Thyroid Cancer Treatment (PDQ®)–Health Professional Version
General Information About Thyroid Cancer
Thyroid cancer includes the following four main types:
- Papillary.
- Follicular.
- Medullary.
- Anaplastic.
For clinical management of the patient, thyroid cancer is generally divided into the following two categories:[1]
- Differentiated thyroid cancer, which includes well-differentiated tumors, poorly differentiated tumors, and undifferentiated tumors (papillary, follicular, or anaplastic).
- Medullary thyroid cancer.
Well-differentiated tumors (papillary and follicular thyroid cancer) are highly treatable and usually curable. Poorly differentiated and undifferentiated thyroid tumors (anaplastic thyroid cancer) are less common, aggressive, metastasize early, and have a poorer prognosis. Medullary thyroid cancer is a neuroendocrine cancer that has an intermediate prognosis.
The thyroid gland may occasionally be the site of other primary tumors, including sarcomas, lymphomas, epidermoid carcinomas, and teratomas. The thyroid may also be the site of metastasis from other cancers, particularly of the lung, breast, and kidney.
Incidence and Mortality
Estimated new cases and deaths from thyroid cancer in the United States in 2025:[2]
- New cases: 44,020.
- Deaths: 2,290.
Thyroid cancer affects women more often than men and usually occurs in people aged 25 to 65 years. The incidence of this malignancy has been increasing over the last decade. Thyroid cancer commonly presents as a so-called cold nodule. It is detected as a palpable thyroid gland during a physical examination and evaluated with iodine I 131 scans. Scintigraphy shows that the isotope is not taken up in an area of the gland. The overall incidence of cancer in a cold nodule is 12% to 15%, but it is higher in people younger than 40 years and in people with calcifications present on preoperative ultrasonography.[3,4]
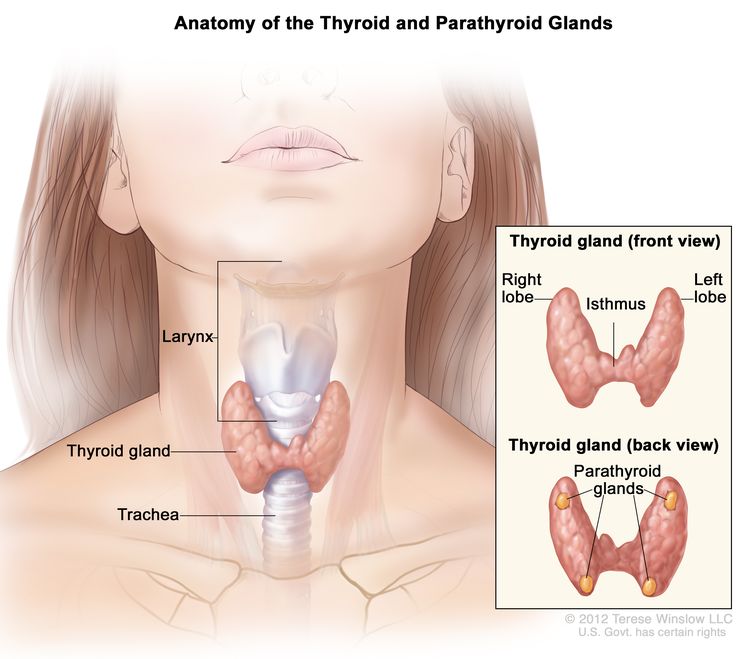
Anatomy
Thyroid gland tissue envelops the upper trachea just below the thyroid and cricoid cartilages that make up the larynx. The gland has an isthmus and often asymmetric right and left lobes; usually four parathyroid glands lie posteriorly. When swallowing, the thyroid may be felt to rise with the larynx—most commonly in the presence of a disease process.
Risk Factors
Patients with a history of radiation therapy administered in infancy or childhood for benign conditions of the head and neck (such as enlarged thymus, tonsils, or adenoids; or acne) have an increased risk of cancer and other abnormalities of the thyroid gland. In this group of patients, malignancies of the thyroid gland appear as early as 5 years after radiation therapy and may appear 20 or more years later.[5] Radiation exposure as a consequence of nuclear fallout has also been associated with a high risk of thyroid cancer, especially in children.[6-8]
Other risk factors for thyroid cancer include:[9]
Diagnostic and Staging Evaluation
The following tests and procedures may be used in the diagnosis and staging of thyroid cancer:
- Physical examination and history.
- Laryngoscopy.
- Blood hormone studies.
- Blood chemistry studies.
- Ultrasonography.
- Computed tomography scan.
- Fine-needle aspiration biopsy of the thyroid.
- Surgical excision.
