
| –pT3b = Macroscopically (extravesical mass). | |||
| T4 = Extravesical tumor directly invades any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, abdominal wall. | |||
| –T4a = Extravesical tumor invades directly into prostatic stroma, uterus, vagina. | |||
| –T4b = Extravesical tumor invades pelvic wall, abdominal wall. | |||
| NX = Lymph nodes cannot be assessed. | |||
| N0 = No lymph node metastasis. | |||
| N1 = Single regional lymph node metastasis in the true pelvis (perivesical, obturator, internal and external iliac, or sacral lymph node). | |||
| N2 = Multiple regional lymph node metastasis in the true pelvis (perivesical, obturator, internal and external iliac, or sacral lymph node metastasis). | |||
| N3 = Lymph node metastasis to the common iliac lymph nodes. | |||
| M0 = No distant metastasis. | |||
| –M1a = Distant metastasis limited to lymph nodes beyond the common iliacs. | |||
| IVB | Any T, Any N, M1b | TX = Primary tumor cannot be assessed. | |
| T0 = No evidence of primary tumor. | |||
| –Ta = Noninvasive papillary carcinoma. | |||
| Tis = Urothelial carcinoma in situ: flat tumor. | |||
| T1 = Tumor invades lamina propria (subepithelial connective tissue). | |||
| T2 = Tumor invades muscularis propria. | |||
| –pT2a = Tumor invades superficial muscularis propria (inner half). | |||
| –pT2b = Tumor invades deep muscularis propria (outer half). | |||
| T3 = Tumor invades perivesical soft tissue. | |||
| –pT3a = Microscopically. | |||
| –pT3b = Macroscopically (extravesical mass). | |||
| T4 = Extravesical tumor directly invades any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, abdominal wall. | |||
| –T4a = Extravesical tumor invades directly into prostatic stroma, uterus, vagina. | |||
| –T4b = Extravesical tumor invades pelvic wall, abdominal wall. | |||
| NX = Lymph nodes cannot be assessed. | |||
| N0 = No lymph node metastasis. | |||
| N1 = Single regional lymph node metastasis in the true pelvis (perivesical, obturator, internal and external iliac, or sacral lymph node). | |||
| N2 = Multiple regional lymph node metastasis in the true pelvis (perivesical, obturator, internal and external iliac, or sacral lymph node metastasis). | |||
| N3 = Lymph node metastasis to the common iliac lymph nodes. | |||
| M1b = Non-lymph node distant metastases. | |||
For urothelial histologies, a low- and high-grade designation is used to match the current World Health Organization/International Society of Urologic Pathology recommended grading system.[3]
For squamous cell carcinoma and adenocarcinoma, the grading schema in Table 6 is recommended.[3]
| G | G Definition |
|---|---|
| aReprinted with permission from AJCC: Urinary bladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 757–65. | |
| GX | Grade cannot be assessed. |
| G1 | Well differentiated. |
| G2 | Moderately differentiated. |
| G3 | Poorly differentiated. |
References
- Cowan NC, Crew JP: Imaging bladder cancer. Curr Opin Urol 20 (5): 409-13, 2010. [PUBMED Abstract]
- Green DA, Durand M, Gumpeni N, et al.: Role of magnetic resonance imaging in bladder cancer: current status and emerging techniques. BJU Int 110 (10): 1463-70, 2012. [PUBMED Abstract]
- Bochner BH, Hansel DE, Efstathiou JA, et al.: Urinary Bladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 757-65.
Treatment Option Overview for Bladder Cancer
Nonmuscle-Invasive Bladder Cancer
Treatment of nonmuscle-invasive bladder cancers (Ta, Tis, T1) is based on risk stratification. Essentially all patients are initially treated with transurethral resection (TUR) of the bladder tumor, followed by a single immediate instillation of intravesical chemotherapy (mitomycin is typically used in the United States).[1-7]
Subsequent therapy is based on risk and typically consists of one of the following:[6-9]
- Surveillance for relapse or recurrence (typically used for tumors with low risk of recurrence or progression).
- A minimum of 1 year of intravesical treatments with bacillus Calmette-Guérin (BCG) plus surveillance for relapse (typically used for tumors at intermediate or high risk of progression to muscle-invasive disease).
- Additional intravesical chemotherapy (typically used for tumors with a high risk of recurrence but low risk of progression to muscle-invasive disease).
Muscle-Invasive Bladder Cancer
Standard treatment for patients with muscle-invasive bladder cancers whose goal is cure is either neoadjuvant multiagent cisplatin–based chemotherapy followed by radical cystectomy and urinary diversion or radiation therapy with concomitant chemotherapy.[10-13] Other treatment approaches include:
Many patients newly diagnosed with bladder cancer are candidates for participation in clinical trials.
Reconstructive techniques that fashion low-pressure storage reservoirs from the reconfigured small and large bowel eliminate the need for external drainage devices and, in many patients, allow voiding per urethra. These techniques are designed to improve the quality of life for patients who require cystectomy.[19]
Fluorouracil Dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[20,21] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[20-22] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient's DPYD genotype and number of functioning DPYD alleles.[23-25] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[26] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[27]
References
- Sylvester RJ, Oosterlinck W, van der Meijden AP: A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 171 (6 Pt 1): 2186-90, quiz 2435, 2004. [PUBMED Abstract]
- Mariappan P, Smith G: A surveillance schedule for G1Ta bladder cancer allowing efficient use of check cystoscopy and safe discharge at 5 years based on a 25-year prospective database. J Urol 173 (4): 1108-11, 2005. [PUBMED Abstract]
- Nieder AM, Brausi M, Lamm D, et al.: Management of stage T1 tumors of the bladder: International Consensus Panel. Urology 66 (6 Suppl 1): 108-25, 2005. [PUBMED Abstract]
- Oosterlinck W, Solsona E, Akaza H, et al.: Low-grade Ta (noninvasive) urothelial carcinoma of the bladder. Urology 66 (6 Suppl 1): 75-89, 2005. [PUBMED Abstract]
- Sylvester RJ, van der Meijden A, Witjes JA, et al.: High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology 66 (6 Suppl 1): 90-107, 2005. [PUBMED Abstract]
- Babjuk M, Oosterlinck W, Sylvester R, et al.: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 54 (2): 303-14, 2008. [PUBMED Abstract]
- Babjuk M, Oosterlinck W, Sylvester R, et al.: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 59 (6): 997-1008, 2011. [PUBMED Abstract]
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al.: Upper urinary tract tumors after primary superficial bladder tumors: prognostic factors and risk groups. J Urol 164 (4): 1183-7, 2000. [PUBMED Abstract]
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al.: Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol 163 (1): 73-8, 2000. [PUBMED Abstract]
- Sauer R, Birkenhake S, Kühn R, et al.: Efficacy of radiochemotherapy with platin derivatives compared to radiotherapy alone in organ-sparing treatment of bladder cancer. Int J Radiat Oncol Biol Phys 40 (1): 121-7, 1998. [PUBMED Abstract]
- Advanced Bladder Cancer Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 361 (9373): 1927-34, 2003. [PUBMED Abstract]
- Winquist E, Kirchner TS, Segal R, et al.: Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol 171 (2 Pt 1): 561-9, 2004. [PUBMED Abstract]
- James ND, Hussain SA, Hall E, et al.: Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366 (16): 1477-88, 2012. [PUBMED Abstract]
- Madersbacher S, Hochreiter W, Burkhard F, et al.: Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol 21 (4): 690-6, 2003. [PUBMED Abstract]
- Stein JP, Dunn MD, Quek ML, et al.: The orthotopic T pouch ileal neobladder: experience with 209 patients. J Urol 172 (2): 584-7, 2004. [PUBMED Abstract]
- Manoharan M, Ayyathurai R, Soloway MS: Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 104 (9): 1227-32, 2009. [PUBMED Abstract]
- Widmark A, Flodgren P, Damber JE, et al.: A systematic overview of radiation therapy effects in urinary bladder cancer. Acta Oncol 42 (5-6): 567-81, 2003. [PUBMED Abstract]
- Holzbeierlein JM, Lopez-Corona E, Bochner BH, et al.: Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol 172 (3): 878-81, 2004. [PUBMED Abstract]
- Hautmann RE, Miller K, Steiner U, et al.: The ileal neobladder: 6 years of experience with more than 200 patients. J Urol 150 (1): 40-5, 1993. [PUBMED Abstract]
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PUBMED Abstract]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PUBMED Abstract]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PUBMED Abstract]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PUBMED Abstract]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PUBMED Abstract]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PUBMED Abstract]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PUBMED Abstract]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PUBMED Abstract]
Treatment of Stage 0 Bladder Cancer
Treatment Options for Stage 0 Bladder Cancer
Patients with stage 0 bladder tumors can be cured by a variety of treatments, even though the tendency for new tumor formation is high. In a series of patients with Ta or T1 tumors who were followed for a minimum of 20 years or until death, the risk of bladder cancer recurrence after initial resection was 80%.[1] Of greater concern than recurrence is the risk of progression to muscle-invasive, locally-advanced, or metastatic bladder cancer. While progression is rare for patients with low-grade tumors, it is common among patients with high-grade cancers.
One series of 125 patients with TaG3 cancers followed for 15 to 20 years reported that 39% progressed to more advanced-stage disease while 26% died of urothelial cancer. In comparison, among 23 patients with TaG1 tumors, none died and 5% progressed.[2] Risk factors for recurrence and progression include:[2-6]
- High-grade disease.
- Presence of carcinoma in situ.
- Tumor larger than 3 cm.
- Multiple tumors.
- History of prior bladder cancer.
Treatment options for stage 0 bladder cancer include:
- Transurethral resection (TUR) with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy.
- TUR with fulguration.
- TUR with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy followed by periodic intravesical instillations of bacillus Calmette-Guérin (BCG).
- TUR with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy followed by intravesical chemotherapy.
- Segmental cystectomy (rarely indicated).
- Radical cystectomy (in rare, highly selected patients with extensive or refractory superficial high-grade tumors).
Transurethral resection (TUR) with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy
TUR and fulguration are the most common and conservative forms of management. Careful surveillance of subsequent bladder tumor progression is important. Because most bladder cancers recur after TUR, one immediate intravesical instillation of chemotherapy is often given after TUR. Numerous randomized controlled trials have evaluated this practice, and a meta-analysis of seven trials reported that a single intravesical treatment with chemotherapy reduced the odds of recurrence by 39% (odds ratio [OR], 0.61; P < .0001).[7,8] However, although a single instillation of chemotherapy lowers the relapse rate in patients with multiple tumors, most still relapse. Such treatment is insufficient by itself for these patients.
One retrospective series addressed the value of performing a second TUR within 2 to 6 weeks of the first TUR.[9][Level of evidence C3] A second TUR performed on 38 patients with Tis or Ta disease revealed that nine patients (24%) had lamina propria invasion (T1) and three patients (8%) had muscle invasion (T2).[9]
Such information may change the definitive management options in these individuals. Patients with extensive multifocal recurrent disease and/or other unfavorable prognostic features require more aggressive forms of treatment.
Evidence (TUR with fulguration followed by immediate postoperative instillation of intravesical chemotherapy):
- A 2004 meta-analysis of seven randomized controlled trials (1,476 patients with stage Ta or stage T1 bladder cancer) compared TUR alone with TUR followed by a single immediate intravesical instillation of chemotherapy.[7]
- The relapse rate was 48% for patients who received TUR alone and 37% for patients who received TUR plus intravesical chemotherapy (OR, 0.61; P < .0001). The risk of recurrence declined for patients with single (OR, 0.61) or multiple (OR, 0.44) tumors, but 65% of those with multiple tumors relapsed despite intravesical chemotherapy.
- Agents studied included epirubicin, mitomycin (MMC), thiotepa, and pirarubicin.
- A subsequent multicenter randomized controlled trial confirmed the reduction in risk of recurrence. A study that included 404 patients reported a relapse rate of 51% for patients who received epirubicin immediately after TUR and 63% for patients who received placebo immediately after TUR (P = .04). However, only small recurrences were prevented in this study, drawing into question the magnitude of benefit.[10]
- Similarly, another multicenter randomized controlled trial confirmed the reduction in risk of recurrence. One study randomly assigned patients (N = 305) to receive either an instillation of epirubicin or no further treatment after TUR.[8]
- The relapse rates were 62% for patients who received epirubicin and 77% for patients in the control arm (P = .016).
- The hazard ratio for recurrence was 0.56 (P = .002) with epirubicin. However, the main benefit was seen in patients at lower risk of relapse. Among patients at intermediate or high risk of relapse, the relapse rates were 81% with epirubicin versus 85% with no further treatment (P = .35).
TUR with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy followed by periodic intravesical instillations of BCG
Intravesical BCG is the treatment of choice for reducing the risk of cancer progression and is mainly used for cancers with an intermediate or high risk of progressing.[6,11-13] An individual patient meta-analysis of randomized trials compared intravesical BCG with intravesical MMC. The meta-analysis reported that there was a 32% reduction in risk of recurrence with BCG but only when the BCG treatment included a maintenance phase whereby BCG was given periodically for at least 1 year (typically an induction phase of six weekly treatments followed by three weekly treatments every 3 months).[12] Intravesical chemotherapy is tolerated better than intravesical BCG.[14-18] Although BCG may not prolong overall survival for Tis disease, it appears to afford complete response rates of about 70%, thereby decreasing the need for salvage cystectomy.[17] Studies show that intravesical BCG delays tumor recurrence and tumor progression.[18,19]
Intravesical therapy with thiotepa, MMC, doxorubicin, or BCG is most often used in patients with multiple tumors or recurrent tumors or as a prophylactic measure in high-risk patients after TUR.[20-22]
Evidence (TUR with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy followed by periodic intravesical instillations of BCG):
Intravesical chemotherapy
- Three meta-analyses of randomized controlled trials that compared TUR alone with TUR followed by intravesical chemotherapy reported that adjuvant therapy was associated with a statistically significant increase in time to recurrence.[23-25] No advantage has been shown with respect to survival or prevention of progression to invasive disease or metastases.
Intravesical BCG with maintenance BCG treatments
- An individual patient meta-analysis of nine randomized trials (2,820 patients with Ta or T1 bladder cancer) that compared intravesical BCG with intravesical MMC was published.[12]
- Among trials in which the BCG treatment included a maintenance component, there was a 32% reduction in risk of recurrence (P < .0001) compared with MMC. BCG was associated with a 28% increase in the risk of recurrence when there was no maintenance BCG administered compared with MMC.
- There were no differences in progression or death.[12]
- A meta-analysis of nine randomized controlled trials (2,410 patients) that compared intravesical BCG with MMC was published.[26]
- Progression was observed in 7.67% of the patients who received BCG and 9.44% of patients who received MMC at a median follow-up of 26 months (P = .08).
- When the analysis was limited to trials in which the BCG arm included a maintenance component, the progression rate was significantly lower in the patients who received BCG (OR, 0.66; 95% confidence interval, 0.47–0.94; P = .02).
- A meta-analysis of the published results of nine randomized controlled trials that compared intravesical BCG with intravesical chemotherapy in 700 patients with carcinoma in situ of the bladder was published.[11]
- With a median follow-up of 3.6 years, 47% of the BCG group had no evidence of disease and 26% of the chemotherapy group had no evidence of disease.
- BCG was superior to MMC at preventing recurrence only when maintenance BCG was part of the treatment.
- A controlled trial evaluated 384 patients randomly assigned to induction intravesical BCG or induction intravesical BCG followed by maintenance intravesical BCG.[27]
- The median recurrence-free survival was 36 months without maintenance BCG and 77 months in the maintenance arm (P < .0001). The risk of disease worsening (progression to T2 or greater disease, use of cystectomy, systemic chemotherapy, or radiation therapy) was greater in the induction arm than in the maintenance arm (P = .04).
- The 5-year overall survival rate was 78% in the induction arm versus 83% in the maintenance arm, but this difference was not statistically significant.