Prognostic Factors for Well-Differentiated Thyroid Cancer
Age appears to be the single most important prognostic factor.[11] The prognosis for differentiated carcinoma (papillary or follicular) without extracapsular extension or vascular invasion is better for patients younger than 40 years.[11-15]
Patients considered at low risk according to age, metastases, extent, and size risk criteria include women younger than 50 years and men younger than 40 years without evidence of distant metastases. The low-risk group also includes older patients with primary papillary tumors smaller than 5 cm without evidence of gross extrathyroidal invasion, and older patients with follicular cancer without major capsular invasion or blood vessel invasion.[13] Using these criteria, a retrospective study of 1,019 patients showed that the 20-year survival rate was 98% for patients with low-risk disease and 50% for patients with high-risk disease.[13]
A retrospective surgical series of 931 previously untreated patients with differentiated thyroid cancer found that age older than 45 years, follicular histology, primary tumor larger than 4 cm (T2–T3), extrathyroidal extension (T4), and distant metastases were adverse prognostic factors.[16,17] Favorable prognostic factors included female sex, multifocality, and regional lymph node involvement.[16] However, other studies have shown that regional lymph node involvement had no effect [18,19] or had an adverse effect on survival.[14,15,20]
Another retrospective series of 1,807 patients found that the presence of distant metastases was most predictive of survival, followed by age.[21] An age cutoff of 55 years was identified as most predictive of survival. This led to an international multi-institutional validation of age 55 years as a cutoff for risk stratification in the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system for well-differentiated thyroid cancer. This analysis of 9,484 patients was responsible for the change in age cutoff from 45 years to 55 years in the AJCC Cancer Staging Manual, 8th edition, using AJCC/UICC staging for well-differentiated thyroid cancer.[22]
The prognostic significance of lymph node status is controversial. Use of sentinel lymph node biopsy may aid in identifying patients with occult metastases who might benefit from central neck dissection.[23]
Diffuse, intense immunostaining for vascular endothelial growth factor in patients with papillary cancer has been associated with a high rate of local recurrence and distant metastases.[24] An elevated serum thyroglobulin level correlates strongly with recurrent tumor when found in patients with differentiated thyroid cancer during postoperative evaluations.[25,26] Serum thyroglobulin levels are most sensitive when patients are hypothyroid and have elevated serum thyroid-stimulating hormone levels.[27] Expression of the TP53 tumor suppressor gene has also been associated with an adverse prognosis for patients with thyroid cancer.[28]
For more information, see the Clinical Features and Prognosis section of the Medullary Thyroid Cancer section and the Clinical Features and Prognosis section of the Anaplastic Thyroid Cancer section.
References
- LiVolsi VA: Pathology of thyroid disease. In: Falk SA: Thyroid Disease: Endocrinology, Surgery, Nuclear Medicine, and Radiotherapy. Lippincott-Raven, 1997, pp 127-175.
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Tennvall J, Biörklund A, Möller T, et al.: Is the EORTC prognostic index of thyroid cancer valid in differentiated thyroid carcinoma? Retrospective multivariate analysis of differentiated thyroid carcinoma with long follow-up. Cancer 57 (7): 1405-14, 1986. [PUBMED Abstract]
- Khoo ML, Asa SL, Witterick IJ, et al.: Thyroid calcification and its association with thyroid carcinoma. Head Neck 24 (7): 651-5, 2002. [PUBMED Abstract]
- Carling T, Udelsman R: Thyroid tumors. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 1457-72.
- Pacini F, Vorontsova T, Molinaro E, et al.: Prevalence of thyroid autoantibodies in children and adolescents from Belarus exposed to the Chernobyl radioactive fallout. Lancet 352 (9130): 763-6, 1998. [PUBMED Abstract]
- Cardis E, Kesminiene A, Ivanov V, et al.: Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst 97 (10): 724-32, 2005. [PUBMED Abstract]
- Tronko MD, Howe GR, Bogdanova TI, et al.: A cohort study of thyroid cancer and other thyroid diseases after the chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst 98 (13): 897-903, 2006. [PUBMED Abstract]
- Iribarren C, Haselkorn T, Tekawa IS, et al.: Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer 93 (5): 745-50, 2001. [PUBMED Abstract]
- Salvatore G, Giannini R, Faviana P, et al.: Analysis of BRAF point mutation and RET/PTC rearrangement refines the fine-needle aspiration diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab 89 (10): 5175-80, 2004. [PUBMED Abstract]
- Mazzaferri EL: Treating differentiated thyroid carcinoma: where do we draw the line? Mayo Clin Proc 66 (1): 105-11, 1991. [PUBMED Abstract]
- Grant CS, Hay ID, Gough IR, et al.: Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery 104 (6): 954-62, 1988. [PUBMED Abstract]
- Sanders LE, Cady B: Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg 133 (4): 419-25, 1998. [PUBMED Abstract]
- Staunton MD: Thyroid cancer: a multivariate analysis on influence of treatment on long-term survival. Eur J Surg Oncol 20 (6): 613-21, 1994. [PUBMED Abstract]
- Mazzaferri EL, Jhiang SM: Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97 (5): 418-28, 1994. [PUBMED Abstract]
- Shah JP, Loree TR, Dharker D, et al.: Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg 164 (6): 658-61, 1992. [PUBMED Abstract]
- Andersen PE, Kinsella J, Loree TR, et al.: Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg 170 (5): 467-70, 1995. [PUBMED Abstract]
- Coburn MC, Wanebo HJ: Prognostic factors and management considerations in patients with cervical metastases of thyroid cancer. Am J Surg 164 (6): 671-6, 1992. [PUBMED Abstract]
- Voutilainen PE, Multanen MM, Leppäniemi AK, et al.: Prognosis after lymph node recurrence in papillary thyroid carcinoma depends on age. Thyroid 11 (10): 953-7, 2001. [PUBMED Abstract]
- Sellers M, Beenken S, Blankenship A, et al.: Prognostic significance of cervical lymph node metastases in differentiated thyroid cancer. Am J Surg 164 (6): 578-81, 1992. [PUBMED Abstract]
- Nixon IJ, Kuk D, Wreesmann V, et al.: Defining a Valid Age Cutoff in Staging of Well-Differentiated Thyroid Cancer. Ann Surg Oncol 23 (2): 410-5, 2016. [PUBMED Abstract]
- Nixon IJ, Wang LY, Migliacci JC, et al.: An International Multi-Institutional Validation of Age 55 Years as a Cutoff for Risk Stratification in the AJCC/UICC Staging System for Well-Differentiated Thyroid Cancer. Thyroid 26 (3): 373-80, 2016. [PUBMED Abstract]
- Cunningham DK, Yao KA, Turner RR, et al.: Sentinel lymph node biopsy for papillary thyroid cancer: 12 years of experience at a single institution. Ann Surg Oncol 17 (11): 2970-5, 2010. [PUBMED Abstract]
- Lennard CM, Patel A, Wilson J, et al.: Intensity of vascular endothelial growth factor expression is associated with increased risk of recurrence and decreased disease-free survival in papillary thyroid cancer. Surgery 129 (5): 552-8, 2001. [PUBMED Abstract]
- van Herle AJ, van Herle KA: Thyroglobulin in benign and malignant thyroid disease. In: Falk SA: Thyroid Disease: Endocrinology, Surgery, Nuclear Medicine, and Radiotherapy. Lippincott-Raven, 1997, pp 601-618.