BCG is associated with a risk of significant toxicity, including rare deaths from BCG sepsis. Compared with MMC, BCG produces more local toxicity (44% with BCG vs. 30% with MMC) and systemic side effects (19% with BCG vs. 12% with MMC). Because of concerns about side effects and toxicity, BCG is not generally used for patients with a low risk of progression to advanced-stage disease.[6,26]
Segmental cystectomy (rarely indicated)
Segmental cystectomy is rarely indicated.[22] It is indicated for relatively few patients because of the tendency of bladder cancer to involve multiple regions of the bladder mucosa and to occur in areas that cannot be segmentally resected. Moreover, cystectomy (whether segmental or radical) is generally not indicated for T0 bladder cancer (see radical cystectomy below).[28,29]
Radical cystectomy (in rare, highly selected patients with extensive or refractory superficial high-grade tumors)
Radical cystectomy is used in selected patients with extensive or refractory superficial tumors,[2,30,31] based on reports that up to 20% of patients with Tis will die of bladder cancer. However, cystectomy (whether segmental or radical) is generally not indicated for patients with Ta or Tis bladder cancer. Patients at high risk of progression, typically those with recurrent high-grade tumors with carcinoma in situ after intravesical therapy with BCG, should consider radical cystectomy.[32-35]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Holmäng S, Hedelin H, Anderström C, et al.: The relationship among multiple recurrences, progression and prognosis of patients with stages Ta and T1 transitional cell cancer of the bladder followed for at least 20 years. J Urol 153 (6): 1823-6; discussion 1826-7, 1995. [PUBMED Abstract]
- Herr HW: Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol 163 (1): 60-1; discussion 61-2, 2000. [PUBMED Abstract]
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al.: Upper urinary tract tumors after primary superficial bladder tumors: prognostic factors and risk groups. J Urol 164 (4): 1183-7, 2000. [PUBMED Abstract]
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al.: Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol 163 (1): 73-8, 2000. [PUBMED Abstract]
- Babjuk M, Oosterlinck W, Sylvester R, et al.: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 54 (2): 303-14, 2008. [PUBMED Abstract]
- Babjuk M, Oosterlinck W, Sylvester R, et al.: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 59 (6): 997-1008, 2011. [PUBMED Abstract]
- Sylvester RJ, Oosterlinck W, van der Meijden AP: A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 171 (6 Pt 1): 2186-90, quiz 2435, 2004. [PUBMED Abstract]
- Gudjónsson S, Adell L, Merdasa F, et al.: Should all patients with non-muscle-invasive bladder cancer receive early intravesical chemotherapy after transurethral resection? The results of a prospective randomised multicentre study. Eur Urol 55 (4): 773-80, 2009. [PUBMED Abstract]
- Herr HW: The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 162 (1): 74-6, 1999. [PUBMED Abstract]
- Berrum-Svennung I, Granfors T, Jahnson S, et al.: A single instillation of epirubicin after transurethral resection of bladder tumors prevents only small recurrences. J Urol 179 (1): 101-5; discussion 105-6, 2008. [PUBMED Abstract]
- Sylvester RJ, van der Meijden AP, Witjes JA, et al.: Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 174 (1): 86-91; discussion 91-2, 2005. [PUBMED Abstract]
- Malmström PU, Sylvester RJ, Crawford DE, et al.: An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol 56 (2): 247-56, 2009. [PUBMED Abstract]
- Stenzl A, Cowan NC, De Santis M, et al.: Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol 59 (6): 1009-18, 2011. [PUBMED Abstract]
- Herr HW, Schwalb DM, Zhang ZF, et al.: Intravesical bacillus Calmette-Guérin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol 13 (6): 1404-8, 1995. [PUBMED Abstract]
- Sarosdy MF, Lamm DL: Long-term results of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. J Urol 142 (3): 719-22, 1989. [PUBMED Abstract]
- Catalona WJ, Hudson MA, Gillen DP, et al.: Risks and benefits of repeated courses of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. J Urol 137 (2): 220-4, 1987. [PUBMED Abstract]
- De Jager R, Guinan P, Lamm D, et al.: Long-term complete remission in bladder carcinoma in situ with intravesical TICE bacillus Calmette Guerin. Overview analysis of six phase II clinical trials. Urology 38 (6): 507-13, 1991. [PUBMED Abstract]
- Herr HW, Wartinger DD, Fair WR, et al.: Bacillus Calmette-Guerin therapy for superficial bladder cancer: a 10-year followup. J Urol 147 (4): 1020-3, 1992. [PUBMED Abstract]
- Lamm DL, Griffith JG: Intravesical therapy: does it affect the natural history of superficial bladder cancer? Semin Urol 10 (1): 39-44, 1992. [PUBMED Abstract]
- Igawa M, Urakami S, Shirakawa H, et al.: Intravesical instillation of epirubicin: effect on tumour recurrence in patients with dysplastic epithelium after transurethral resection of superficial bladder tumour. Br J Urol 77 (3): 358-62, 1996. [PUBMED Abstract]
- Lamm DL, Blumenstein BA, Crawford ED, et al.: A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Engl J Med 325 (17): 1205-9, 1991. [PUBMED Abstract]
- Soloway MS: The management of superficial bladder cancer. In: Javadpour N, ed.: Principles and Management of Urologic Cancer. 2nd ed. Williams and Wilkins, 1983, pp 446-467.
- Pawinski A, Sylvester R, Kurth KH, et al.: A combined analysis of European Organization for Research and Treatment of Cancer, and Medical Research Council randomized clinical trials for the prophylactic treatment of stage TaT1 bladder cancer. European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council Working Party on Superficial Bladder Cancer. J Urol 156 (6): 1934-40, discussion 1940-1, 1996. [PUBMED Abstract]
- Huncharek M, Geschwind JF, Witherspoon B, et al.: Intravesical chemotherapy prophylaxis in primary superficial bladder cancer: a meta-analysis of 3703 patients from 11 randomized trials. J Clin Epidemiol 53 (7): 676-80, 2000. [PUBMED Abstract]
- Huncharek M, McGarry R, Kupelnick B: Impact of intravesical chemotherapy on recurrence rate of recurrent superficial transitional cell carcinoma of the bladder: results of a meta-analysis. Anticancer Res 21 (1B): 765-9, 2001 Jan-Feb. [PUBMED Abstract]
- Böhle A, Bock PR: Intravesical bacille Calmette-Guérin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology 63 (4): 682-6; discussion 686-7, 2004. [PUBMED Abstract]
- Lamm DL, Blumenstein BA, Crissman JD, et al.: Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 163 (4): 1124-9, 2000. [PUBMED Abstract]
- Holzbeierlein JM, Lopez-Corona E, Bochner BH, et al.: Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol 172 (3): 878-81, 2004. [PUBMED Abstract]
- Fahmy N, Aprikian A, Tanguay S, et al.: Practice patterns and recurrence after partial cystectomy for bladder cancer. World J Urol 28 (4): 419-23, 2010. [PUBMED Abstract]
- Amling CL, Thrasher JB, Frazier HA, et al.: Radical cystectomy for stages Ta, Tis and T1 transitional cell carcinoma of the bladder. J Urol 151 (1): 31-5; discussion 35-6, 1994. [PUBMED Abstract]
- Sylvester RJ, van der Meijden A, Witjes JA, et al.: High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology 66 (6 Suppl 1): 90-107, 2005. [PUBMED Abstract]
- Madersbacher S, Hochreiter W, Burkhard F, et al.: Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol 21 (4): 690-6, 2003. [PUBMED Abstract]
- Stein JP, Dunn MD, Quek ML, et al.: The orthotopic T pouch ileal neobladder: experience with 209 patients. J Urol 172 (2): 584-7, 2004. [PUBMED Abstract]
- Lerner SP, Tangen CM, Sucharew H, et al.: Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancer. Urol Oncol 27 (2): 155-9, 2009 Mar-Apr. [PUBMED Abstract]
- Manoharan M, Ayyathurai R, Soloway MS: Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 104 (9): 1227-32, 2009. [PUBMED Abstract]
Treatment of Stage I Bladder Cancer
Treatment Options for Stage I Bladder Cancer
Patients with stage I bladder tumors are unlikely to die of bladder cancer, but the tendency for new tumor formation is high. In a series of patients with Ta or T1 tumors who were followed for a minimum of 20 years or until death, the risk of bladder recurrence after initial resection was 80%.[1] Of greater concern than recurrence is the risk of progression to muscle-invasive, locally-advanced, or metastatic bladder cancer. While progression is rare for low-grade tumors, it is common among high-grade cancers.
One series of 125 patients with TaG3 cancers followed for 15 to 20 years reported that 39% progressed to more advanced stage disease, while 26% died of urothelial cancer. In comparison, among 23 patients with TaG1 tumors, none died and 5% progressed.[2] Risk factors for recurrence and progression include:[2-6]
- High-grade disease.
- Presence of carcinoma in situ.
- Tumor larger than 3 cm.
- Multiple tumors.
- History of prior bladder cancer.
Treatment options for stage I bladder cancer include:
- Transurethral resection (TUR) with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy.
- TUR with fulguration.
- TUR with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy followed by periodic intravesical instillations of bacillus Calmette-Guérin (BCG).
- TUR with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy followed by intravesical chemotherapy.
- Segmental cystectomy (rarely indicated).
- Radical cystectomy (in selected patients with extensive or refractory superficial tumors).
TUR with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy
TUR and fulguration are the most common and conservative forms of management. Careful surveillance of subsequent bladder tumor progression is important. Because most bladder cancers recur after TUR, one immediate intravesical instillation of chemotherapy after TUR is widely used. Numerous randomized controlled trials have evaluated this practice, and a meta-analysis of seven trials reported that a single intravesical treatment with chemotherapy reduced the odds of recurrence by 39% (odds ratio [OR], 0.61; P < .0001).[7,8]
TUR with fulguration
Staging a bladder cancer via TUR is based on the extent of invasion. To assess whether cancer has invaded the muscle, muscularis propria must be present in the resected tissue. While a repeat TUR is generally considered mandatory for T1 and high-grade noninvasive bladder cancers if no muscularis propria is present in the resected tissue from the first TUR, many experts recommend that a second TUR be routinely performed within 2 to 6 weeks of the first TUR to confirm staging and achieve a more complete resection. The rationale for this derives from numerous findings, including:
- The risk of local recurrence after TUR is high.
- Residual cancer is often found when a repeat TUR is performed.
- More-advanced–stage cancer is sometimes found with repeat TUR.
- Patients undergoing radical cystectomy for nonmuscle-invasive bladder cancer are often found to have T2 or greater disease when the cystectomy specimen is examined.
- A substantial number of patients with high-grade nonmuscle-invasive bladder cancer subsequently die of their disease.
Evidence (routine repeat TUR):
- A review of more than 2,400 patients from over 60 different institutions reported a 3-month recurrence rate of roughly 14% to 20% after TUR, while a literature review reported that up to 10% of patients who underwent a second TUR for Ta to T1 cancer were upstaged to T2.[9] The likelihood of being upstaged to T2 is much higher when no muscularis propria is present in the initial TUR tissue.[10]
- One retrospective series of 38 patients with Tis or Ta disease who underwent a second TUR found that nine patients (24%) had lamina propria invasion (T1) and three patients (8%) had muscle invasion (T2).[11]
- A subsequent study from a different institution reported that among 214 patients with Ta to T1 cancers who underwent a second TUR, 27% of Ta and 37% of T1 patients had residual cancer detected.[12]
- A review of other published papers reported that residual tumor was present in 27% to 62% of cases, and muscle-invasive disease was discovered in 1% to 10% of case series with at least 50 patients.[10]
Repeat TUR has not been shown to reduce relapse rates or prolong survival, but there is a clear rationale for seeking accurate staging information on which to base treatment decisions. Such information may change the definitive management options for patients and identify patients who are more likely to benefit from more aggressive treatment.
TUR with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy followed by periodic intravesical instillations of BCG
Intravesical BCG is the treatment of choice for reducing the risk of cancer progression and is mainly used for cancers with an intermediate or high risk of progressing.[6,13-15] An individual patient meta-analysis of randomized trials that compared intravesical BCG with intravesical mitomycin (MMC) reported that there was a 32% reduction in risk of recurrence with BCG but only when the BCG treatment included a maintenance phase whereby BCG was given periodically for at least 1 year (typically an induction phase of six weekly treatments followed by three weekly treatments every 3 months).[14] Intravesical chemotherapy is tolerated better than intravesical BCG.[16-20] Although BCG may not prolong overall survival for Tis disease, it appears to afford complete response rates of about 70%, thereby decreasing the need for salvage cystectomy.[19] Studies show that intravesical BCG delays tumor recurrence and tumor progression.[20,21]
Evidence (immediate intravesical chemotherapy after transurethral resection):
- A 2004 meta-analysis of seven randomized controlled trials (1,476 patients with Ta or T1 bladder cancer) compared TUR alone with TUR followed by a single immediate intravesical instillation of chemotherapy. Agents studied included epirubicin, MMC, thiotepa, and pirarubicin.[7]
- The relapse rates were 48% for patients who received TUR alone and 37% for patients who received TUR plus intravesical chemotherapy (OR, 0.61; P < .0001). The risk of recurrence declined for patients with single (OR, 0.61) or multiple (OR, 0.44) tumors, but 65% of those with multiple tumors relapsed despite intravesical chemotherapy.
- A subsequent multicenter randomized controlled trial confirmed the reduction in risk of recurrence. A study that included 404 patients reported a relapse rate of 51% for patients who received epirubicin immediately after TUR and 63% for patients who received placebo immediately after TUR (P = .04). However, only small recurrences were prevented in this study, drawing into question the magnitude of benefit.[22]
- Similarly, another multicenter randomized controlled trial confirmed the reduction in risk of recurrence. One study randomly assigned patients (N = 305) to receive either an instillation of epirubicin or no further treatment after TUR.[8]
- The relapse rates were 62% for patients who received epirubicin and 77% for patients in the control arm (P = .016).
- The hazard ratio for recurrence was 0.56 (P = .002) with epirubicin. However, the main benefit was seen in patients at lower risk of relapse. Among patients at intermediate or high risk of relapse, the relapse rates were 81% with epirubicin versus 85% with no further treatment (P = .35).
Evidence (intravesical BCG with maintenance BCG treatments):
- An individual patient meta-analysis of nine randomized trials (2,820 patients with Ta or T1 bladder cancer) that compared intravesical BCG with intravesical MMC was published.[14]
- Among trials in which the BCG treatment included a maintenance component, there was a 32% reduction in risk of recurrence (P < .0001) compared with MMC. BCG was associated with a 28% increase in the risk of recurrence when no maintenance BCG was given compared with MMC.
- There were no differences in progression or death.
- A meta-analysis of nine randomized controlled trials (2,410 patients) that compared intravesical BCG with MMC was published.[23]
- Progression was seen in 7.67% of the patients who received BCG and 9.44% of patients who received MMC at a median follow-up of 26 months (P = .08).
- When the analysis was limited to trials in which the BCG arm included a maintenance component, the progression rate was significantly lower in the patients who received BCG (OR, 0.66; 95% confidence interval, 0.47–0.94; P = .02).
- A meta-analysis of the published results of nine randomized controlled trials that compared intravesical BCG with intravesical chemotherapy in 700 patients with carcinoma in situ of the bladder was published.[13]
- With a median follow-up of 3.6 years, 47% of the BCG group had no evidence of disease and 26% of the chemotherapy group had no evidence of disease.
- In this meta-analysis, BCG was superior to MMC at preventing recurrence only when maintenance BCG was part of the treatment.
- A controlled trial evaluated 384 patients randomly assigned to induction intravesical BCG or induction intravesical BCG followed by maintenance intravesical BCG.[24]
- Median recurrence-free survival was 36 months without maintenance BCG and 77 months in the maintenance arm (P < .0001). The risk of disease worsening (progression to T2 or greater disease, use of cystectomy, systemic chemotherapy, or radiation therapy) was greater in the induction arm than in the maintenance arm (P = .04).
- The 5-year overall survival rate was 78% in the induction arm versus 83% in the maintenance arm, but this difference was not statistically significant.
BCG is associated with a risk of significant toxicity, including rare deaths from BCG sepsis. Compared with MMC, BCG produces more local toxicity (44% with BCG vs. 30% with MMC) and systemic side effects (19% with BCG vs. 12% with MMC). Because of concerns about side effects and toxicity, BCG is not generally used for patients with a low risk of progression to more-advanced–stage disease.[6,23]
Evidence (two treatment courses of intravesical BCG):
- Two nonconsecutive 6-week courses with BCG may be necessary to obtain optimal response.[25] Patients with a T1 tumor at the 3-month evaluation after a 6-week course of BCG and patients with Tis that persists after a second 6-week BCG course have a high likelihood of developing muscle-invasive disease and should be considered for cystectomy.[18,25,26]
TUR with fulguration followed by an immediate postoperative instillation of intravesical chemotherapy followed by intravesical chemotherapy
Intravesical therapy with thiotepa, MMC, doxorubicin, or BCG is most often used in patients with multiple tumors or recurrent tumors or as a prophylactic measure in high-risk patients after TUR.[27,28]
Evidence (intravesical chemotherapy):
- Three meta-analyses of randomized controlled trials that compared TUR alone with TUR followed by intravesical chemotherapy reported that adjuvant therapy was associated with a statistically significant increase in time to recurrence. No advantage has been shown with respect to survival or prevention of progression to invasive disease or metastases.[29-31]
- One analysis of eight studies with a combined total of 1,609 patients reported that intravesical chemotherapy reduced the risk of relapse at 1 year by 38% and by as much as 70% at 3 years, depending on which drugs were used.[30]
- Another analysis of 11 studies that enrolled a total of 3,703 patients reported a 44% reduction in 1-year recurrence rates.[30]
- An earlier study of 2,535 patients enrolled in six different randomized controlled trials reported a decreased risk of recurrence but not significant benefit with regard to risk of progression to more-advanced–stage disease or survival.[29]
Segmental cystectomy (rarely indicated)
Segmental cystectomy is rarely indicated.[32] It is indicated for relatively few patients because of the tendency of bladder carcinoma to involve multiple regions of the bladder mucosa and to occur in areas that cannot be segmentally resected. Moreover, cystectomy (whether segmental or radical) is generally not indicated for patients with T0 bladder cancer.[33,34]
Radical cystectomy in selected patients with extensive or refractory superficial tumors
Radical cystectomy is used in selected patients with extensive or refractory superficial tumors.[35-43] Patients at high risk of progression, typically those with recurrent high-grade tumors with carcinoma in situ after intravesical therapy with BCG, should consider radical cystectomy. Other risk factors include multiple tumors and tumors larger than 3 cm.
Certain patients with nonmuscle-invasive bladder cancer face a substantial risk of progression and death from their cancers.
Evidence (radical cystectomy):
- One analysis of 307 patients enrolled in studies of intravesical BCG in the 1980s reported that among 85 patients with T1 recurrence, 60 progressed to at least stage II disease. Five years after T1 recurrence, 71% had progressed and 48% had died of their cancer.[44]
- By comparison, in another cohort of 589 patients treated with BCG between 1992 and 2004, 65 of the 120 patients with T1 recurrence underwent immediate cystectomy. Among all patients with T1 recurrence, 28% progressed to more-advanced–stage disease and 31% died of their cancer. While these data confirm that patients with recurring cancer after intravesical BCG face a substantial risk of dying of their disease, they do not provide strong evidence that immediate cystectomy results in a lower risk of death or progression.[44]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Holmäng S, Hedelin H, Anderström C, et al.: The relationship among multiple recurrences, progression and prognosis of patients with stages Ta and T1 transitional cell cancer of the bladder followed for at least 20 years. J Urol 153 (6): 1823-6; discussion 1826-7, 1995. [PUBMED Abstract]
- Herr HW: Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol 163 (1): 60-1; discussion 61-2, 2000. [PUBMED Abstract]
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al.: Upper urinary tract tumors after primary superficial bladder tumors: prognostic factors and risk groups. J Urol 164 (4): 1183-7, 2000. [PUBMED Abstract]
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al.: Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol 163 (1): 73-8, 2000. [PUBMED Abstract]
- Babjuk M, Oosterlinck W, Sylvester R, et al.: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 54 (2): 303-14, 2008. [PUBMED Abstract]
- Babjuk M, Oosterlinck W, Sylvester R, et al.: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 59 (6): 997-1008, 2011. [PUBMED Abstract]
- Sylvester RJ, Oosterlinck W, van der Meijden AP: A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 171 (6 Pt 1): 2186-90, quiz 2435, 2004. [PUBMED Abstract]
- Gudjónsson S, Adell L, Merdasa F, et al.: Should all patients with non-muscle-invasive bladder cancer receive early intravesical chemotherapy after transurethral resection? The results of a prospective randomised multicentre study. Eur Urol 55 (4): 773-80, 2009. [PUBMED Abstract]
- Brausi M, Collette L, Kurth K, et al.: Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol 41 (5): 523-31, 2002. [PUBMED Abstract]
- Jakse G, Algaba F, Malmström PU, et al.: A second-look TUR in T1 transitional cell carcinoma: why? Eur Urol 45 (5): 539-46; discussion 546, 2004. [PUBMED Abstract]
- Herr HW: The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 162 (1): 74-6, 1999. [PUBMED Abstract]
- Zurkirchen MA, Sulser T, Gaspert A, et al.: Second transurethral resection of superficial transitional cell carcinoma of the bladder: a must even for experienced urologists. Urol Int 72 (2): 99-102, 2004. [PUBMED Abstract]
- Sylvester RJ, van der Meijden AP, Witjes JA, et al.: Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 174 (1): 86-91; discussion 91-2, 2005. [PUBMED Abstract]
- Malmström PU, Sylvester RJ, Crawford DE, et al.: An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol 56 (2): 247-56, 2009. [PUBMED Abstract]
- Stenzl A, Cowan NC, De Santis M, et al.: Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol 59 (6): 1009-18, 2011. [PUBMED Abstract]
- Herr HW, Schwalb DM, Zhang ZF, et al.: Intravesical bacillus Calmette-Guérin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol 13 (6): 1404-8, 1995. [PUBMED Abstract]
- Sarosdy MF, Lamm DL: Long-term results of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. J Urol 142 (3): 719-22, 1989. [PUBMED Abstract]
- Catalona WJ, Hudson MA, Gillen DP, et al.: Risks and benefits of repeated courses of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. J Urol 137 (2): 220-4, 1987. [PUBMED Abstract]
- De Jager R, Guinan P, Lamm D, et al.: Long-term complete remission in bladder carcinoma in situ with intravesical TICE bacillus Calmette Guerin. Overview analysis of six phase II clinical trials. Urology 38 (6): 507-13, 1991. [PUBMED Abstract]
- Herr HW, Wartinger DD, Fair WR, et al.: Bacillus Calmette-Guerin therapy for superficial bladder cancer: a 10-year followup. J Urol 147 (4): 1020-3, 1992. [PUBMED Abstract]
- Lamm DL, Griffith JG: Intravesical therapy: does it affect the natural history of superficial bladder cancer? Semin Urol 10 (1): 39-44, 1992. [PUBMED Abstract]
- Berrum-Svennung I, Granfors T, Jahnson S, et al.: A single instillation of epirubicin after transurethral resection of bladder tumors prevents only small recurrences. J Urol 179 (1): 101-5; discussion 105-6, 2008. [PUBMED Abstract]
- Böhle A, Bock PR: Intravesical bacille Calmette-Guérin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology 63 (4): 682-6; discussion 686-7, 2004. [PUBMED Abstract]
- Lamm DL, Blumenstein BA, Crissman JD, et al.: Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 163 (4): 1124-9, 2000. [PUBMED Abstract]
- Coplen DE, Marcus MD, Myers JA, et al.: Long-term followup of patients treated with 1 or 2, 6-week courses of intravesical bacillus Calmette-Guerin: analysis of possible predictors of response free of tumor. J Urol 144 (3): 652-7, 1990. [PUBMED Abstract]
- Herr HW: Progression of stage T1 bladder tumors after intravesical bacillus Calmette-Guerin. J Urol 145 (1): 40-3; discussion 43-4, 1991. [PUBMED Abstract]
- Igawa M, Urakami S, Shirakawa H, et al.: Intravesical instillation of epirubicin: effect on tumour recurrence in patients with dysplastic epithelium after transurethral resection of superficial bladder tumour. Br J Urol 77 (3): 358-62, 1996. [PUBMED Abstract]
- Rintala E, Jauhiainen K, Kaasinen E, et al.: Alternating mitomycin C and bacillus Calmette-Guerin instillation prophylaxis for recurrent papillary (stages Ta to T1) superficial bladder cancer. Finnbladder Group. J Urol 156 (1): 56-9; discussion 59-60, 1996. [PUBMED Abstract]
- Pawinski A, Sylvester R, Kurth KH, et al.: A combined analysis of European Organization for Research and Treatment of Cancer, and Medical Research Council randomized clinical trials for the prophylactic treatment of stage TaT1 bladder cancer. European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council Working Party on Superficial Bladder Cancer. J Urol 156 (6): 1934-40, discussion 1940-1, 1996. [PUBMED Abstract]
- Huncharek M, Geschwind JF, Witherspoon B, et al.: Intravesical chemotherapy prophylaxis in primary superficial bladder cancer: a meta-analysis of 3703 patients from 11 randomized trials. J Clin Epidemiol 53 (7): 676-80, 2000. [PUBMED Abstract]
- Huncharek M, McGarry R, Kupelnick B: Impact of intravesical chemotherapy on recurrence rate of recurrent superficial transitional cell carcinoma of the bladder: results of a meta-analysis. Anticancer Res 21 (1B): 765-9, 2001 Jan-Feb. [PUBMED Abstract]
- Soloway MS: The management of superficial bladder cancer. In: Javadpour N, ed.: Principles and Management of Urologic Cancer. 2nd ed. Williams and Wilkins, 1983, pp 446-467.
- Holzbeierlein JM, Lopez-Corona E, Bochner BH, et al.: Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol 172 (3): 878-81, 2004. [PUBMED Abstract]
- Fahmy N, Aprikian A, Tanguay S, et al.: Practice patterns and recurrence after partial cystectomy for bladder cancer. World J Urol 28 (4): 419-23, 2010. [PUBMED Abstract]
- Lambert EH, Pierorazio PM, Olsson CA, et al.: The increasing use of intravesical therapies for stage T1 bladder cancer coincides with decreasing survival after cystectomy. BJU Int 100 (1): 33-6, 2007. [PUBMED Abstract]
- Skinner EC: The best treatment for high-grade T1 bladder cancer is cystectomy. Urol Oncol 25 (6): 523-5, 2007 Nov-Dec. [PUBMED Abstract]
- Stein JP, Penson DF: Invasive T1 bladder cancer: indications and rationale for radical cystectomy. BJU Int 102 (3): 270-5, 2008. [PUBMED Abstract]
- Boström PJ, Alkhateeb S, van Rhijn BW, et al.: Optimal timing of radical cystectomy in T1 high-grade bladder cancer. Expert Rev Anticancer Ther 10 (12): 1891-902, 2010. [PUBMED Abstract]
- Fritsche HM, Burger M, Svatek RS, et al.: Characteristics and outcomes of patients with clinical T1 grade 3 urothelial carcinoma treated with radical cystectomy: results from an international cohort. Eur Urol 57 (2): 300-9, 2010. [PUBMED Abstract]
- Ali-El-Dein B, Al-Marhoon MS, Abdel-Latif M, et al.: Survival after primary and deferred cystectomy for stage T1 transitional cell carcinoma of the bladder. Urol Ann 3 (3): 127-32, 2011. [PUBMED Abstract]
- Canter D, Egleston B, Wong YN, et al.: Use of radical cystectomy as initial therapy for the treatment of high-grade T1 urothelial carcinoma of the bladder: a SEER database analysis. Urol Oncol 31 (6): 866-70, 2013. [PUBMED Abstract]
- Chalasani V, Kassouf W, Chin JL, et al.: Radical cystectomy for the treatment of T1 bladder cancer: the Canadian Bladder Cancer Network experience. Can Urol Assoc J 5 (2): 83-7, 2011. [PUBMED Abstract]
- Segal R, Yafi FA, Brimo F, et al.: Prognostic factors and outcome in patients with T1 high-grade bladder cancer: can we identify patients for early cystectomy? BJU Int 109 (7): 1026-30, 2012. [PUBMED Abstract]
- Raj GV, Herr H, Serio AM, et al.: Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. J Urol 177 (4): 1283-6; discussion 1286, 2007. [PUBMED Abstract]
Treatment of Stages II and III Bladder Cancer
Treatment Options for Stages II and III Bladder Cancer
Treatment options for stage II bladder cancer and stage III bladder cancer include:
- Radical cystectomy.
- Neoadjuvant chemoimmunotherapy followed by radical cystectomy followed by adjuvant immunotherapy.
- Neoadjuvant combination chemotherapy followed by radical cystectomy.
- Radical cystectomy followed by adjuvant chemotherapy or immunotherapy.
- External-beam radiation therapy (EBRT) with or without concomitant chemotherapy.
- Segmental cystectomy (in selected patients).
- Transurethral resection (TUR) with fulguration (in selected patients).
The most common treatments for muscle-invasive bladder cancer are radical cystectomy and radiation therapy. There is no strong evidence from randomized controlled trials to determine whether surgery or radiation therapy is more effective. There is strong evidence that both therapies are more effective when combined with chemotherapy. The treatments with the highest levels of evidence supporting their effectiveness are radical cystectomy preceded by multiagent cisplatin-based chemotherapy and radiation therapy with concomitant chemotherapy.
Because bladder cancer commonly recurs with distant metastases, studies have evaluated giving systemic immunotherapy or chemotherapy before or after cystectomy as a means of improving outcomes.
Radical cystectomy
Radical cystectomy is a standard treatment option for stage II and stage III bladder cancer, and its effectiveness at prolonging survival increases if it is preceded by cisplatin-based multiagent chemotherapy.[1-4] Radical cystectomy is accompanied by pelvic lymph node dissection and includes removal of the bladder, perivesical tissues, prostate, and seminal vesicles in men and removal of the uterus, fallopian tubes, ovaries, anterior vaginal wall, and urethra in women.[5-8] Studies of outcomes after radical cystectomy report increased survival in patients who had more, rather than fewer, lymph nodes resected. It is unknown whether this represents a therapeutic benefit of resecting additional nodes or stage migration.[9] There are no randomized controlled trials evaluating the therapeutic benefit of lymph node dissection in this setting.
Radical cystectomy is a major operation with a perioperative mortality rate of 2% to 3% when performed at centers of excellence.[6-8] Postoperative complications include ileus. Most men have erectile dysfunction after radical cystectomy. Sexual dysfunction after this operation is also common in women.[10-12]
One study of 27 women who underwent radical cystectomy reported diminished ability to have orgasm in 45%, decreased lubrication in 41%, decreased sexual desire in 37%, and pain with vaginal intercourse in 22%. Fewer than one-half were able to have successful vaginal intercourse and most reported decreased satisfaction with their sexual lives after surgery.[12] Studies suggest that radical cystectomy with preservation of sexual function can be performed in some men. In addition, new forms of urinary diversion can obviate the need for an external urinary appliance.[13-16]
In a retrospective analysis from a single institution, older patients (≥70 years) in good general health were found to have clinical and functional results after radical cystectomy similar to younger patients.[17]
After radical cystectomy, however, an approximate 30% to 40% risk of recurrence still exists for patients with muscle-invasive disease, even at centers of excellence.[6-8] The 5-year overall survival (OS) rates range from 50% to 60%, but these rates vary by cancer stage.[4] The addition of preoperative radiation therapy to radical cystectomy did not result in any survival advantage when compared with radical cystectomy alone in a prospective randomized trial.[18]
Neoadjuvant chemoimmunotherapy followed by radical cystectomy followed by adjuvant immunotherapy
In patients eligible for immunotherapy, adding perioperative immunotherapy to standard-of-care cisplatin-based combination chemotherapy before radical cystectomy may further improve survival.
Evidence (neoadjuvant chemoimmunotherapy followed by radical cystectomy followed by adjuvant immunotherapy):
- A randomized controlled trial (NIAGARA [NCT03732677]) compared neoadjuvant durvalumab plus gemcitabine and cisplatin followed by radical cystectomy and adjuvant durvalumab (durvalumab arm) with neoadjuvant gemcitabine and cisplatin followed by radical cystectomy (comparison arm). The study included 1,063 patients with clinical stage T2 or higher muscle-invasive bladder cancer. Patients were eligible to receive cisplatin if they had a creatinine clearance of at least 40 mL/min. Four cycles of neoadjuvant durvalumab (1,500 mg) were given every 3 weeks, and up to eight cycles of adjuvant durvalumab (1,500 mg) were given every 4 weeks.[19][Level of evidence A1]
- At 24 months, the OS rate was 82.2% (95% confidence interval [CI], 78.7%–85.2%) in the durvalumab arm and 75.2% (95% CI, 71.3%–78.8%) in the comparison arm (hazard ratio [HR], 0.75; 95% CI, 0.59–0.93).
- At 24 months, the event-free survival (EFS) rate was 67.8% (95% CI, 63.6%–71.7%) in the durvalumab arm and 59.8% (95% CI, 55.4%–64.0%) in the comparison arm (HR, 0.68; 95% CI, 0.56–0.82).
- Grade 3 or higher treatment-related adverse events occurred in 40.6% of patients in the durvalumab arm and 40.9% of patients in the comparison arm.
- Radical cystectomy was performed in 88.0% of patients in the durvalumab arm and 83.2% of patients in the comparison arm.
Neoadjuvant combination chemotherapy followed by radical cystectomy
Giving chemotherapy before cystectomy (i.e., neoadjuvant chemotherapy) may be preferable to postoperative treatment because tumor downstaging from chemotherapy may enhance resectability; occult metastatic disease may be treated as early as possible; and chemotherapy may be better tolerated. Currently, the body of evidence supporting preoperative chemotherapy is much stronger than the evidence supporting postoperative chemotherapy.
Evidence (neoadjuvant combination chemotherapy followed by radical cystectomy):
- A controlled trial of preoperative chemotherapy conducted by the Medical Research Council and the European Organisation for Research and Treatment of Cancer included 976 patients with locally advanced (T3 or T4a) or high-grade muscle-invasive (T2) bladder cancer. Patients were randomly assigned to undergo either definitive treatment immediately or definitive treatment preceded by three cycles of neoadjuvant cisplatin, vinblastine, and methotrexate.[20,21] In this study, definitive treatment consisted of radical cystectomy (n = 428), radiation therapy (n = 403), or preoperative radiation therapy followed by radical cystectomy (n = 66).
- At a median follow-up of 8.0 years for patients still alive, OS was significantly greater in the arm randomly assigned to receive neoadjuvant chemotherapy (HR, 0.84; 95% CI, 0.72–0.99; P = .037). The survival benefit from neoadjuvant chemotherapy compared with definitive treatment alone conferred a 6% absolute increase in the likelihood of being alive at 3 years (56% vs. 50%), 5 years (49% vs. 43%), and 10 years (36% vs. 30%).[21][Level of evidence A1]
- A randomized study conducted by the Southwest Oncology Group compared three cycles of neoadjuvant cisplatin, methotrexate, vinblastine, and doxorubicin administered before cystectomy with cystectomy alone in 317 patients with stage T2 to stage T4a bladder cancer.[22]
- The study showed that the 5-year survival rate was 57% in the group that received neoadjuvant chemotherapy and 43% in the group treated with cystectomy alone. This difference was of borderline statistical significance (two-sided P value = .06 by stratified log-rank test).
- No deaths were associated with neoadjuvant chemotherapy, and there was no difference in the rate or severity of postoperative complications in patients who received immediate surgery and in those who received preoperative chemotherapy. Cystectomy was performed as planned for 82% of patients assigned to preoperative chemotherapy and 81% of those assigned to cystectomy alone. This study provided evidence that preoperative chemotherapy does not prevent patients from undergoing cystectomy and does not increase the risk of perioperative complications.
- Thirty-eight percent of patients who received neoadjuvant chemotherapy had a pathological complete response at the time of surgery, and 85% of those who achieved a pathological complete response were alive at 5 years.
- A meta-analysis of ten randomized trials of neoadjuvant chemotherapy included updated data for 2,688 individual patients.[2]
- Cisplatin-based combination chemotherapy was associated with a significant 13% relative reduction in the risk of death and resulted in an improvement in 5-year survival rates from 45% to 50% (P = .016).
- Neoadjuvant single-agent cisplatin was not associated with any survival benefit in the meta-analysis.
- A subsequent meta-analysis evaluated a nearly identical body of data (11 randomized controlled trials enrolling a total of 2,605 patients) and reached similar conclusions. When the analysis was limited to the eight trials that used multiagent, cisplatin-based chemotherapy, neoadjuvant chemotherapy compared with cystectomy alone was associated with a 6.5% absolute benefit in the 5-year OS rate (56.5% vs. 50%; P = .006).[4]
Most patients included in these studies received cisplatin, methotrexate, and vinblastine with or without doxorubicin. It is not known whether the doublet regimen of cisplatin plus gemcitabine offers any benefit when given in the preoperative setting, nor is there any evidence of benefit for carboplatin-based chemotherapy regimens.
Based on these findings, preoperative cisplatin-based combination chemotherapy followed by radical cystectomy represents a standard therapeutic option for patients with muscle-invasive bladder cancer who are fit for chemotherapy and for whom the priority is to maximize survival.
Radical cystectomy followed by adjuvant chemotherapy or immunotherapy
Adjuvant chemotherapy
Numerous trials have investigated whether giving chemotherapy after radical cystectomy can improve progression-free survival (PFS) and OS. Although several trials showed a PFS benefit from postoperative cisplatin-based chemotherapy, the trials tended to be small and underpowered, and no trial demonstrated a persuasive benefit in OS.
Evidence (adjuvant chemotherapy):
- A systematic review and meta-analysis evaluated nine randomized controlled trials that enrolled 945 patients. A variety of different cisplatin-based chemotherapy regimens were used.[23][Level of evidence A2]
- This analysis reported a significant OS benefit for patients who received adjuvant chemotherapy (HR, 0.77; 95% CI, 0.49–0.99).
- The disease-free survival (DFS) benefit was similar (HR, 0.66; 95% CI, 0.45–0.91).