- Ruiz-Garcia J, Ruiz de Almodóvar JM, Olea N, et al.: Thyroglobulin level as a predictive factor of tumoral recurrence in differentiated thyroid cancer. J Nucl Med 32 (3): 395-8, 1991. [PUBMED Abstract]
- Duren M, Siperstein AE, Shen W, et al.: Value of stimulated serum thyroglobulin levels for detecting persistent or recurrent differentiated thyroid cancer in high- and low-risk patients. Surgery 126 (1): 13-9, 1999. [PUBMED Abstract]
- Godballe C, Asschenfeldt P, Jørgensen KE, et al.: Prognostic factors in papillary and follicular thyroid carcinomas: p53 expression is a significant indicator of prognosis. Laryngoscope 108 (2): 243-9, 1998. [PUBMED Abstract]
Cellular Classification of Thyroid Cancer
In thyroid cancer, cell type is an important determinant of prognosis and treatment. The thyroid has two cell types: follicular cells and parafollicular C cells. The management of thyroid cancer depends on the cell of origin and how well the integrity of the cell type is maintained. The four main types of thyroid cancer are divided into the following two categories for clinical management:[1]
Differentiated (follicular cell) thyroid cancers.
- Well-differentiated.
- Poorly differentiated.
- Undifferentiated.
- Anaplastic thyroid carcinoma.
Parafollicular C cell thyroid cancers.
- Medullary thyroid carcinoma.
Other types (not derived from thyroid cells).
- Lymphoma.
- Sarcoma.
- Carcinosarcoma.
References
- LiVolsi VA: Pathology of thyroid disease. In: Falk SA: Thyroid Disease: Endocrinology, Surgery, Nuclear Medicine, and Radiotherapy. Lippincott-Raven, 1997, pp 127-175.
- Kushchayeva Y, Duh QY, Kebebew E, et al.: Comparison of clinical characteristics at diagnosis and during follow-up in 118 patients with Hurthle cell or follicular thyroid cancer. Am J Surg 195 (4): 457-62, 2008. [PUBMED Abstract]
- Mills SC, Haq M, Smellie WJ, et al.: Hürthle cell carcinoma of the thyroid: Retrospective review of 62 patients treated at the Royal Marsden Hospital between 1946 and 2003. Eur J Surg Oncol 35 (3): 230-4, 2009. [PUBMED Abstract]
Stage Information for Thyroid Cancer
Definitions of TNM
The American Joint Committee on Cancer has designated staging by the TNM (tumor, node, metastasis) classification to define thyroid cancer.[1,2] For definitions of TNM stages for each type of thyroid cancer, see the following sections:
References
- Tuttle RM, Morris LF, Haugen BR, et al.: Thyroid--Differentiated and Anaplastic Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 873-90.
- Rosen Je, Lloyd RV, Brierley JD, et al.: Thyroid--Medullary. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 891-901.
Treatment Option Overview for Thyroid Cancer
Treatment options for thyroid cancer are described in Table 1.
| Type of Thyroid Cancer | Disease Status | Treatment Options | |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastases; EBRT = external-beam radiation therapy; RAI = radioactive iodine. | |||
| Papillary and follicular | Localized/regional | Surgery | |
| –Total thyroidectomy | |||
| –Lobectomy | |||
| RAI therapy | |||
| Thyroid-suppression therapy | |||
| EBRT | |||
| Papillary and follicular | Metastatic | Iodine-sensitive: | |
| RAI therapy | |||
| Thyroid-suppression therapy | |||
| Iodine-resistant: | |||
| Thyroid-suppression therapy | |||
| Targeted therapy | |||
| Surgery | |||
| EBRT | |||
| Chemotherapy (under clinical evaluation) | |||
| Clinical trials | |||
| Recurrent papillary and follicular thyroid cancer | Surgery with or without postoperative RAI therapy | ||
| Targeted therapy | |||
| EBRT | |||
| Chemotherapy | |||
| Clinical trials | |||
| Medullary thyroid cancer | Localized disease | Total thyroidectomy | |
| EBRT | |||
| Locally advanced and metastatic disease | Targeted therapy | ||
| Palliative chemotherapy | |||
| Anaplastic thyroid cancer | Surgery | ||
| EBRT | |||
| Systemic therapy | |||
Papillary and Follicular Thyroid Cancer
Clinical Features and Prognosis
The clinical features and prognosis of well-differentiated thyroid tumors vary by stage.
Most papillary cancers have some follicular elements. These follicular elements may outnumber the papillary formations, but they do not change the prognosis.
Follicular adenomas, which are characterized by their lack of invasion through the capsule into the surrounding thyroid tissue, must be distinguished from follicular thyroid carcinoma. While follicular cancer has a good prognosis, it is less favorable than that of papillary carcinoma. The 10-year survival is better for patients with follicular carcinoma without vascular invasion than it is for patients with vascular invasion.
Papillary carcinomas metastasize more frequently to regional lymph nodes than to distant sites. Follicular carcinomas more commonly invade blood vessels and metastasize hematogenously to the lungs and to the bone rather than through the lymphatic system. When metastases occur, treatment with radioiodine is initially effective, but prognosis worsens as resistance to radioiodine ensues.
Staging and prognosis of papillary and follicular thyroid cancer are determined by the patient's age and site of the disease. Patients with papillary thyroid cancer have the following clinical features and prognoses:
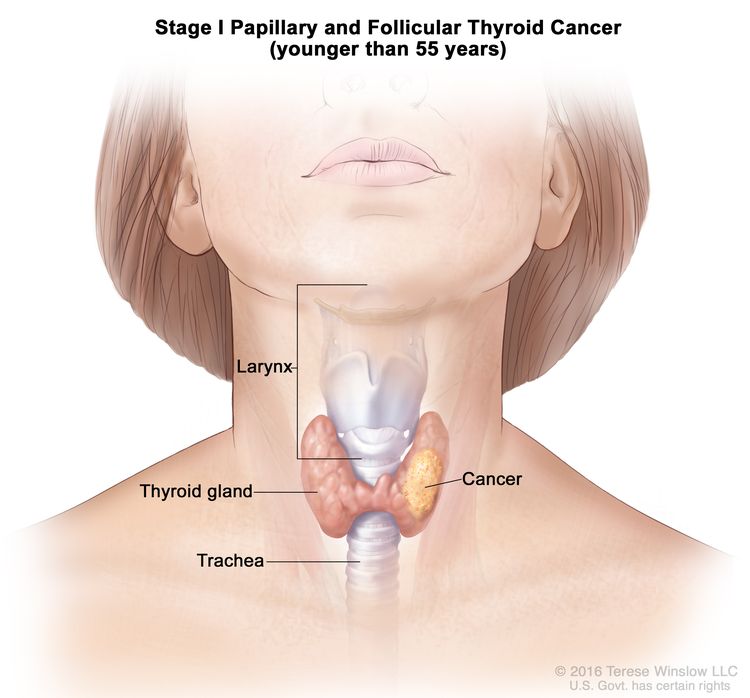
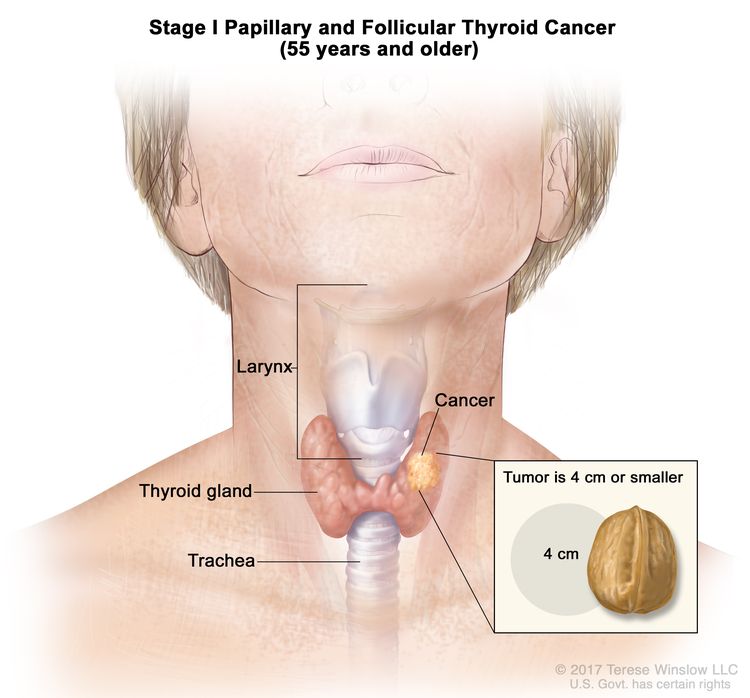
- Stage I papillary or follicular thyroid cancer is localized to the thyroid gland in patients aged 55 years or older. In those younger than 55 years, the cancer may have spread to nearby tissues and lymph nodes but not to other parts of the body. In as many as 50% of the cases, papillary thyroid cancer is multifocal.