Adjuvant immunotherapy
Evidence (adjuvant immunotherapy):
- A randomized controlled trial (Checkmate 274 [NCT02632409]) compared nivolumab with placebo in 709 patients with pathological T3, T4, or node-positive muscle-invasive urothelial carcinoma who had undergone radical cystectomy. Of note, the patients in this trial had either received preoperative cisplatin-based chemotherapy, refused postoperative cisplatin-based chemotherapy, or been deemed unfit for cisplatin-based chemotherapy.[24][Level of evidence A1]
- At a median follow-up of 36.1 months, the OS was 69.6 months (95% CI, 58.1–not estimable [NE]) in the nivolumab arm and 50.1 months (95% CI, 38.2–NE) in the placebo arm (HR, 0.76; 95% CI, 0.61–0.96). In patients with programmed death-ligand 1 (PD-L1) expression of 1% or higher (approximately 40% of all enrolled patients), the median OS was not reached in either arm (HR, 0.56; 95% CI, 0.36–0.86).
- The median DFS was 22.0 months (95% CI, 18.8–36.9) in the nivolumab arm and 10.9 months (95% CI, 8.3–15.2) in the placebo arm (HR, 0.71; 95% CI, 0.58–0.86). In patients with PD-L1 expression of 1% or greater, the median DFS was 52.6 months (95% CI, 25.8–NE) in the nivolumab arm and 8.4 months (95% CI, 5.6–17.9) in the placebo arm (HR, 0.52; 95% CI, 0.37–0.72).
- In patients with PD-L1 expression of less than 1% in the intent-to-treat population, the HR for median DFS for nivolumab compared to placebo was 0.84 (95% CI, 0.66–1.06). In patients with PD-L1 expression of less than 1% and muscle-invasive bladder cancer (79% of enrolled patients), the median DFS was 18.3 months (95% CI, 14.1–22.4) in the nivolumab arm and 9.7 months (95% CI, 7.4–16.6) in the placebo arm (HR, 0.74; 95% CI, 0.56–0.97). OS data were not included in the supplement for patients with PD-L1 expression of less than 1%.
- Grade 3 or higher adverse events were seen in 42.7% of patients in the nivolumab arm and 36.8% of patients in the placebo arm. High-grade treatment-related adverse events were seen in 18.2% of patients in the nivolumab arm and 7.2% of patients in the placebo arm. The most common adverse events were pruritus, fatigue, and diarrhea, while the most common grade 3 or higher adverse events were elevated lipase or amylase, diarrhea, colitis, and pneumonitis. Two patients in the nivolumab arm died of pneumonitis, and one patient died of a bowel perforation.
- A randomized controlled trial (AMBASSADOR [NCT03244384]) compared adjuvant pembrolizumab with observation in 702 patients. Patients had undergone radical cystectomy and had positive margins, node-positive disease, and either pathological T2 or higher stage disease (if neoadjuvant cisplatin-based chemotherapy was received) or T3 or higher disease (if no neoadjuvant chemotherapy had been received).[25][Level of evidence B1]
- At a median follow-up of 44.8 months, the DFS was 26.9 months (95% CI, 20.0–40.7) in the pembrolizumab arm and 14.2 months (95% CI, 11.0–20.2) in the observation arm (HR, 0.73; 95% CI, 0.59–0.90).
- In patients with a PD-L1 combined positive score (CPS) of at least 10 (57.5% of all enrolled patients), the median DFS was 36.9 months (95% CI, 27.2–NE) in the pembrolizumab arm and 21.0 months (95% CI, 13.6–53.3) in the observation arm (HR, 0.81; 95% CI, 0.61–1.08). In patients with a PD–L1 CPS less than 10 (42.5% of all enrolled patients), the median DFS was 17.3 months (95% CI, 13.2–32.0) in the pembrolizumab arm and 9.0 months (95% CI, 6.9–15.3) in the observation arm (HR, 0.71; 95% CI, 0.53–0.95).
- The 3-year OS rate was not significantly different, at 60.5% for patients in the pembrolizumab arm and 61.9% for patients in the observation arm (HR, 0.98; 95% CI, 0.76–1.26).
- Grade 3 or higher adverse events occurred in 50.6% of patients in the pembrolizumab arm and 31.6% of patients in the observation arm.
EBRT with or without concomitant chemotherapy
Definitive radiation therapy is a standard option that yields 5-year survival rates of approximately 30% to 40%.[26] When radiation therapy and chemotherapy are administered concomitantly, the results are better. However, while the addition of chemotherapy to radiation therapy has been shown to reduce local relapse rates, it has not been shown to result in increased survival, decreased mortality, or improved quality of life.
Most protocols for bladder preservation that use combined chemotherapy and radiation therapy have followed a relatively complex algorithm. After the initial stage TUR of the bladder tumor, patients undergo a repeat TUR to maximally resect the tumor. The patient is then treated with synchronous chemoradiation therapy to a dose of roughly 40 Gy followed by a repeat cystoscopy with biopsies to assess for residual cancer. If residual cancer is detected histopathologically, then the chemoradiation therapy is judged to have failed and the patient is advised to undergo a radical cystectomy. If the biopsies at 40 Gy are benign, then chemoradiation therapy is completed to a dose of about 65 Gy.
With definitive radiation therapy, best results are seen in patients with solitary lesions and without carcinoma in situ or hydronephrosis.
After radiation therapy, approximately 50% of patients have dysuria and urinary frequency during treatment, which resolves several weeks after treatment, and 15% report acute toxic effects of the bowel.
Randomized trials that directly compare the bladder-preserving chemoradiation therapy approach with radical cystectomy have not been performed. The relative effectiveness of these two treatments is thus unknown.
Evidence (EBRT with or without concomitant chemotherapy):
TUR followed by chemoradiation therapy
- A multicenter phase III trial randomly assigned 360 patients with muscle-invasive bladder cancer to radiation therapy with or without synchronous chemotherapy using fluorouracil and mitomycin.[26]
- The 2-year locoregional DFS rate was higher in the chemoradiation therapy group (67% vs. 54%; HR, 0.68; 95% CI, 0.48–0.96; P = .03). The 5-year OS rate was 48% in the chemoradiation therapy group and 35% in the radiation therapy group, but the difference was not statistically significant (P = .16).
- Similarly, synchronous chemoradiation therapy using other chemotherapy regimens, such as cisplatin alone or combined with fluorouracil, have reported 5-year OS rates of 50% to 60% and survival with an intact bladder in 40% to 45% of patients. These figures are higher than what has generally been reported in studies of radiation therapy alone.[27]
TUR followed by chemoradiation therapy
Radiation therapy and chemotherapy
- A randomized controlled trial randomly assigned 99 patients with T2 to T4b urothelial carcinoma of the bladder to radiation therapy with or without three 14-day cycles of cisplatin (100 mg/m2 on day 1). Patients and their physicians chose whether the radiation therapy was definitive or given as precystectomy treatment.[31]
- The pelvic relapse rate was reduced (multivariable regression model HR, 0.50; 90% CI, 0.29–0.86; P = .036) using concurrent cisplatin, but there was no difference in the occurrence of distant metastases or OS.
- The reduction in pelvic relapse was similar in patients who received definitive radiation therapy and precystectomy radiation therapy.
Neoadjuvant chemotherapy followed by chemoradiation therapy
- In a phase III study (RTOG-8903), the Radiation Therapy Oncology Group evaluated the potential benefit of adding two cycles of neoadjuvant methotrexate, cisplatin, and vinblastine before concurrent cisplatin and radiation therapy.[32]
- Neoadjuvant chemotherapy was associated with increased hematologic toxic effects and yielded no improvement in response rate, freedom from distant metastases, or OS compared with chemoradiation therapy alone.
Segmental cystectomy (in selected patients)
Segmental cystectomy is appropriate only in very selected patients.[1] There are no randomized controlled trials comparing segmental cystectomy with radical cystectomy. Only patients with adenocarcinomas of the urachus are routinely treated with segmental cystectomy. These tumors typically are mucinous adenocarcinomas occurring at the dome of the bladder and are treated with an en bloc resection of the bladder dome and urachal remnant, including the umbilicus.[33-36]
TUR with fulguration (in selected patients)
TUR may control stage II bladder cancer in some patients. However, more aggressive forms of treatment are often dictated by recurrent tumor or by the large size, multiple foci, or undifferentiated grade of the neoplasm.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Richie JP: Surgery for invasive bladder cancer. Hematol Oncol Clin North Am 6 (1): 129-45, 1992. [PUBMED Abstract]
- Advanced Bladder Cancer Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 361 (9373): 1927-34, 2003. [PUBMED Abstract]
- Sherif A, Holmberg L, Rintala E, et al.: Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol 45 (3): 297-303, 2004. [PUBMED Abstract]
- Winquist E, Kirchner TS, Segal R, et al.: Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol 171 (2 Pt 1): 561-9, 2004. [PUBMED Abstract]
- Olsson CA: Management of invasive carcinoma of the bladder. In: deKernion JB, Paulson DF, eds.: Genitourinary Cancer Management. Lea and Febiger, 1987, pp 59-94.
- Stein JP, Lieskovsky G, Cote R, et al.: Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 19 (3): 666-75, 2001. [PUBMED Abstract]
- Madersbacher S, Hochreiter W, Burkhard F, et al.: Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol 21 (4): 690-6, 2003. [PUBMED Abstract]
- Manoharan M, Ayyathurai R, Soloway MS: Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 104 (9): 1227-32, 2009. [PUBMED Abstract]
- Ather MH, Fatima S, Sinanoglu O: Extent of lymphadenectomy in radical cystectomy for bladder cancer. World J Surg Oncol 3: 43, 2005. [PUBMED Abstract]
- Hart S, Skinner EC, Meyerowitz BE, et al.: Quality of life after radical cystectomy for bladder cancer in patients with an ileal conduit, cutaneous or urethral kock pouch. J Urol 162 (1): 77-81, 1999. [PUBMED Abstract]
- Miyao N, Adachi H, Sato Y, et al.: Recovery of sexual function after nerve-sparing radical prostatectomy or cystectomy. Int J Urol 8 (4): 158-64, 2001. [PUBMED Abstract]
- Zippe CD, Raina R, Shah AD, et al.: Female sexual dysfunction after radical cystectomy: a new outcome measure. Urology 63 (6): 1153-7, 2004. [PUBMED Abstract]
- Brendler CB, Steinberg GD, Marshall FF, et al.: Local recurrence and survival following nerve-sparing radical cystoprostatectomy. J Urol 144 (5): 1137-40; discussion 1140-1, 1990. [PUBMED Abstract]
- Skinner DG, Boyd SD, Lieskovsky G: Clinical experience with the Kock continent ileal reservoir for urinary diversion. J Urol 132 (6): 1101-7, 1984. [PUBMED Abstract]
- Fowler JE: Continent urinary reservoirs and bladder substitutes in the adult: part I. Monographs in Urology 8 (2): 1987.
- Fowler JE: Continent urinary reservoirs and bladder substitutes in the adult: part II. Monographs in Urology 8 (3): 1987.
- Figueroa AJ, Stein JP, Dickinson M, et al.: Radical cystectomy for elderly patients with bladder carcinoma: an updated experience with 404 patients. Cancer 83 (1): 141-7, 1998. [PUBMED Abstract]
- Smith JA, Crawford ED, Paradelo JC, et al.: Treatment of advanced bladder cancer with combined preoperative irradiation and radical cystectomy versus radical cystectomy alone: a phase III intergroup study. J Urol 157 (3): 805-7; discussion 807-8, 1997. [PUBMED Abstract]
- Powles T, Catto JWF, Galsky MD, et al.: Perioperative Durvalumab with Neoadjuvant Chemotherapy in Operable Bladder Cancer. N Engl J Med 391 (19): 1773-1786, 2024. [PUBMED Abstract]
- Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet 354 (9178): 533-40, 1999. [PUBMED Abstract]
- Griffiths G, Hall R, Sylvester R, et al.: International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 29 (16): 2171-7, 2011. [PUBMED Abstract]
- Grossman HB, Natale RB, Tangen CM, et al.: Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349 (9): 859-66, 2003. [PUBMED Abstract]
- Leow JJ, Martin-Doyle W, Rajagopal PS, et al.: Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol 66 (1): 42-54, 2014. [PUBMED Abstract]
- Galsky MD, Witjes JA, Gschwend JE, et al.: Adjuvant Nivolumab in High-Risk Muscle-Invasive Urothelial Carcinoma: Expanded Efficacy From CheckMate 274. J Clin Oncol 43 (1): 15-21, 2025. [PUBMED Abstract]
- Apolo AB, Ballman KV, Sonpavde G, et al.: Adjuvant Pembrolizumab versus Observation in Muscle-Invasive Urothelial Carcinoma. N Engl J Med 392 (1): 45-55, 2025. [PUBMED Abstract]
- James ND, Hussain SA, Hall E, et al.: Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366 (16): 1477-88, 2012. [PUBMED Abstract]
- Efstathiou JA, Zietman AL, Kaufman DS, et al.: Bladder-sparing approaches to invasive disease. World J Urol 24 (5): 517-29, 2006. [PUBMED Abstract]
- Kachnic LA, Kaufman DS, Heney NM, et al.: Bladder preservation by combined modality therapy for invasive bladder cancer. J Clin Oncol 15 (3): 1022-9, 1997. [PUBMED Abstract]
- Housset M, Maulard C, Chretien Y, et al.: Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: a prospective study. J Clin Oncol 11 (11): 2150-7, 1993. [PUBMED Abstract]
- Rödel C, Grabenbauer GG, Kühn R, et al.: Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol 20 (14): 3061-71, 2002. [PUBMED Abstract]
- Coppin CM, Gospodarowicz MK, James K, et al.: Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 14 (11): 2901-7, 1996. [PUBMED Abstract]
- Shipley WU, Winter KA, Kaufman DS, et al.: Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol 16 (11): 3576-83, 1998. [PUBMED Abstract]
- Ashley RA, Inman BA, Sebo TJ, et al.: Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer 107 (4): 712-20, 2006. [PUBMED Abstract]
- Siefker-Radtke A: Urachal carcinoma: surgical and chemotherapeutic options. Expert Rev Anticancer Ther 6 (12): 1715-21, 2006. [PUBMED Abstract]
- Herr HW, Bochner BH, Sharp D, et al.: Urachal carcinoma: contemporary surgical outcomes. J Urol 178 (1): 74-8; discussion 78, 2007. [PUBMED Abstract]
- Bruins HM, Visser O, Ploeg M, et al.: The clinical epidemiology of urachal carcinoma: results of a large, population based study. J Urol 188 (4): 1102-7, 2012. [PUBMED Abstract]
Treatment of Stage IV Bladder Cancer
Few patients with stage IV bladder cancer can be cured, and for many patients, the emphasis is on palliation of symptoms. The potential for cure is restricted to patients with stage IV disease with involvement of pelvic organs by direct extension or metastases to regional lymph nodes.[1]
Treatment Options for Stage IV Bladder Cancer
Treatment options for patients with T4b, N0, M0 disease
Treatment options for patients with T4b, N0, M0 disease include:
- Enfortumab vedotin plus pembrolizumab.
- Chemotherapy plus immunotherapy.
- Chemotherapy alone.
- Systemic therapy followed by radical cystectomy.
- External-beam radiation therapy (EBRT) with or without concomitant chemotherapy.
- Urinary diversion or cystectomy for palliation.
Enfortumab vedotin plus pembrolizumab
Enfortumab vedotin is an antibody-drug conjugate, combining an antibody that binds to nectin-4 with a microtubule inhibitor. The U.S. Food and Drug Administration (FDA) approved enfortumab vedotin as monotherapy for patients with previously treated metastatic urothelial carcinoma. Pembrolizumab is an anti−programmed death-1 (PD-1) antibody that the FDA has approved as monotherapy for patients with metastatic bladder cancer. The combination of the two agents showed a promising response rate and duration of response, leading to a single-arm phase II trial and then a comparison with chemotherapy in a randomized, controlled, phase III trial.[2-4] While results cannot be extrapolated across trials, enfortumab vedotin plus pembrolizumab is a first-line therapy option for patients without a contraindication.
Evidence (enfortumab vedotin plus pembrolizumab):
- In a phase III trial (EV-302 [NCT04223856]), 886 patients were randomly assigned to receive either enfortumab vedotin plus pembrolizumab (n = 442) or gemcitabine plus either cisplatin or carboplatin (n = 444). The median follow-up was 17.2 months.[4][Level of evidence A1]
- Overall survival (OS) and progression-free survival (PFS) were longer for patients in the enfortumab vedotin-plus-pembrolizumab arm than in the chemotherapy arm.
- The median OS was 31.5 months for patients who received enfortumab vedotin plus pembrolizumab and 16.1 months for patients who received chemotherapy (hazard ratio [HR], 0.47; 95% confidence interval [CI], 0.38–0.58; P < .001).
- PFS was 12.5 months for patients who received enfortumab vedotin plus pembrolizumab and 6.3 months for patients who received chemotherapy (HR, 0.45; 95% CI, 0.38–0.54; P < .001).
- Grade 3 or higher adverse events occurred in 55.9% of patients who received enfortumab vedotin plus pembrolizumab and 69.5% of patients who received chemotherapy.
- In the experimental arm, the most common high-grade adverse events were skin reactions, peripheral neuropathy, hyperglycemia, and neutropenia.
Chemotherapy plus immunotherapy
For many years, cisplatin-based chemotherapy was the standard-of-care first-line systemic treatment for patients with stage IV urothelial carcinoma who were eligible to receive cisplatin. A randomized, controlled, phase III trial reported longer OS when cisplatin-based chemotherapy was given with nivolumab, compared with chemotherapy alone.[5]
Evidence (chemotherapy plus immunotherapy):
- A phase III, randomized controlled trial (CheckMate901 [NCT03036098]) included 608 patients (304 to each arm). Patients were randomly assigned to receive either nivolumab plus gemcitabine and cisplatin or gemcitabine and cisplatin alone. Patients received up to six cycles of chemotherapy and up to 2 years of nivolumab. The median follow-up was 33.6 months.[5]
- The median OS was 21.7 months for patients who received nivolumab and 18.9 months for patients who received chemotherapy alone (HRdeath, 0.78; 95% CI, 0.63–0.96; P = .02).[5][Level of evidence A1]
- The median PFS was 7.9 months for patients who received nivolumab and 7.6 months for patients who received chemotherapy alone (HRprogression or death, 0.72; 95% CI, 0.59–0.88; P = .001).
- At 1 year, the PFS rate was 34.2% in the nivolumab arm and 21.8% in the chemotherapy-alone arm.