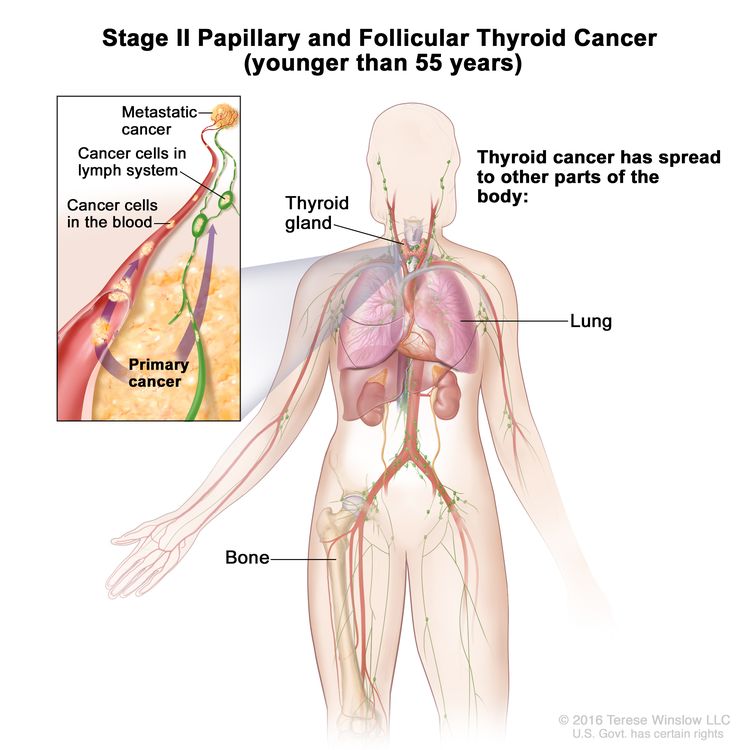
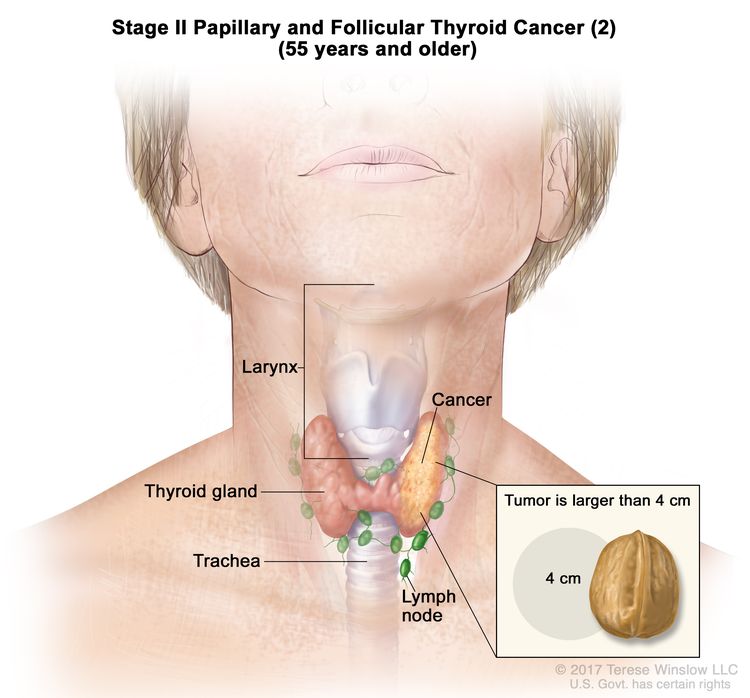
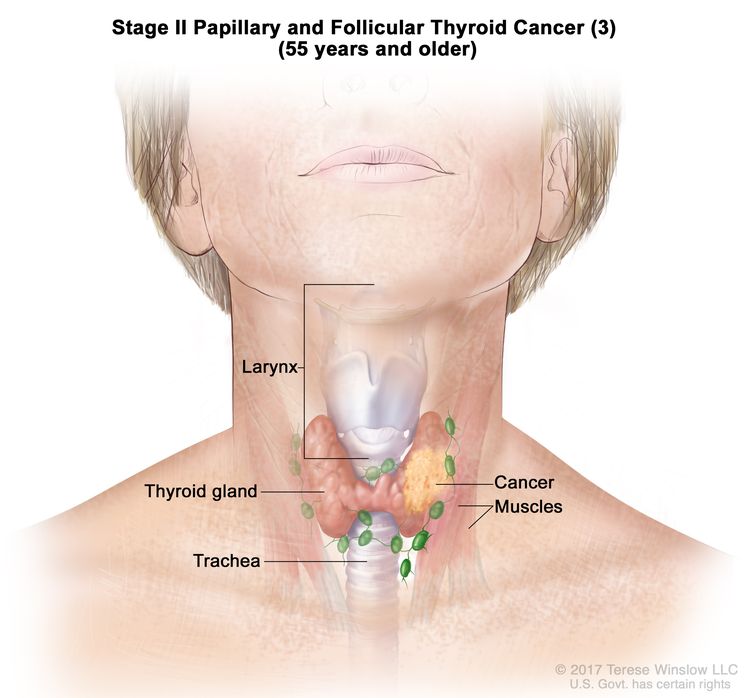
- Stage II papillary or follicular thyroid cancer is defined by either of the following descriptions: (1) patients are younger than 55 years and the tumor has spread from the thyroid to distant parts of the body, or (2) patients are aged 55 years or older: and the tumor is confined to or has limited invasion outside of the thyroid, with or without lymph node involvement, but without spread to distant parts of the body. Stage II is the most advanced stage possible in a patient younger than 55 years.
- Stage III papillary or follicular thyroid cancer is only possible in patients aged 55 years or older. The thyroid tumor demonstrates extension into surrounding soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve. There may or may not be lymph node involvement, but there is no distant spread to other parts of the body.
- Stage IVA papillary or follicular thyroid cancer is only applicable for patients aged 55 years or older. The thyroid tumor demonstrates extension into surrounding soft tissues that includes either prevertebral fascia or encases the carotid artery or mediastinal vessels. There may or may not be lymph node involvement, but there is no distant spread to other parts of the body.
- Stage IVB papillary or follicular thyroid cancer is only applicable for patients aged 55 years or older. It is defined by the presence of distant spread to other parts of the body. The lungs and bones are the most frequent sites of distant spread.
Hürthle cell carcinoma is a variant of follicular carcinoma with a similar prognosis and is treated in the same way as equivalent stage non-Hürthle cell follicular carcinoma.[1]
Stage Information for Papillary and Follicular Thyroid Cancer
| Stage | TbNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Thyroid--Differentiated and Anaplastic Carcinoma. In: Amin, MB, Edge Sb, Greene FL et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 873–90. | |||
| bAll categories may be subdivided: (s) solitary tumor and (m) multifocal tumor (the largest determines the classification). | |||
| Age at diagnosis is <55 years: | |||
| I | Any T, Any N, M0 | TX = Primary tumor cannot be assessed. |
 |
| T0 = No evidence of primary tumor. | |||
| T1 = Tumor ≤2 cm in greatest dimension, limited to the thyroid. | |||
| –T1a = Tumor ≤1 cm in greatest dimension, limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension, limited to the thyroid. | |||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension, limited to the thyroid. | |||
| T3 = Tumor >4 cm limited to the thyroid, or gross extrathyroidal extension invading only strap muscles. | |||
| –T3a = Tumor >4 cm limited to the thyroid. | |||
| –T3b = Gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles) from a tumor of any size. | |||
| T4 = Includes gross extrathyroidal extension beyond the strap muscles. | |||
| –T4a = Gross extrathyroidal extension invading subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve from a tumor of any size. | |||
| –T4b = Gross extrathyroidal extension invading prevertebral fascia or encasing the carotid artery or mediastinal vessels from a tumor of any size. | |||
| NX = Regional lymph nodes cannot be assessed. | |||
| N0 = No evidence of locoregional lymph node metastasis. | |||
| –N0a = One or more cytologically or histologically confirmed benign lymph nodes. | |||
| –N0b = No radiological or clinical evidence of locoregional lymph node metastasis. | |||
| N1 = Metastasis to regional nodes. | |||