- The overall response rate was 57.6% in the nivolumab arm and 21.7% in the chemotherapy-alone arm. The complete response rate was 21.7% in the nivolumab arm and 11.8% in the chemotherapy-alone arm.
- The duration of complete response was 37.1 months in the nivolumab arm and 13.2 months in the chemotherapy-alone arm.
- Grade 3 or higher adverse events occurred in 61.8% of patients in the nivolumab arm and 51.7% of patients in the chemotherapy-alone arm.
Chemotherapy alone
Cisplatin-based combination chemotherapy regimens are a standard-of-care option for patients with stage IV bladder cancer.[6-10] The only chemotherapy regimens that have been shown to result in longer survival in randomized controlled trials are methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC); high-dose MVAC; and cisplatin, methotrexate, and vinblastine (CMV). Gemcitabine plus cisplatin (GC) was compared with MVAC in a randomized controlled trial that reported no difference in response rate or survival. Of note, patients with good performance status and lymph node–only disease have a low but significant rate of achieving a durable complete remission with MVAC or GC. For example, in a large, randomized controlled trial that compared MVAC with GC, the 5-year OS rate was 20.9% in patients with lymph node–only disease.[11]
Single-agent cisplatin and multiagent regimens that do not include cisplatin have never resulted in improved survival in a randomized controlled trial. For patients who are not candidates for cisplatin-based multiagent chemotherapy regimens, there is no regimen that has been shown to prolong survival. However, many regimens have demonstrated radiologically measurable responses.
These regimens include carboplatin plus paclitaxel,[12] carboplatin plus gemcitabine,[13-15] paclitaxel plus gemcitabine,[16-18] single-agent gemcitabine,[19,20] and single-agent paclitaxel.[21-23] Regimens of carboplatin, methotrexate, and vinblastine; carboplatin, epirubicin, methotrexate, and vinblastine; and paclitaxel, gemcitabine, and carboplatin have been studied but are not widely used.[24-27]
Evidence (chemotherapy alone):
- Results from a randomized controlled trial that compared MVAC with docetaxel plus cisplatin in 220 patients reported that MVAC was associated with longer OS (median survival, 14.2 months vs. 9.3 months; P = .026).[28]
- A randomized trial that compared MVAC with cisplatin, cyclophosphamide, and doxorubicin demonstrated improved response and median survival rates (48 weeks vs. 36 weeks; P = .003) with the MVAC regimen.[29]
- Results from a randomized trial that compared MVAC with single-agent cisplatin in advanced bladder cancer also showed a significant advantage with MVAC in both response rate and median survival (12.5 months vs. 8.2 months; P = .002).[30]
- A multicenter randomized controlled trial compared CMV with methotrexate plus vinblastine without cisplatin in 214 patients. The relative risk of dying was 0.68 (95% CI, 0.51–0.90; P = .0065) in favor of CMV. The median survival was 7 months with CMV and 4.5 months with methotrexate plus vinblastine.[31]
- The European Organisation for Research and Treatment of Cancer (EORTC) conducted a randomized trial that included 263 patients with advanced bladder cancer. The study evaluated the efficacy of a high-dose intensity MVAC regimen given every 2 weeks with granulocyte colony-stimulating factor (G-CSF) versus a classic MVAC regimen given every 4 weeks.[32]
- There was no significant difference in OS at a median follow-up of 3.2 years (HR, 0.80; 95% CI, 0.60–1.06; P = .122). However, at a median follow-up of 7.3 years, the high-dose intensity MVAC regimen was associated with improved OS (HR, 0.76; 95% CI, 0.58–0.99; P = .042), with a 5-year survival rate of 22% compared with 14% in patients treated with the classic MVAC regimen.
- The high-dose intensity MVAC regimen was also associated with higher response rates (72% vs. 58%; P = .016), improved median PFS (9.5 months vs. 8.1 months; P = .017), and decreased neutropenic fever (10% vs. 26%; P < .001), although only 19% of patients treated with a classic MVAC regimen ever received G-CSF.[32][Level of evidence A1] An imbalance in baseline prognostic factors (i.e., visceral metastases were found in 37 patients randomly assigned to the high-dose MVAC regimen and 47 patients assigned to the classic MVAC regimen) may account, in part, for these results.
- A multicenter, randomized, phase III trial compared GC with MVAC in 405 patients with advanced or metastatic bladder cancer.
- GC yielded response rates, time-to-progression, and OS (HR, 1.04; 95% CI, 0.82–1.32; P = .75) similar to MVAC, but GC had a better safety profile and was better tolerated than MVAC.[33][Level of evidence A1]
- Although this study was not designed to show the equivalence of the two regimens, the similar efficacy and reduced toxic effects of GC make it a reasonable alternative in patients who may not tolerate the MVAC regimen.
External-beam radiation therapy (EBRT) with or without concomitant chemotherapy
Definitive radiation therapy with or without concurrent chemotherapy, evaluated mainly in patients with locally advanced (T2–T4) disease, appears to have minimal curative potential in patients with regional lymph node metastases.[34,35] Patients with evidence of lymph node metastases have generally been excluded from phase III trials of radiation therapy.[36,37]
Urinary diversion or cystectomy for palliation
Urinary diversion may be indicated, not only for palliation of urinary symptoms but also for preservation of renal function in patients who are candidates for chemotherapy.
Treatment options for patients with any T, any N, M1 disease
Treatment options for patients with any T, any N, M1 disease include:
- Enfortumab vedotin plus pembrolizumab.
- Chemotherapy plus immunotherapy.
- Chemotherapy alone or as an adjunct to local treatment.
- Immunotherapy.
- EBRT for palliation.
- Urinary diversion or cystectomy for palliation.
- Other chemotherapy agents with activity in metastatic bladder cancer, such as paclitaxel, docetaxel, and ifosfamide (under clinical evaluation).[38,39][Level of evidence C3]
- Clinical trials.
Enfortumab vedotin plus pembrolizumab
Enfortumab vedotin is an antibody-drug conjugate, combining an antibody that binds to nectin-4 with a microtubule inhibitor. The FDA approved enfortumab vedotin as monotherapy for patients with previously treated metastatic urothelial carcinoma. Pembrolizumab is an anti–PD-1 antibody that has been approved as monotherapy for patients with metastatic bladder cancer. The combination of the two agents showed a promising response rate and duration of response, leading to a single-arm phase II trial and then a comparison with chemotherapy in a phase III, randomized controlled trial.[2-4] While results cannot be extrapolated across trials, enfortumab vedotin plus pembrolizumab is a first-line therapy option for patients without a contraindication.
Evidence (enfortumab vedotin plus pembrolizumab):
- In a phase III trial (EV-302 [NCT04223856]), 886 patients were randomly assigned to receive either enfortumab vedotin plus pembrolizumab (n = 442) or gemcitabine plus either cisplatin or carboplatin (n = 444). The median follow-up was 17.2 months.[4][Level of evidence A1]
- OS and PFS were longer for patients in the enfortumab vedotin-plus-pembrolizumab arm than in the chemotherapy arm.
- The median OS was 31.5 months for patients who received enfortumab vedotin plus pembrolizumab and 16.1 months for patients who received chemotherapy (HR, 0.47; 95% CI, 0.38–0.58; P < .001).
- PFS was 12.5 months for patients who received enfortumab vedotin plus pembrolizumab and 6.3 months for patients who received chemotherapy (HR, 0.45; 95% CI, 0.38–0.54; P < .001).
- Grade 3 or higher adverse events occurred in 55.9% of patients who received enfortumab vedotin plus pembrolizumab and 69.5% of patients who received chemotherapy.
- In the experimental arm, the most common high-grade adverse events were skin reactions, peripheral neuropathy, hyperglycemia, and neutropenia.
Chemotherapy plus immunotherapy
For many years, cisplatin-based chemotherapy was the standard-of-care first-line systemic treatment for patients with stage IV urothelial carcinoma who were eligible to receive cisplatin. A randomized, controlled, phase III trial reported longer OS when cisplatin-based chemotherapy was given with nivolumab, compared with chemotherapy alone.[5]
Evidence (chemotherapy plus immunotherapy):
- A phase III, randomized controlled trial (CheckMate901 [NCT03036098]) included 608 patients (304 to each arm). Patients were randomly assigned to receive either nivolumab plus gemcitabine and cisplatin or gemcitabine and cisplatin alone. Patients received up to six cycles of chemotherapy and up to 2 years of nivolumab. The median follow-up was 33.6 months.[5]
- The median OS was 21.7 months for patients who received nivolumab and 18.9 months for patients who received chemotherapy alone (HRdeath, 0.78; 95% CI, 0.63–0.96; P = .02).[5][Level of evidence A1]
- The median PFS was 7.9 months for patients who received nivolumab and 7.6 months for patients who received chemotherapy alone (HRprogression or death, 0.72; 95% CI, 0.59–0.88; P = .001).
- At 1 year, the PFS rate was 34.2% in the nivolumab arm and 21.8% in the chemotherapy-alone arm.
- The overall response rate was 57.6% in the nivolumab arm and 21.7% in the chemotherapy-alone arm. The complete response rate was 21.7% in the nivolumab arm and 11.8% in the chemotherapy-alone arm.
- The duration of complete response was 37.1 months in the nivolumab arm and 13.2 months in the chemotherapy-alone arm.
- Grade 3 or higher adverse events occurred in 61.8% of patients in the nivolumab arm and 51.7% of patients in the chemotherapy-alone arm.
Chemotherapy alone or as an adjunct to local treatment
Cisplatin-based combination chemotherapy regimens are the standard of care for first-line therapy for stage IV bladder cancer in patients who can tolerate it.[6-10] The only chemotherapy regimens that have been shown to result in longer survival in randomized controlled trials are MVAC, dose-dense MVAC, and CMV. GC was compared with MVAC in a randomized controlled trial and neither regimen was associated with a statistically significant difference in response rate or survival. The two regimens are generally considered equivalent, but they have never been compared in a noninferiority trial. Of note, patients with good performance status and lymph node–only disease have a low but significant rate of achieving a durable complete remission with MVAC or GC. For example, in a large, randomized controlled trial that compared MVAC with GC, the 5-year OS rate was 20.9% in patients with lymph node–only disease.[11] Dose-dense MVAC and standard-dose MVAC were compared in a randomized controlled trial, and dose-dense MVAC was associated with longer survival.
Single-agent cisplatin and multiagent regimens that do not include cisplatin have never resulted in improved survival in a randomized controlled trial. For patients who are not candidates for cisplatin-based multiagent chemotherapy regimens, there is no regimen that has been shown to prolong survival. However, many regimens have demonstrated radiologically measurable responses.
These regimens include carboplatin plus paclitaxel,[12] carboplatin plus gemcitabine,[13-15] paclitaxel plus gemcitabine,[16-18] single-agent gemcitabine,[19,20] and single-agent paclitaxel.[21-23] The regimens of carboplatin, methotrexate, and vinblastine; carboplatin, epirubicin, methotrexate, and vinblastine; and paclitaxel, gemcitabine, and carboplatin have been studied but are not widely used.[24-27]
Ongoing studies are evaluating new chemotherapy combinations.
Evidence (chemotherapy):
- A prospective randomized trial that compared MVAC with cisplatin, cyclophosphamide, and doxorubicin demonstrated improved response rate and longer median survival (48 weeks vs. 36 weeks; P = .003) with the MVAC regimen.[29]
- Results from a randomized trial that compared MVAC with single-agent cisplatin in advanced bladder cancer also showed a significant advantage with MVAC in both response rate and median survival (12.5 months vs. 8.2 months; P = .002).[30]
- A multicenter randomized controlled trial compared CMV with methotrexate plus vinblastine without cisplatin in 214 patients. The relative risk of dying was 0.68 (95% CI, 0.51–0.90; P = .0065) in favor of CMV. The median survival was 7 months with CMV compared with 4.5 months for methotrexate plus vinblastine.[31]
- The EORTC conducted a randomized trial that included 263 patients with advanced bladder cancer. The study evaluated the efficacy of a high-dose intensity MVAC regimen given every 2 weeks with G-CSF compared with a classic MVAC regimen given every 4 weeks.[32]
- There was no significant difference in OS at a median follow-up of 3.2 years (HR, 0.80; 95% CI, 0.60–1.06; P = .122). However, at a median follow-up of 7.3 years, the high-dose intensity MVAC regimen was associated with improved OS (HR, 0.76; 95% CI, 0.58–0.99; P = .042), with a 5-year survival rate of 22%, compared with 14% in patients treated with the classic MVAC regimen.
- The high-dose intensity MVAC regimen was also associated with higher response rates (72% vs. 58%; P = .016), improved median PFS (9.5 months vs. 8.1 months; P = .017), and decreased neutropenic fever (10% vs. 26%, P < .001), although only 19% of patients treated with a classic MVAC regimen ever received G-CSF.[32][Level of evidence A1] An imbalance in baseline prognostic factors (i.e., visceral metastases were found in 37 patients randomly assigned to the high-dose MVAC regimen and 47 patients assigned to the classic MVAC regimen) may account, in part, for these results.
- A multicenter, randomized, phase III trial compared GC with MVAC in 405 patients with advanced or metastatic bladder cancer.
- GC yielded response rates, time-to-progression, and OS (HR, 1.04; 95% CI, 0.82–1.32; P = .75) similar to MVAC, but GC had a better safety profile and was better tolerated than MVAC.[33][Level of evidence A1]
- Although this study was not designed to show the equivalence of the two regimens, the similar efficacy and reduced toxic effects of GC make it a reasonable alternative in patients who may not tolerate the MVAC regimen.
- Other chemotherapy regimens that have shown activity in metastatic bladder cancer include single-agent paclitaxel, single-agent gemcitabine, single-agent pemetrexed, carboplatin combined with either gemcitabine or paclitaxel, and gemcitabine combined with paclitaxel. There are no phase III trials demonstrating a survival or quality-of-life benefit from second-line chemotherapy.[13,16,18,19,27,40-52]
Immunotherapy
Immunotherapy has emerged as a treatment alternative for patients with stage IV bladder cancer. Anti−PD-1 or anti−programmed death-ligand 1 (PD-L1) immune checkpoint inhibitors have activity against urothelial carcinoma in patients who have previously been treated with, or who are ineligible for, platinum-based chemotherapy.[53-57] There are several approved agents. However, pembrolizumab has the most robust data in terms of level of evidence and survival rates.
Pembrolizumab
Pembrolizumab is a humanized monoclonal antibody that binds to PD-1. In patients previously treated with platinum-based chemotherapy, pembrolizumab has prolonged OS compared with second-line chemotherapy. As a result, the FDA approved pembrolizumab for patients with locally advanced or metastatic urothelial carcinoma who fall into one of the following three categories:
- Cisplatin-ineligible and have tumors that express PD-L1 (combined positive score [CPS] of at least 10).
- Cisplatin- and carboplatin-ineligible.
- Progression of disease after treatment with platinum-based chemotherapy.
Evidence (pembrolizumab):
- An open-label, international, randomized controlled, phase III trial (NCT02256436) included patients whose disease had progressed after treatment with platinum-based chemotherapy. Patients received pembrolizumab (200 mg intravenously [IV] every 21 days) or second-line chemotherapy (investigator’s choice of paclitaxel, docetaxel, or vinflunine).[58]
- The median OS was longer with pembrolizumab (10.3 months vs. 7.4 months; HR, 0.73; 95% CI, 0.59–0.91).
- In patients with a PD-L1 CPS of at least 10, the median OS was 8.0 months with pembrolizumab compared with 5.2 months of chemotherapy (HR, 0.57; 95% CI, 0.37–0.88).
- The PFS rate at 12 months was 16.8% (95% CI, 12.3%–22.0%) in the pembrolizumab arm and 6.2% (95% CI, 3.3%–10.2%) in the chemotherapy arm.
- The objective response rate was 21.1% in the pembrolizumab arm and 11.4% in the chemotherapy arm. Among those who responded, the median duration of response was longer than 18 months with pembrolizumab compared with a response of 4.3 months with chemotherapy.
- Pembrolizumab was associated with a lower rate of treatment-related adverse events than chemotherapy (60.9% vs. 90.2%) and a lower rate of high-grade adverse events (15.0% vs. 49.4%). The most common adverse events with pembrolizumab were pruritus, fatigue, nausea, diarrhea, and decreased appetite.[58][Level of evidence A1]
- A single-arm, phase II trial (NCT02335424) of pembrolizumab included 370 treatment-naïve, cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma.[59]
- The overall response rate was 29%. In patients with a PD-L1 CPS of at least 10, the overall response rate was 51%.[59][Level of evidence C3]
- The median duration of response had not been reached. Eighty-two percent of responses lasted at least 6 months.
- Ten percent of patients had a serious treatment-related adverse event, and one patient died of treatment-related adverse events. The most common high-grade adverse events were fatigue (2%), elevated alkaline phosphatase (1%), colitis (1%), and muscle weakness (1%).
Atezolizumab
Atezolizumab is a humanized monoclonal antibody that binds to PD-L1 and prevents it from binding to its receptors, PD-1 or B7-1. Atezolizumab was voluntarily withdrawn in 2022 as a first-line treatment option for locally advanced or metastatic urothelial cancer. The withdrawal was based on the results of the randomized IMvigor130 trial (NCT02807636) which showed no significant difference in OS. No survival improvement was seen in two analyses: atezolizumab plus platinum-based chemotherapy (group A) versus placebo plus platinum-based chemotherapy (group C),[60][Level of evidence A1] and atezolizumab monotherapy (group B) versus group C.[61][Level of evidence A1]
The trials below examined the use of atezolizumab as a first-line therapy in cisplatin-eligible patients.
Evidence (atezolizumab):
- A multicenter single-arm trial (NCT0108652) included 315 cisplatin-ineligible patients or patients who were previously treated with platinum-based chemotherapy and who had inoperable locally advanced or metastatic urothelial carcinoma. Patients received atezolizumab (1,200 mg IV every 21 days).[56][Level of evidence C2]
- The overall response rate was 15%.
- With a median follow-up of 11.7 months, 38 of 45 responders (84%) had an ongoing response.
- The response rate was 27% in patients with PD-L1 expression of at least 5%, 10% in those with PD-L1 expression of 1% to 5%, and 8% in those with PD-L1 expression of less than 1%.
- Grade 3 to 4 adverse events were reported in 16% of patients, and high-grade, immune-related adverse events were reported in 5% of patients.