| –N1a = Metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes. This can be unilateral or bilateral disease. | |||
| –N1b = Metastasis to unilateral, bilateral, or contralateral cervical neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes. | |||
| M0 = No distant metastasis. | |||
| Age at diagnosis is ≥55 years: | |||
| I | T1, N0/NX, M0 | T1 = Tumor ≤2 cm in greatest dimension, limited to the thyroid. |
 |
| N0 = No evidence of locoregional lymph node metastasis. | |||
| NX = Regional lymph nodes cannot be assessed. | |||
| M0 = No distant metastasis. | |||
| T2, N0/NX, M0 | T2 = Tumor >2 cm but ≤4 cm in greatest dimension, limited to the thyroid. | ||
| N0 = No evidence of locoregional lymph node metastasis. | |||
| NX = Regional lymph nodes cannot be assessed. | |||
| M0 = No distant metastasis. | |||
| Stage | TbNM | Description | Illustration |
|---|---|---|---|
| Age at diagnosis is <55 years: | |||
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Thyroid--Differentiated and Anaplastic Carcinoma. In: Amin, MB, Edge Sb, Greene FL et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 873–90. | |||
| bAll categories may be subdivided: (s) solitary tumor and (m) multifocal tumor (the largest determines the classification). | |||
| II | Any T, Any N, M1 | Any T = See descriptions in Table 2. |
 |
| Any N = See descriptions in Table 2. | |||
| Age at diagnosis is ≥55 years: | |||
| II | T1, N1, M0 | T1 = Tumor ≤2 cm in greatest dimension, limited to the thyroid. |
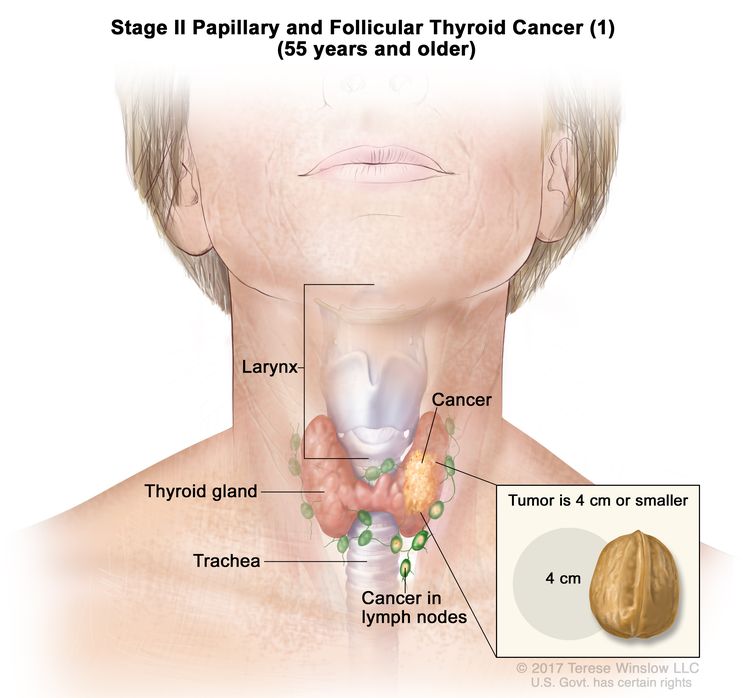   |
| –T1a = Tumor ≤1 cm in greatest dimension, limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension, limited to the thyroid. | |||
| N1 = Metastasis to regional nodes. | |||
| –N1a = Metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes. This can be unilateral or bilateral disease. | |||
| –N1b = Metastasis to unilateral, bilateral, or contralateral cervical neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T2, N1, M0 | T2 = Tumor >2 cm but ≤4 cm in greatest dimension, limited to the thyroid. | ||
| N1 = Metastasis to regional nodes. | |||
| –N1a = Metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes. This can be unilateral or bilateral disease. | |||
| –N1b = Metastasis to unilateral, bilateral, or contralateral cervical neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T3a/T3b, Any N, M0 | –T3a = Tumor >4 cm limited to the thyroid. | ||
| –T3b = Gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles) from a tumor of any size. | |||
| Any N = See descriptions in Table 2. | |||
| M0 = No distant metastasis. | |||
| Stage | TbNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Thyroid--Differentiated and Anaplastic Carcinoma. In: Amin, MB, Edge Sb, Greene FL et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 873–90. | |||