- Similarly, a study (NCT02108652) of 123 previously untreated patients with cisplatin-ineligible, locally advanced metastatic urothelial carcinoma who were treated with atezolizumab reported that at a median follow-up of 17.2 months, the objective response rate was 23%, and 9% had complete responses.[57][Level of evidence C2]
- Of the 27 responses, 19 were ongoing at the time of data analysis. Median OS was 15.9 months.
- High-grade adverse events were reported in 16% of patients, 8% of patients discontinued treatment because of the adverse events, and there was one treatment-related death.
Nivolumab
Nivolumab is a fully human immunoglobulin G4 PD-1 immune checkpoint inhibitor antibody that blocks interaction between PD-L1 and PD-L2 with PD-1. There are no published controlled trials and thus no data regarding whether nivolumab results in longer survival or improved quality of life.
Evidence (nivolumab):
- A multicenter trial (NCT01928394) included 86 patients with metastatic urothelial carcinoma whose disease progressed after platinum-based chemotherapy. Patients received nivolumab (3 mg/kg IV every 2 weeks).[55][Level of evidence B4]
- With a minimum follow-up of 9 months and a median follow-up of 15 months, 24% of patients had an objective response, and 22% had grade 3 or 4 adverse events.
- The most common adverse events were elevated lipase or amylase, fatigue, rash, dyspnea, lymphopenia, and neutropenia.
- Another multicenter study (NCT02387996) of 270 patients with locally advanced or metastatic urothelial carcinoma that had progressed after treatment with platinum-based chemotherapy reported an objective response rate of 20%.[54][Level of evidence B4]
- When evaluated by PD-L1 expression, the objective response rate was 28% in those with PD-L1 expression of 5% or greater; 24% in those with PD-L1 expression of 1% or greater; and 16% in those with PD-L1 expression of less than 1%.
- Grade 3 to 4 adverse events were reported in 18% of patients.
- There were three treatment-related deaths.
Avelumab
Avelumab is a monoclonal anti–PD-L1 antibody that has shown activity against urothelial carcinoma. A randomized controlled trial reported an OS benefit from maintenance avelumab when given after platinum-based chemotherapy in patients whose cancer did not progress during chemotherapy.
Evidence (avelumab):
- A phase III study (NCT02603432) evaluated maintenance avelumab (10 mg/kg IV every 14 days) in patients with metastatic urothelial carcinoma who had not progressed during treatment with first-line chemotherapy with gemcitabine combined with either cisplatin or carboplatin. After four to six cycles of chemotherapy, 700 patients were randomly assigned to receive either best supportive care (control arm) or best supportive care plus maintenance avelumab (avelumab arm).[62][Level of evidence A1]
- The 1-year OS rate was 71.3% in the avelumab arm compared with 58.4% in the control arm (HR, 0.69; 95% CI, 0.56–0.86).
- The OS benefit was limited to patients whose tumors were PD-L1 positive. The OS in patients with PD-L1–negative tumors was not significantly different between the two arms.
- Patients with PD-L1–positive tumors had a 1-year OS rate of 79.1% in the avelumab arm and 60.4% in the control arm (HR, 0.56; 95% CI, 0.40–0.79).
- In the overall study population, the median PFS was 3.7 months for patients in the avelumab arm and 2.0 months for patients in the control arm (HR, 0.62; 95% CI, 0.52–0.75). In patients with PD-L1–positive tumors, the median PFS was 5.7 months with avelumab and 2.1 months with best supportive care alone (HR, 0.56; 95% CI, 0.43–0.73).
- Grade 3 or higher adverse events were reported for 47.4% of patients in the avelumab arm and 25.2% of patients in the control arm. Most adverse events with avelumab were immune related, and 9% of patients who received avelumab were treated with high-dose glucocorticoids for these events.
- A study (NCT01772004) of avelumab (10 mg/kg IV every 14 days) included 249 patients with metastatic urothelial carcinoma who had progressed after treatment with platinum-based chemotherapy.[63,64][Level of evidence C3]
- Among the 161 patients with at least 6 months of follow-up, the overall response rate was 17%, and response was ongoing in 23 of 28 responders with a median follow-up of 7.3 months. Six percent of the patients had a complete response.
- The overall response rate was 25.0% in patients with PD-L1 expression of at least 5% and 14.7% for those with less than 5% PD-L1 positivity.
- Median PFS was 6.3 weeks; 23% of the patients were progression free at 24 weeks.
- A treatment-related adverse event was reported in 66.7% of patients, including 8.4% of patients with high-grade adverse events. There was one treatment-related death from pneumonitis. High-grade immune-related adverse events were reported in 2.4% of patients.
Durvalumab
Durvalumab is an anti–PD-L1 monoclonal antibody with activity when given perioperatively with neoadjuvant chemotherapy in patients with resectable disease. However, durvalumab was voluntarily withdrawn in 2021 as a first-line therapy for locally advanced or metastatic urothelial cancer. The withdrawal was based on the randomized DANUBE trial (NCT02516241) which showed no significant improvement in OS.[65][Level of evidence A1]
EBRT for palliation
Definitive radiation therapy with or without concurrent chemotherapy, evaluated mainly in patients with locally advanced (T2–T4) disease, appears to have minimal curative potential in patients with regional lymph node metastases.
Urinary diversion or cystectomy for palliation
Urinary diversion may be indicated, not only for palliation of urinary symptoms, but also for preservation of renal function in candidates for chemotherapy.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Vieweg J, Gschwend JE, Herr HW, et al.: The impact of primary stage on survival in patients with lymph node positive bladder cancer. J Urol 161 (1): 72-6, 1999. [PUBMED Abstract]
- Hoimes CJ, Flaig TW, Milowsky MI, et al.: Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J Clin Oncol 41 (1): 22-31, 2023. [PUBMED Abstract]
- O'Donnell PH, Milowsky MI, Petrylak DP, et al.: Enfortumab Vedotin With or Without Pembrolizumab in Cisplatin-Ineligible Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol 41 (25): 4107-4117, 2023. [PUBMED Abstract]
- Powles T, Valderrama BP, Gupta S, et al.: Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med 390 (10): 875-888, 2024. [PUBMED Abstract]
- van der Heijden MS, Sonpavde G, Powles T, et al.: Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med 389 (19): 1778-1789, 2023. [PUBMED Abstract]
- Kachnic LA, Kaufman DS, Heney NM, et al.: Bladder preservation by combined modality therapy for invasive bladder cancer. J Clin Oncol 15 (3): 1022-9, 1997. [PUBMED Abstract]
- Tester W, Porter A, Asbell S, et al.: Combined modality program with possible organ preservation for invasive bladder carcinoma: results of RTOG protocol 85-12. Int J Radiat Oncol Biol Phys 25 (5): 783-90, 1993. [PUBMED Abstract]
- Logothetis CJ, Johnson DE, Chong C, et al.: Adjuvant chemotherapy of bladder cancer: a preliminary report. J Urol 139 (6): 1207-11, 1988. [PUBMED Abstract]
- Skinner DG, Daniels JR, Russell CA, et al.: The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol 145 (3): 459-64; discussion 464-7, 1991. [PUBMED Abstract]
- Scher HI: Chemotherapy for invasive bladder cancer: neoadjuvant versus adjuvant. Semin Oncol 17 (5): 555-65, 1990. [PUBMED Abstract]
- von der Maase H, Sengelov L, Roberts JT, et al.: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23 (21): 4602-8, 2005. [PUBMED Abstract]
- Vaishampayan UN, Faulkner JR, Small EJ, et al.: Phase II trial of carboplatin and paclitaxel in cisplatin-pretreated advanced transitional cell carcinoma: a Southwest Oncology Group study. Cancer 104 (8): 1627-32, 2005. [PUBMED Abstract]
- Carles J, Nogué M: Gemcitabine/carboplatin in advanced urothelial cancer. Semin Oncol 28 (3 Suppl 10): 19-24, 2001. [PUBMED Abstract]
- Linardou H, Aravantinos G, Efstathiou E, et al.: Gemcitabine and carboplatin combination as first-line treatment in elderly patients and those unfit for cisplatin-based chemotherapy with advanced bladder carcinoma: Phase II study of the Hellenic Co-operative Oncology Group. Urology 64 (3): 479-84, 2004. [PUBMED Abstract]
- De Santis M, Bellmunt J, Mead G, et al.: Randomized phase II/III trial assessing gemcitabine/ carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer "unfit" for cisplatin-based chemotherapy: phase II--results of EORTC study 30986. J Clin Oncol 27 (33): 5634-9, 2009. [PUBMED Abstract]
- Sternberg CN, Calabrò F, Pizzocaro G, et al.: Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer 92 (12): 2993-8, 2001. [PUBMED Abstract]
- Kaufman DS, Carducci MA, Kuzel TM, et al.: A multi-institutional phase II trial of gemcitabine plus paclitaxel in patients with locally advanced or metastatic urothelial cancer. Urol Oncol 22 (5): 393-7, 2004 Sep-Oct. [PUBMED Abstract]
- Calabrò F, Lorusso V, Rosati G, et al.: Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer 115 (12): 2652-9, 2009. [PUBMED Abstract]
- Stadler WM, Kuzel T, Roth B, et al.: Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol 15 (11): 3394-8, 1997. [PUBMED Abstract]
- Lorusso V, Pollera CF, Antimi M, et al.: A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. Eur J Cancer 34 (8): 1208-12, 1998. [PUBMED Abstract]
- Roth BJ, Dreicer R, Einhorn LH, et al.: Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: a phase II trial of the Eastern Cooperative Oncology Group. J Clin Oncol 12 (11): 2264-70, 1994. [PUBMED Abstract]
- Dreicer R, Gustin DM, See WA, et al.: Paclitaxel in advanced urothelial carcinoma: its role in patients with renal insufficiency and as salvage therapy. J Urol 156 (5): 1606-8, 1996. [PUBMED Abstract]
- Vaughn DJ, Broome CM, Hussain M, et al.: Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 20 (4): 937-40, 2002. [PUBMED Abstract]
- Bellmunt J, Albanell J, Gallego OS, et al.: Carboplatin, methotrexate, and vinblastine in patients with bladder cancer who were ineligible for cisplatin-based chemotherapy. Cancer 70 (7): 1974-9, 1992. [PUBMED Abstract]
- Bellmunt J, Ribas A, Albanell J, et al.: M-CAVI, a neoadjuvant carboplatin-based regimen for the treatment of T2-4N0M0 carcinoma of the bladder. Am J Clin Oncol 19 (4): 344-8, 1996. [PUBMED Abstract]
- Skarlos DV, Aravantinos G, Linardou E, et al.: Chemotherapy with methotrexate, vinblastine, epirubicin and carboplatin (Carbo-MVE) in transitional cell urothelial cancer. A Hellenic Co-Operative Oncology Group study. Eur Urol 31 (4): 420-7, 1997. [PUBMED Abstract]
- Hainsworth JD, Meluch AA, Litchy S, et al.: Paclitaxel, carboplatin, and gemcitabine in the treatment of patients with advanced transitional cell carcinoma of the urothelium. Cancer 103 (11): 2298-303, 2005. [PUBMED Abstract]
- Bamias A, Aravantinos G, Deliveliotis C, et al.: Docetaxel and cisplatin with granulocyte colony-stimulating factor (G-CSF) versus MVAC with G-CSF in advanced urothelial carcinoma: a multicenter, randomized, phase III study from the Hellenic Cooperative Oncology Group. J Clin Oncol 22 (2): 220-8, 2004. [PUBMED Abstract]
- Logothetis CJ, Dexeus FH, Finn L, et al.: A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol 8 (6): 1050-5, 1990. [PUBMED Abstract]
- Loehrer PJ, Einhorn LH, Elson PJ, et al.: A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 10 (7): 1066-73, 1992. [PUBMED Abstract]
- Mead GM, Russell M, Clark P, et al.: A randomized trial comparing methotrexate and vinblastine (MV) with cisplatin, methotrexate and vinblastine (CMV) in advanced transitional cell carcinoma: results and a report on prognostic factors in a Medical Research Council study. MRC Advanced Bladder Cancer Working Party. Br J Cancer 78 (8): 1067-75, 1998. [PUBMED Abstract]
- Sternberg CN, de Mulder P, Schornagel JH, et al.: Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 42 (1): 50-4, 2006. [PUBMED Abstract]
- von der Maase H, Hansen SW, Roberts JT, et al.: Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18 (17): 3068-77, 2000. [PUBMED Abstract]
- Jahnson S, Pedersen J, Westman G: Bladder carcinoma--a 20-year review of radical irradiation therapy. Radiother Oncol 22 (2): 111-7, 1991. [PUBMED Abstract]
- Coppin CM, Gospodarowicz MK, James K, et al.: Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 14 (11): 2901-7, 1996. [PUBMED Abstract]
- Shipley WU, Winter KA, Kaufman DS, et al.: Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol 16 (11): 3576-83, 1998. [PUBMED Abstract]
- James ND, Hussain SA, Hall E, et al.: Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366 (16): 1477-88, 2012. [PUBMED Abstract]
- Raghavan D, Huben R: Management of bladder cancer. Curr Probl Cancer 19 (1): 1-64, 1995 Jan-Feb. [PUBMED Abstract]
- Sweeney CJ, Roth BJ, Kabbinavar FF, et al.: Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol 24 (21): 3451-7, 2006. [PUBMED Abstract]
- Vaughn DJ, Malkowicz SB, Zoltick B, et al.: Paclitaxel plus carboplatin in advanced carcinoma of the urothelium: an active and tolerable outpatient regimen. J Clin Oncol 16 (1): 255-60, 1998. [PUBMED Abstract]
- Zielinski CC, Schnack B, Grbovic M, et al.: Paclitaxel and carboplatin in patients with metastatic urothelial cancer: results of a phase II trial. Br J Cancer 78 (3): 370-4, 1998. [PUBMED Abstract]
- Bajorin DF, McCaffrey JA, Dodd PM, et al.: Ifosfamide, paclitaxel, and cisplatin for patients with advanced transitional cell carcinoma of the urothelial tract: final report of a phase II trial evaluating two dosing schedules. Cancer 88 (7): 1671-8, 2000. [PUBMED Abstract]
- Carles J, Nogué M, Domènech M, et al.: Carboplatin-gemcitabine treatment of patients with transitional cell carcinoma of the bladder and impaired renal function. Oncology 59 (1): 24-7, 2000. [PUBMED Abstract]
- Dreicer R, Manola J, Roth BJ, et al.: Phase II study of cisplatin and paclitaxel in advanced carcinoma of the urothelium: an Eastern Cooperative Oncology Group Study. J Clin Oncol 18 (5): 1058-61, 2000. [PUBMED Abstract]
- Hussain M, Vaishampayan U, Du W, et al.: Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancer. J Clin Oncol 19 (9): 2527-33, 2001. [PUBMED Abstract]
- Meluch AA, Greco FA, Burris HA, et al.: Paclitaxel and gemcitabine chemotherapy for advanced transitional-cell carcinoma of the urothelial tract: a phase II trial of the Minnie Pearl cancer research network. J Clin Oncol 19 (12): 3018-24, 2001. [PUBMED Abstract]
- Albers P, Siener R, Härtlein M, et al.: Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma - prognostic factors for response and improvement of quality of life. Onkologie 25 (1): 47-52, 2002. [PUBMED Abstract]
- Li J, Juliar B, Yiannoutsos C, et al.: Weekly paclitaxel and gemcitabine in advanced transitional-cell carcinoma of the urothelium: a phase II Hoosier Oncology Group study. J Clin Oncol 23 (6): 1185-91, 2005. [PUBMED Abstract]
- Lorusso V, Crucitta E, Silvestris N, et al.: Randomised, open-label, phase II trial of paclitaxel, gemcitabine and cisplatin versus gemcitabine and cisplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium. Oncol Rep 13 (2): 283-7, 2005. [PUBMED Abstract]
- Fechner G, Siener R, Reimann M, et al.: Randomised phase II trial of gemcitabine and paclitaxel second-line chemotherapy in patients with transitional cell carcinoma (AUO Trial AB 20/99). Int J Clin Pract 60 (1): 27-31, 2006. [PUBMED Abstract]
- Galsky MD, Mironov S, Iasonos A, et al.: Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs 25 (3): 265-70, 2007. [PUBMED Abstract]
- Chee CE, Jett JR, Bernath AM, et al.: Phase 2 trial of pemetrexed disodium and carboplatin in previously untreated extensive-stage small cell lung cancer, N0423. Cancer 116 (10): 2382-9, 2010. [PUBMED Abstract]
- Plimack ER, Bellmunt J, Gupta S, et al.: Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 18 (2): 212-220, 2017. [PUBMED Abstract]
- Sharma P, Retz M, Siefker-Radtke A, et al.: Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 18 (3): 312-322, 2017. [PUBMED Abstract]
- Sharma P, Callahan MK, Bono P, et al.: Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 17 (11): 1590-1598, 2016. [PUBMED Abstract]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al.: Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387 (10031): 1909-20, 2016. [PUBMED Abstract]
- Balar AV, Galsky MD, Rosenberg JE, et al.: Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389 (10064): 67-76, 2017. [PUBMED Abstract]
- Bellmunt J, de Wit R, Vaughn DJ, et al.: Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 376 (11): 1015-1026, 2017. [PUBMED Abstract]
- Balar AV, Castellano D, O'Donnell PH, et al.: First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 18 (11): 1483-1492, 2017. [PUBMED Abstract]
- Grande E, Arranz JÁ, De Santis M, et al.: Atezolizumab plus chemotherapy versus placebo plus chemotherapy in untreated locally advanced or metastatic urothelial carcinoma (IMvigor130): final overall survival analysis results from a randomised, controlled, phase 3 study. Lancet Oncol 25 (1): 29-45, 2024. [PUBMED Abstract]
- Bamias A, Davis ID, Galsky MD, et al.: Atezolizumab monotherapy versus chemotherapy in untreated locally advanced or metastatic urothelial carcinoma (IMvigor130): final overall survival analysis from a randomised, controlled, phase 3 study. Lancet Oncol 25 (1): 46-61, 2024. [PUBMED Abstract]
- Powles T, Park SH, Voog E, et al.: Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 383 (13): 1218-1230, 2020. [PUBMED Abstract]
- Apolo AB, Ellerton JA, Infante JR, et al.: Updated efficacy and safety of avelumab in metastatic urothelial carcinoma (mUC): pooled analysis from 2 cohorts of the phase 1b Javelin solid tumor study. [Abstract] J Clin Oncol 35 (Suppl 15): A-4529, 2017.
- Patel MR, Ellerton J, Infante JR, et al.: Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 19 (1): 51-64, 2018. [PUBMED Abstract]
- Powles T, van der Heijden MS, Castellano D, et al.: Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 21 (12): 1574-1588, 2020. [PUBMED Abstract]
Treatment of Recurrent Bladder Cancer
The prognosis for any patient with progressive or recurrent invasive bladder cancer is generally poor. Management of recurrence depends on previous therapy, sites of recurrence, and individual patient considerations.
Treatment Options for Recurrent Bladder Cancer
Treatment options for patients with recurrent bladder cancer include:
- Combination chemotherapy.
- Immunotherapy.
- Targeted therapy.
- Surgery for new superficial or localized tumors.
- Palliative therapy.
- Clinical trials.
Combination chemotherapy
Patients who have not received previous chemotherapy for urothelial carcinoma should consider chemotherapy as described above for stage IV disease.
In patients with recurrent transitional cell carcinoma, combination chemotherapy has produced high response rates, with occasional complete responses seen.[1,2]
Cisplatin-based combination chemotherapy regimens are the standard of care for first-line therapy for stage IV bladder cancer in patients who can tolerate it.[3-7] The only chemotherapy regimens that have been shown to result in longer survival in randomized controlled trials are MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin), dose-dense MVAC, and CMV (cisplatin, methotrexate, and vinblastine).
GC (gemcitabine and cisplatin) was compared with MVAC in a randomized controlled trial, and neither regimen was associated with a statistically significant difference in response rate or survival. The two regimens are generally considered equivalent, but they have never been compared in a noninferiority trial. Of note, patients with good performance status and lymph node–only disease have a low but significant rate of achieving a durable complete remission with MVAC or GC. For example, in a large, randomized controlled trial comparing MVAC with GC, the 5-year overall survival (OS) rate was 20.9% in patients with lymph node–only disease.[8] Dose-dense MVAC and standard-dose MVAC were compared in a randomized controlled trial, and dose-dense MVAC was associated with longer survival.
Single-agent cisplatin and multiagent regimens that do not include cisplatin have never resulted in improved survival in a randomized controlled trial. For patients who are not candidates for cisplatin-based multiagent chemotherapy regimens, there is no regimen that has been shown to prolong survival. However, many regimens have demonstrated radiologically measurable responses.
These regimens include carboplatin plus paclitaxel,[9] carboplatin plus gemcitabine,[10-12] paclitaxel plus gemcitabine,[13-15] single-agent gemcitabine,[16,17] and single-agent paclitaxel.[18-20] The regimens of carboplatin, methotrexate, and vinblastine; carboplatin, epirubicin, methotrexate, and vinblastine; and paclitaxel, gemcitabine, and carboplatin have been studied but are not widely used.[21-24]
Evidence (combination chemotherapy):
- In a prospective randomized trial that compared MVAC with cisplatin, cyclophosphamide, and doxorubicin, the MVAC regimen demonstrated improved response rate and longer median survival (48 weeks vs. 36 weeks; P = .003).[25]
- Results from a randomized trial that compared MVAC with single-agent cisplatin in advanced bladder cancer also showed a significant advantage with MVAC in both response rate and median survival (12.5 months vs. 8.2 months; P = .002).[26]
- A multicenter randomized controlled trial compared CMV with methotrexate plus vinblastine without cisplatin in 214 patients.[27]
- The relative risk of dying was 0.68 (95% confidence interval [CI], 0.51–0.90; P = .0065) in favor of CMV.
- The median survival was 7 months with CMV compared with 4.5 months for methotrexate plus vinblastine.
- The European Organisation for Research and Treatment of Cancer (EORTC) conducted a randomized trial that included 263 patients with advanced bladder cancer. The study evaluated the efficacy of a high-dose intensity MVAC regimen given every 2 weeks with granulocyte colony-stimulating factor (G-CSF) versus a classic MVAC regimen given every 4 weeks.[28]
- Although there was no significant difference in OS at a median follow-up of 3.2 years (hazard ratio [HR], 0.80; 95% CI, 0.60–1.06; P = .122), an update at a median follow-up of 7.3 years reported that the high-dose intensity MVAC regimen was associated with improved OS (HR, 0.76; 95% CI, 0.58–0.99; P = .042), with a 5-year survival rate of 22% compared with 14% in patients treated with the classic MVAC regimen.
- The high-dose intensity MVAC regimen was also associated with higher response rates (72% vs. 58%; P = .016), improved median progression-free survival (PFS) (9.5 months vs. 8.1 months; P = .017), and decreased neutropenic fever (10% vs. 26%; P < .001), although only 19% of patients treated with a classic MVAC regimen ever received G-CSF.[28][Level of evidence A1] An imbalance in baseline prognostic factors (i.e., visceral metastases were found in 37 patients randomly assigned to the high-dose MVAC regimen and 47 patients assigned to the classic MVAC regimen) may account, in part, for these results.
- A multicenter, randomized, phase III trial compared GC with MVAC in 405 patients with advanced or metastatic bladder cancer.
- GC yielded response rates, time-to-progression, and OS (HR, 1.04; 95% CI, 0.82–1.32; P = .75) similar to MVAC, but GC had a better safety profile and was better tolerated than MVAC.[29][Level of evidence A1]
Although this study was not designed to show the equivalence of the two regimens, the similar efficacy and reduced toxic effects of GC make it a reasonable alternative in patients who may not tolerate the MVAC regimen.
- Other chemotherapy regimens that have shown activity in metastatic bladder cancer include single-agent paclitaxel, single-agent gemcitabine, single-agent pemetrexed, carboplatin combined with either gemcitabine or paclitaxel, and gemcitabine combined with paclitaxel. There are no phase III trials demonstrating a survival or quality-of-life benefit from second-line chemotherapy.[30-37]
Immunotherapy
Immunotherapy has emerged as a treatment alternative for patients with recurrent bladder cancer. Anti−programmed death-1 (PD-1) or anti−programmed death-ligand 1 (PD-L1) immune checkpoint inhibitors have activity against urothelial carcinoma in patients who have previously been treated with, or who are ineligible for, cisplatin-based chemotherapy.[38-42] There are several approved agents. However, pembrolizumab has the most robust data in terms of level of evidence and survival.
Pembrolizumab
Pembrolizumab is a humanized monoclonal antibody that binds to PD-1. In patients previously treated with platinum-based chemotherapy, pembrolizumab has prolonged OS compared with second-line chemotherapy. As a result, the U.S. Food and Drug Administration (FDA) approved pembrolizumab for patients with locally advanced or metastatic urothelial carcinoma who fall into one of the following three categories:
- Cisplatin-ineligible and have tumors that express PD-L1 (combined positive score [CPS] of at least 10).
- Cisplatin- and carboplatin-ineligible.
- Progression of disease after treatment with platinum-based chemotherapy.
Evidence (pembrolizumab):
- An open-label, international, phase III, randomized controlled trial (NCT02256436) included patients whose disease had progressed after treatment with platinum-based chemotherapy. Patients received pembrolizumab (200 mg intravenously [IV] every 21 days) or second-line chemotherapy (investigator’s choice of paclitaxel, docetaxel, or vinflunine).[43]
- The median OS was longer with pembrolizumab (10.3 months vs.7.4 months; HR, 0.73; 95% CI, 0.59–0.91).
- In patients with a PD-L1 CPS of at least 10, median OS was 8.0 months with pembrolizumab compared with 5.2 months of chemotherapy (HR, 0.57; 95% CI, 0.37–0.88).
- The PFS rate at 12 months was 16.8% (95% CI, 12.3%–22.0%) in the pembrolizumab arm and 6.2% (95% CI, 3.3%–10.2%) in the chemotherapy arm.
- The objective response rate was 21.1% in the pembrolizumab arm and 11.4% in the chemotherapy arm. Among those who responded, the median duration of response was longer than 18 months with pembrolizumab compared with a response of 4.3 months with chemotherapy.
- Pembrolizumab was associated with a lower rate of treatment-related adverse events than chemotherapy (60.9% vs. 90.2%) and a lower rate of high-grade adverse events (15.0% vs. 49.4%). The most common adverse events with pembrolizumab were pruritus, fatigue, nausea, diarrhea, and decreased appetite.[43][Level of evidence A1]
- A single-arm phase II trial (NCT02335424) of pembrolizumab included 370 treatment-naïve, cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma.[44]
- The overall response rate was 29%. In patients with a PD-L1 CPS of at least 10, the overall response rate was 51%.
- The median duration of response had not been reached. Eighty-two percent of responses lasted at least 6 months.
- Ten percent of patients had a serious treatment-related adverse event, and one patient died of treatment-related adverse events. The most common high-grade adverse events were fatigue (2%), elevated alkaline phosphatase (1%), colitis (1%), and muscle weakness (1%).[44][Level of evidence C3]
Atezolizumab
Atezolizumab is a humanized monoclonal antibody that binds to PD-L1 and prevents it from binding to its receptors PD-1 or B7-1. Atezolizumab was voluntarily withdrawn in 2022 as a first-line treatment option for locally advanced or metastatic urothelial cancer. The withdrawal was based on the results of the randomized IMvigor130 trial (NCT02807636) which showed no significant difference in OS.[45][Level of evidence A1]; [46][Level of evidence A1] Atezolizumab has been studied as a second-line therapy after platinum-based chemotherapy.
Evidence (atezolizumab):
- The IMvigor211 trial (NCT02302807) is a randomized controlled trial that compared atezolizumab with second-line chemotherapy (docetaxel, paclitaxel, or vinflunine) in 931 patients with locally advanced or metastatic urothelial carcinoma who were previously treated with platinum-containing chemotherapy.[47] The trial design specified that OS would be primarily assessed in patients with PD-L1 expression of at least 5%.
- The median OS was 11.1 months for atezolizumab versus 10.6 months for chemotherapy in patients with PD-L1 expression of at least 5% (P = .41).[47][Level of evidence A1]
- Response rates were also similar: 23% with atezolizumab and 22% with chemotherapy.
- Patients receiving atezolizumab had a lower rate of high-grade toxicity (20% vs. 43%) and a lower rate of treatment discontinuation resulting from adverse events (7% vs. 18%).
Nivolumab
Nivolumab is a fully human immunoglobulin G4 PD-1 immune checkpoint inhibitor antibody that blocks interaction between PD-L1 and PD-L2 with PD-1. There are no published controlled trials and thus no data regarding whether nivolumab results in longer survival or improved quality of life.
Evidence (nivolumab):
- A multicenter trial (NCT01928394) included 86 patients with metastatic urothelial carcinoma whose disease progressed after platinum-based chemotherapy. Patients received nivolumab (3 mg/kg IV every 2 weeks).[40][Level of evidence B4]
- With a minimum follow-up of 9 months and a median follow-up of 15 months, 24% had an objective response, and 22% had grade 3 or 4 adverse events.
- The most common adverse events were elevated lipase or amylase, fatigue, rash, dyspnea, lymphopenia, and neutropenia.
- Another multicenter study (NCT02387996) of 270 patients with locally advanced or metastatic urothelial carcinoma that had progressed after treatment with platinum-based chemotherapy reported an objective response rate of 20%.[39][Level of evidence B4]
- When evaluated by PD-L1 expression, the objective response rate was 28% in those with PD-L1 expression of 5% or greater; 24% in those with PD-L1 expression of 1% or greater; and 16% in those with PD-L1 expression of less than 1%.
- Grade 3 to 4 adverse events were reported in 18% of patients.
- There were three treatment-related deaths.
Avelumab
Avelumab is a monoclonal anti–PD-L1 antibody that has shown activity against urothelial carcinoma. A randomized controlled trial reported an OS benefit from maintenance avelumab when given after platinum-based chemotherapy in patients whose cancer did not progress during chemotherapy.
Evidence (avelumab):
- A phase III study (NCT02603432) evaluated maintenance avelumab (10 mg/kg IV every 14 days) in patients with metastatic urothelial carcinoma who had not progressed during treatment with first-line chemotherapy with gemcitabine combined with either cisplatin or carboplatin. After four to six cycles of chemotherapy, 700 patients were randomly assigned to receive either best supportive care (control arm) or best supportive care plus maintenance avelumab (avelumab arm).[48][Level of evidence A1]
- The 1-year OS rate was 71.3% in the avelumab arm compared with 58.4% in the control arm (HR, 0.69; 95% CI, 0.56–0.86).
- The OS benefit was limited to patients whose tumors were PD-L1-positive. The OS in patients with PD-L1–negative tumors was not significantly different between the two arms.
- Patients with PD-L1–positive tumors had a 1-year OS rate of 79.1% in the avelumab arm and 60.4% in the control arm (HR, 0.56; 95% CI, 0.40–0.79).
- In the overall study population, the median PFS was 3.7 months for patients in the avelumab arm and 2.0 months for patients in the control arm (HR, 0.62; 95% CI, 0.52–0.75). In patients with PD-L1–positive tumors, the median PFS was 5.7 months with avelumab and 2.1 months with best supportive care alone (HR, 0.56; 95% CI, 0.43–0.73).
- Grade 3 or higher adverse events were reported for 47.4% of patients in the avelumab arm and 25.2% of patients in the control arm. Most adverse events with avelumab were immune related, and 9% of patients who received avelumab were treated with high-dose glucocorticoids for these events.
- A study (NCT01772004) of avelumab (10 mg/kg IV every 14 days) in 249 patients with metastatic urothelial carcinoma who had progressed after treatment with platinum-based chemotherapy reported the following results:[49,50][Level of evidence C3]
- Among the 161 patients with at least 6 months of follow-up, the overall response rate was 17%, and response was ongoing in 23 of 28 responders with a median follow-up of 7.3 months. Six percent of the patients had a complete response.
- The overall response rate was 25.0% in patients with PD-L1 expression of at least 5% and 14.7% for those with PD-L1 expression of less than 5%.
- The median progression-free survival was 6.3 weeks; 23% of the patients were progression free at 24 weeks.
- A treatment-related adverse event was reported in 66.7% of patients, including 8.4% of patients with high-grade adverse events. There was one treatment-related death from pneumonitis. High-grade immune-related adverse events were reported in 2.4% of patients.
Durvalumab
Durvalumab is an anti–PD-L1 monoclonal antibody with activity when given perioperatively with neoadjuvant chemotherapy in patients with resectable disease. However, durvalumab was voluntarily withdrawn in 2021 as a first-line therapy for locally advanced or metastatic urothelial cancer. The withdrawal was based on the randomized DANUBE trial (NCT02516241) which showed no significant improvement in OS.[51][Level of evidence A1]
Durvalumab was assessed as second-line therapy after platinum-based chemotherapy or for cisplatin-eligible patients.
Evidence (durvalumab):
- The efficacy of durvalumab (10 mg/kg IV every 14 days) was assessed in a study (NCT01693562) of 191 patients with locally advanced or metastatic urothelial carcinoma whose disease had progressed while they were on chemotherapy or who were either ineligible for or unwilling to be treated with chemotherapy.[52][Level of evidence C3]
- With a median follow-up of 5.78 months, the overall response rate was 17.8%.
- The response rate was 27.6% in patients with high expression of PD-L1 compared with 5.1% in patients with low or no PD-L1 expression.
- High-grade adverse events were reported in 6.8% of patients, including 2.1% with high-grade immune-mediated adverse events.
Targeted therapy
Ramucirumab
Ramucirumab is an immunoglobulin G1 monoclonal antibody that blocks the vascular endothelial growth factor receptor-2 (VEGFR-2). The FDA approved ramucirumab for patients with gastric carcinoma and gastroesophageal junction adenocarcinoma, but not for patients with bladder cancer.
Evidence (ramucirumab):
- Docetaxel chemotherapy plus ramucirumab was compared with docetaxel plus placebo in a double-blind, multicenter, randomized controlled phase III trial in 530 patients with metastatic urothelial carcinoma that had progressed after treatment with platinum-based chemotherapy. Forty-five patients (8.5%) had previously been treated with immunotherapy.[53][Level of evidence B1]
- OS was not significantly different between the two arms.
- The median PFS was 1.3 months (39 days) longer in the ramucirumab arm compared with the placebo arm (4.1 months vs. 2.8 months; HR, 0.696; 95% CI, 0.573–0.845, P = .0002).
- The overall response rate was 26% in the ramucirumab arm and 14% in the placebo arm.
- Treatment was discontinued because of adverse events in 19% of patients who received docetaxel plus ramucirumab and 8% of patients who received docetaxel plus placebo.
- Grade 3 or higher adverse events were reported in 48% of patients in the ramucirumab arm and 41% of patients in the placebo arm. Adverse events included febrile neutropenia, epistaxis, hypertension, hematuria, and proteinuria. Sepsis occurred in 2% of patients who received ramucirumab, and no patients who received placebo.
Enfortumab vedotin
Enfortumab vedotin is a type of targeted therapy called an antibody-drug conjugate. Antibody-drug conjugates consist of a monoclonal antibody chemically linked to a drug. The monoclonal antibody part of enfortumab vedotin binds to a protein called nectin-4, which is found on the surface of most bladder cancer cells. The antibody is chemically linked to monomethyl auristatin E, or MMAE, a type of chemotherapy drug called a microtubule inhibitor. Once the conjugate is taken up by cells, the drug stops them from dividing and leads to their death.
The FDA approved enfortumab vedotin for patients with metastatic urothelial carcinoma that has progressed after treatment with both platinum-based first-line chemotherapy and second-line therapy with an immune checkpoint inhibitor. The approval was based on a single-arm trial of 125 patients with metastatic urothelial carcinoma.[54]
Evidence (enfortumab vedotin):
- In a single-arm trial, 125 patients with metastatic urothelial carcinoma were treated with enfortumab vedotin.[54]
- There was a 44% overall response rate and a 12% complete response rate.
- The median duration of response was 7.6 months.
- Adverse events included fatigue (50%), peripheral neuropathy (50%), alopecia (49%), rash (48%), decreased appetite (44%), and dysgeusia (40%).
Because there was no control arm, this study could not demonstrate an improvement in OS or quality of life. A follow-up phase III trial is under way to assess the benefits of this medication.
Erdafitinib
Erdafitinib (JNJ-42756493) is a potent FGFR 1–4 tyrosine kinase inhibitor. The FDA approved erdafitinib for patients with urothelial carcinoma and a variant in one of the four FGFR genes and disease that has progressed after prior chemotherapy.
Roughly 20% of metastatic urothelial carcinomas of the bladder have FGFR variants, as do 35% of urothelial carcinomas of the ureters and renal pelvis.
Evidence (erdafitinib):
- A phase II trial included patients with metastatic urothelial carcinoma whose disease had progressed during or after systemic chemotherapy.[55] To be eligible for the study, patients were required to have tumors with an FGFR3 variant or an FGFR2::FGFR3 gene fusion. The trial was originally designed as a randomized phase II trial comparing continuous with intermittent treatment, but after an interim safety analysis, the trial was converted to a single-arm study of continuous treatment with dose escalation. The published analysis focuses on this latter phase of the study, referred to as the “selected-regimen group.” The selected-regimen group consisted of 99 patients, 74 with an FGFR3 variant and 25 with an FGFR2::FGFR3 gene fusion.
- The response rate by independent radiological review was 34%.
- The response rate was higher among the patients with FGFR3 variants than among the patients with FGFR2::FGFR3 gene fusions.
- Grade 3 adverse events that affected at least 10% of patients included stomatitis and hyponatremia. The most common adverse events of any grade were hypophosphatemia (77%), stomatitis (58%), diarrhea (51%), dry mouth (46%), decreased appetite (38%), and dysgeusia (37%).
- A randomized controlled trial (THOR [NCT03390504]) included 266 patients with locally advanced or metastatic urothelial carcinoma who had received anti–PD-1 or anti–PD-L1 therapy. Patients had FGFR2 or FGFR3 pathogenic variants or gene fusions. The study compared erdafitinib with chemotherapy.[56][Level of evidence A1]
- At a median follow-up of 15.9 months, the OS was 12.1 months in the erdafitinib arm and 7.8 months in the chemotherapy arm (HR, 0.64; 95% CI, 0.47–0.88).
- The median PFS was 5.6 months in the erdafitinib arm and 2.7 months in the chemotherapy arm (HR, 0.58; 95% CI, 0.44–0.78).
- Grade 3 or higher treatment-related adverse events occurred in 45.9% of patients in the erdafitinib arm and 46.4% of patients in the chemotherapy arm. The most common grade 3 or higher events were hand-foot syndrome (9.6%), stomatitis (8.1%), onycholysis (5.9%), and hyperphosphatemia (5.2%) in the erdafitinib arm and neutropenia (14.3%) in the chemotherapy arm.
- Erdafitinib caused central serous retinopathy (CSR) of any grade in 17.0% of patients. CSR was grade 3 or higher in 2.2% of patients, which is consistent with prior safety data. Monthly ophthalmological examinations are recommended for the first 4 months of treatment and then every 3 months thereafter, as CSR may necessitate dose interruptions, dose reductions, and, sometimes, permanent erdafitinib discontinuation.
Surgery for new superficial or localized tumors
For information about the treatment of new superficial or locally invasive tumors that develop in patients who received previous conservative therapy for superficial bladder neoplasia, see the Treatment Options for Stage I Bladder Cancer section.
Palliative therapy
Patients with symptomatic tumors should consider palliative radiation therapy.
Clinical trials
Recurrent or progressive disease in distant sites or after definitive local therapy has an extremely poor prognosis, and patients should consider clinical trials whenever possible.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Sternberg CN, Yagoda A, Scher HI, et al.: Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer 64 (12): 2448-58, 1989. [PUBMED Abstract]
- Harker WG, Meyers FJ, Freiha FS, et al.: Cisplatin, methotrexate, and vinblastine (CMV): an effective chemotherapy regimen for metastatic transitional cell carcinoma of the urinary tract. A Northern California Oncology Group study. J Clin Oncol 3 (11): 1463-70, 1985. [PUBMED Abstract]
- Kachnic LA, Kaufman DS, Heney NM, et al.: Bladder preservation by combined modality therapy for invasive bladder cancer. J Clin Oncol 15 (3): 1022-9, 1997. [PUBMED Abstract]
- Tester W, Porter A, Asbell S, et al.: Combined modality program with possible organ preservation for invasive bladder carcinoma: results of RTOG protocol 85-12. Int J Radiat Oncol Biol Phys 25 (5): 783-90, 1993. [PUBMED Abstract]
- Logothetis CJ, Johnson DE, Chong C, et al.: Adjuvant chemotherapy of bladder cancer: a preliminary report. J Urol 139 (6): 1207-11, 1988. [PUBMED Abstract]
- Skinner DG, Daniels JR, Russell CA, et al.: The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol 145 (3): 459-64; discussion 464-7, 1991. [PUBMED Abstract]
- Scher HI: Chemotherapy for invasive bladder cancer: neoadjuvant versus adjuvant. Semin Oncol 17 (5): 555-65, 1990. [PUBMED Abstract]
- von der Maase H, Sengelov L, Roberts JT, et al.: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23 (21): 4602-8, 2005. [PUBMED Abstract]
- Vaishampayan UN, Faulkner JR, Small EJ, et al.: Phase II trial of carboplatin and paclitaxel in cisplatin-pretreated advanced transitional cell carcinoma: a Southwest Oncology Group study. Cancer 104 (8): 1627-32, 2005. [PUBMED Abstract]
- Carles J, Nogué M: Gemcitabine/carboplatin in advanced urothelial cancer. Semin Oncol 28 (3 Suppl 10): 19-24, 2001. [PUBMED Abstract]
- Linardou H, Aravantinos G, Efstathiou E, et al.: Gemcitabine and carboplatin combination as first-line treatment in elderly patients and those unfit for cisplatin-based chemotherapy with advanced bladder carcinoma: Phase II study of the Hellenic Co-operative Oncology Group. Urology 64 (3): 479-84, 2004. [PUBMED Abstract]
- De Santis M, Bellmunt J, Mead G, et al.: Randomized phase II/III trial assessing gemcitabine/ carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer "unfit" for cisplatin-based chemotherapy: phase II--results of EORTC study 30986. J Clin Oncol 27 (33): 5634-9, 2009. [PUBMED Abstract]
- Sternberg CN, Calabrò F, Pizzocaro G, et al.: Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer 92 (12): 2993-8, 2001. [PUBMED Abstract]
- Kaufman DS, Carducci MA, Kuzel TM, et al.: A multi-institutional phase II trial of gemcitabine plus paclitaxel in patients with locally advanced or metastatic urothelial cancer. Urol Oncol 22 (5): 393-7, 2004 Sep-Oct. [PUBMED Abstract]
- Calabrò F, Lorusso V, Rosati G, et al.: Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer 115 (12): 2652-9, 2009. [PUBMED Abstract]
- Stadler WM, Kuzel T, Roth B, et al.: Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol 15 (11): 3394-8, 1997. [PUBMED Abstract]
- Lorusso V, Pollera CF, Antimi M, et al.: A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. Eur J Cancer 34 (8): 1208-12, 1998. [PUBMED Abstract]
- Roth BJ, Dreicer R, Einhorn LH, et al.: Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: a phase II trial of the Eastern Cooperative Oncology Group. J Clin Oncol 12 (11): 2264-70, 1994. [PUBMED Abstract]
- Dreicer R, Gustin DM, See WA, et al.: Paclitaxel in advanced urothelial carcinoma: its role in patients with renal insufficiency and as salvage therapy. J Urol 156 (5): 1606-8, 1996. [PUBMED Abstract]
- Vaughn DJ, Broome CM, Hussain M, et al.: Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 20 (4): 937-40, 2002. [PUBMED Abstract]
- Bellmunt J, Albanell J, Gallego OS, et al.: Carboplatin, methotrexate, and vinblastine in patients with bladder cancer who were ineligible for cisplatin-based chemotherapy. Cancer 70 (7): 1974-9, 1992. [PUBMED Abstract]
- Bellmunt J, Ribas A, Albanell J, et al.: M-CAVI, a neoadjuvant carboplatin-based regimen for the treatment of T2-4N0M0 carcinoma of the bladder. Am J Clin Oncol 19 (4): 344-8, 1996. [PUBMED Abstract]
- Skarlos DV, Aravantinos G, Linardou E, et al.: Chemotherapy with methotrexate, vinblastine, epirubicin and carboplatin (Carbo-MVE) in transitional cell urothelial cancer. A Hellenic Co-Operative Oncology Group study. Eur Urol 31 (4): 420-7, 1997. [PUBMED Abstract]
- Hainsworth JD, Meluch AA, Litchy S, et al.: Paclitaxel, carboplatin, and gemcitabine in the treatment of patients with advanced transitional cell carcinoma of the urothelium. Cancer 103 (11): 2298-303, 2005. [PUBMED Abstract]
- Logothetis CJ, Dexeus FH, Finn L, et al.: A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol 8 (6): 1050-5, 1990. [PUBMED Abstract]
- Loehrer PJ, Einhorn LH, Elson PJ, et al.: A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 10 (7): 1066-73, 1992. [PUBMED Abstract]
- Mead GM, Russell M, Clark P, et al.: A randomized trial comparing methotrexate and vinblastine (MV) with cisplatin, methotrexate and vinblastine (CMV) in advanced transitional cell carcinoma: results and a report on prognostic factors in a Medical Research Council study. MRC Advanced Bladder Cancer Working Party. Br J Cancer 78 (8): 1067-75, 1998. [PUBMED Abstract]
- Sternberg CN, de Mulder P, Schornagel JH, et al.: Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 42 (1): 50-4, 2006. [PUBMED Abstract]
- von der Maase H, Hansen SW, Roberts JT, et al.: Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18 (17): 3068-77, 2000. [PUBMED Abstract]
- Roth BJ: Preliminary experience with paclitaxel in advanced bladder cancer. Semin Oncol 22 (3 Suppl 6): 1-5, 1995. [PUBMED Abstract]
- Witte RS, Elson P, Bono B, et al.: Eastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J Clin Oncol 15 (2): 589-93, 1997. [PUBMED Abstract]
- Einhorn LH, Roth BJ, Ansari R, et al.: Phase II trial of vinblastine, ifosfamide, and gallium combination chemotherapy in metastatic urothelial carcinoma. J Clin Oncol 12 (11): 2271-6, 1994. [PUBMED Abstract]
- Pollera CF, Ceribelli A, Crecco M, et al.: Weekly gemcitabine in advanced bladder cancer: a preliminary report from a phase I study. Ann Oncol 5 (2): 182-4, 1994. [PUBMED Abstract]
- Seidman AD, Scher HI, Heinemann MH, et al.: Continuous infusion gallium nitrate for patients with advanced refractory urothelial tract tumors. Cancer 68 (12): 2561-5, 1991. [PUBMED Abstract]
- Roth BJ: Ifosfamide in the treatment of bladder cancer. Semin Oncol 23 (3 Suppl 6): 50-5, 1996. [PUBMED Abstract]
- Bajorin DF: Paclitaxel in the treatment of advanced urothelial cancer. Oncology (Huntingt) 14 (1): 43-52, 57; discussion 58, 61-2, 2000. [PUBMED Abstract]
- Sweeney CJ, Roth BJ, Kabbinavar FF, et al.: Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol 24 (21): 3451-7, 2006. [PUBMED Abstract]
- Plimack ER, Bellmunt J, Gupta S, et al.: Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 18 (2): 212-220, 2017. [PUBMED Abstract]
- Sharma P, Retz M, Siefker-Radtke A, et al.: Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 18 (3): 312-322, 2017. [PUBMED Abstract]
- Sharma P, Callahan MK, Bono P, et al.: Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 17 (11): 1590-1598, 2016. [PUBMED Abstract]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al.: Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387 (10031): 1909-20, 2016. [PUBMED Abstract]
- Balar AV, Galsky MD, Rosenberg JE, et al.: Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389 (10064): 67-76, 2017. [PUBMED Abstract]
- Bellmunt J, de Wit R, Vaughn DJ, et al.: Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 376 (11): 1015-1026, 2017. [PUBMED Abstract]
- Balar AV, Castellano D, O'Donnell PH, et al.: First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 18 (11): 1483-1492, 2017. [PUBMED Abstract]
- Grande E, Arranz JÁ, De Santis M, et al.: Atezolizumab plus chemotherapy versus placebo plus chemotherapy in untreated locally advanced or metastatic urothelial carcinoma (IMvigor130): final overall survival analysis results from a randomised, controlled, phase 3 study. Lancet Oncol 25 (1): 29-45, 2024. [PUBMED Abstract]
- Bamias A, Davis ID, Galsky MD, et al.: Atezolizumab monotherapy versus chemotherapy in untreated locally advanced or metastatic urothelial carcinoma (IMvigor130): final overall survival analysis from a randomised, controlled, phase 3 study. Lancet Oncol 25 (1): 46-61, 2024. [PUBMED Abstract]
- Powles T, Durán I, van der Heijden MS, et al.: Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391 (10122): 748-757, 2018. [PUBMED Abstract]
- Powles T, Park SH, Voog E, et al.: Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 383 (13): 1218-1230, 2020. [PUBMED Abstract]
- Apolo AB, Ellerton JA, Infante JR, et al.: Updated efficacy and safety of avelumab in metastatic urothelial carcinoma (mUC): pooled analysis from 2 cohorts of the phase 1b Javelin solid tumor study. [Abstract] J Clin Oncol 35 (Suppl 15): A-4529, 2017.
- Patel MR, Ellerton J, Infante JR, et al.: Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 19 (1): 51-64, 2018. [PUBMED Abstract]
- Powles T, van der Heijden MS, Castellano D, et al.: Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 21 (12): 1574-1588, 2020. [PUBMED Abstract]
- Powles T, O'Donnell PH, Massard C, et al.: Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol 3 (9): e172411, 2017. [PUBMED Abstract]
- Petrylak DP, de Wit R, Chi KN, et al.: Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): overall survival and updated results of a randomised, double-blind, phase 3 trial. Lancet Oncol 21 (1): 105-120, 2020. [PUBMED Abstract]
- Rosenberg JE, O'Donnell PH, Balar AV, et al.: Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol 37 (29): 2592-2600, 2019. [PUBMED Abstract]
- Loriot Y, Necchi A, Park SH, et al.: Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 381 (4): 338-348, 2019. [PUBMED Abstract]
- Loriot Y, Matsubara N, Park SH, et al.: Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 389 (21): 1961-1971, 2023. [PUBMED Abstract]
Latest Updates to This Summary (05/02/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Treatment Option Overview for Bladder Cancer
Revised Table 7, Treatment Options for Bladder Cancer, to include neoadjuvant chemoimmunotherapy followed by radical cystectomy followed by adjuvant immunotherapy as a treatment option for stages II and III bladder cancer.
Treatment of Stages II and III Bladder Cancer
Added Neoadjuvant chemoimmunotherapy followed by radical cystectomy followed by adjuvant immunotherapy as a new subsection.
Revised text about the results of a randomized controlled trial that compared nivolumab with placebo in 709 patients with pathological T3, T4, or node-positive muscle-invasive urothelial carcinoma who had undergone radical cystectomy (cited Galsky et al. as reference 24 and level of evidence A1).
Added text about a randomized controlled trial that compared adjuvant pembrolizumab with observation in 702 patients. Patients had undergone radical cystectomy and had positive margins, node-positive disease, and either pathological T2 or higher stage disease (if neoadjuvant cisplatin-based chemotherapy was received) or T3 or higher disease (if no neoadjuvant chemotherapy had been received) (cited Apolo et al. as reference 25 and level of evidence B1).
Treatment of Stage IV Bladder Cancer
Revised text in the Treatment options for patients with T4b, N0, M0 disease and Treatment options for patients with any T, any N, M1 disease subsections about the results of a phase III trial of 886 patients who were randomly assigned to receive either enfortumab vedotin plus pembrolizumab or gemcitabine plus either cisplatin or carboplatin (cited 2024 Powles et al. as reference 4 and level of evidence A1).
Revised text to state that atezolizumab was voluntarily withdrawn in 2022 as a first-line treatment option for locally advanced or metastatic urothelial cancer. The withdrawal was based on the results of the randomized IMvigor130 trial which showed no significant difference in two overall survival (OS) analyses (cited Grande et al. as reference 60 and Bamias et al. as reference 61, both level of evidence A1).
Revised text to state that durvalumab is an anti–programmed death-ligand 1 (PD-L1) monoclonal antibody with activity when given perioperatively with neoadjuvant chemotherapy in patients with resectable disease. However, durvalumab was voluntarily withdrawn in 2021 as a first-line therapy for locally advanced or metastatic urothelial cancer. The withdrawal was based on the randomized DANUBE trial which showed no significant improvement in OS (cited 2020 Powles et al. as reference 65 and level of evidence A1).
Treatment of Recurrent Bladder Cancer
Revised text to state that atezolizumab was voluntarily withdrawn in 2022 as a first-line treatment option for locally advanced or metastatic urothelial cancer. The withdrawal was based on the results of the randomized IMvigor130 trial which showed no significant difference in OS (cited Grande et al. as reference 45 and Bamias et al. as reference 46, both level of evidence A1).
Revised text to state that durvalumab is an anti–PD-L1 monoclonal antibody with activity when given perioperatively with neoadjuvant chemotherapy in patients with resectable disease. However, durvalumab was voluntarily withdrawn in 2021 as a first-line therapy for locally advanced or metastatic urothelial cancer. The withdrawal was based on the randomized DANUBE trial which showed no significant improvement in OS (cited Powles et al. as reference 51 and level of evidence A1).
Added text about a randomized controlled trial of 266 patients with locally advanced or metastatic urothelial carcinoma who had received anti–programmed death-1 or anti–PD-L1 therapy. Patients had FGFR2 or FGFR3 pathogenic variants or gene fusions. The study compared erdafitinib with chemotherapy (cited Loriot et al. as reference 56 and level of evidence A1).
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of bladder cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Bladder Cancer Treatment are:
- Juskaran S. Chadha, DO (Moffitt Cancer Center)
- Jad Chahoud, MD, MPH (Moffitt Cancer Center)
- Timothy Gilligan, MD (Cleveland Clinic Taussig Cancer Institute)
- Joseph J. Park, MD (Duke University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Bladder Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/bladder/hp/bladder-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389399]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.