| bAll categories may be subdivided: (s) solitary tumor and (m) multifocal tumor (the largest determines the classification). | |||
| Age at diagnosis is ≥55 years: | |||
| III | T4a, Any N, M0 | –T4a = Gross extrathyroidal extension invading subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve from a tumor of any size. |
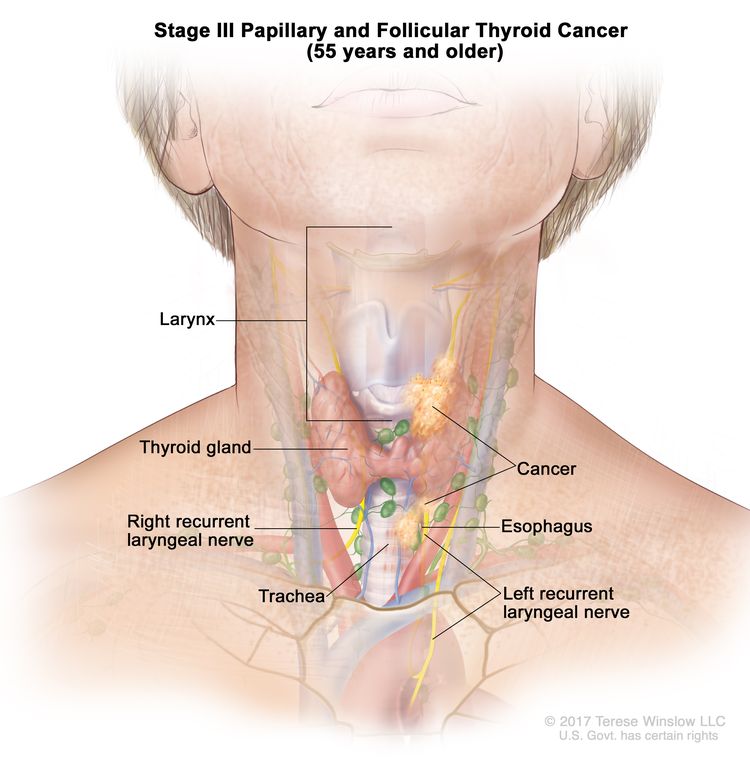 |
| Any N = See descriptions in Table 2. | |||
| M0 = No distant metastasis. | |||
| Stage | TbNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Thyroid--Differentiated and Anaplastic Carcinoma. In: Amin, MB, Edge Sb, Greene FL et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 873–90. | |||
| bAll categories may be subdivided: (s) solitary tumor and (m) multifocal tumor (the largest determines the classification). | |||
| Age at diagnosis is ≥55 years: | |||
| IVA | T4b, Any N, M0 | –T4b = Gross extrathyroidal extension invading prevertebral fascia or encasing the carotid artery or mediastinal vessels from a tumor of any size. |
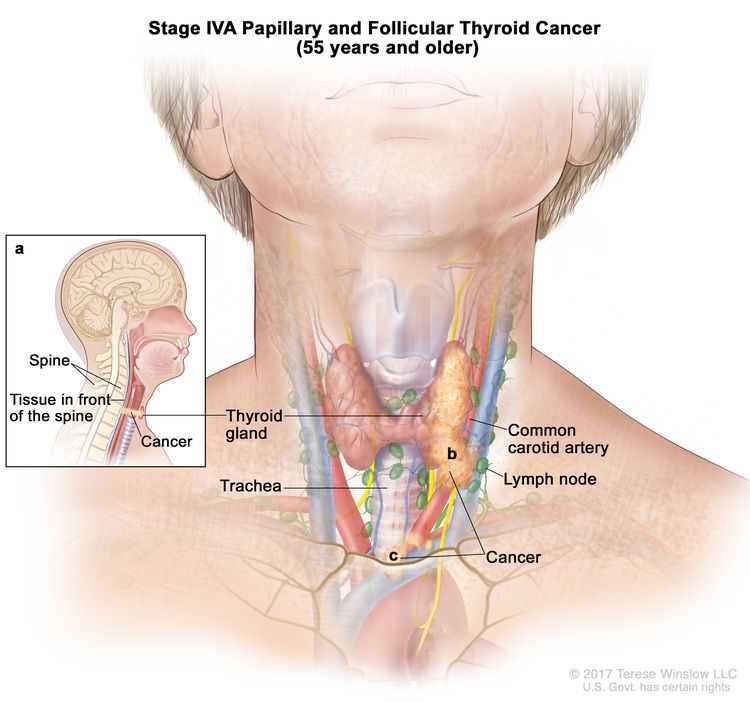 |
| Any N = See descriptions in Table 2. | |||
| M0 = No distant metastasis. | |||
| IVB | Any T, Any N, M1 | Any T = See descriptions in Table 2. |
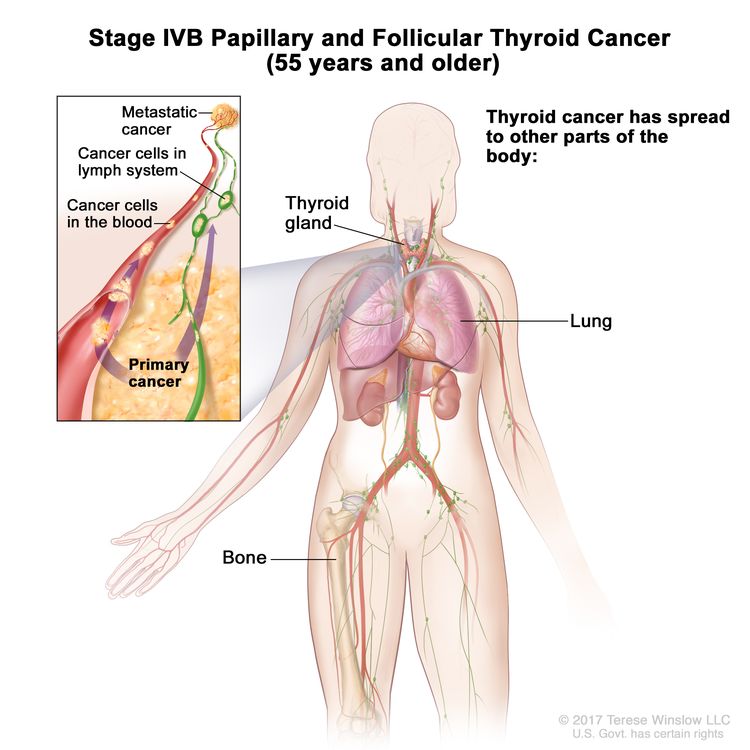 |
| Any N = See descriptions in Table 2. | |||
| M1 = Distant metastasis. | |||
| Stage | TbNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Thyroid--Differentiated and Anaplastic Carcinoma. In: Amin, MB, Edge Sb, Greene FL et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 873–90. | |||
| bAll categories may be subdivided: (s) solitary tumor and (m) multifocal tumor (the largest determines the classification). | |||
| IVA | T1–T3a, N0/NX, M0 | T1 = Tumor ≤2 cm in greatest dimension, limited to the thyroid. |
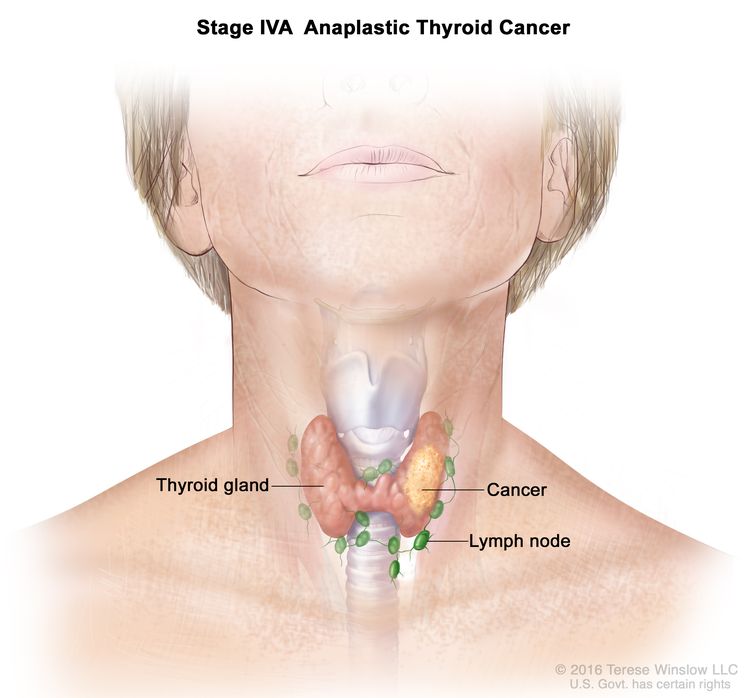 |
| –T1a = Tumor ≤1 cm in greatest dimension, limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension, limited to the thyroid. | |||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension, limited to the thyroid. | |||
| T3 = Tumor >4 cm limited to the thyroid, or gross extrathyroidal extension invading only strap muscles. | |||
| –T3a = Tumor >4 cm limited to the thyroid. | |||
| N0 = No evidence of locoregional lymph node metastasis. | |||
| –N0a = One or more cytologically or histologically confirmed benign lymph nodes. | |||
| –N0b = No radiological or clinical evidence of locoregional lymph node metastasis. | |||
| NX = Regional lymph nodes cannot be assessed. | |||
| M0 = No distant metastasis | |||
| IVB | T1–T3a, N1, M0 | T1 = Tumor ≤2 cm in greatest dimension, limited to the thyroid. |
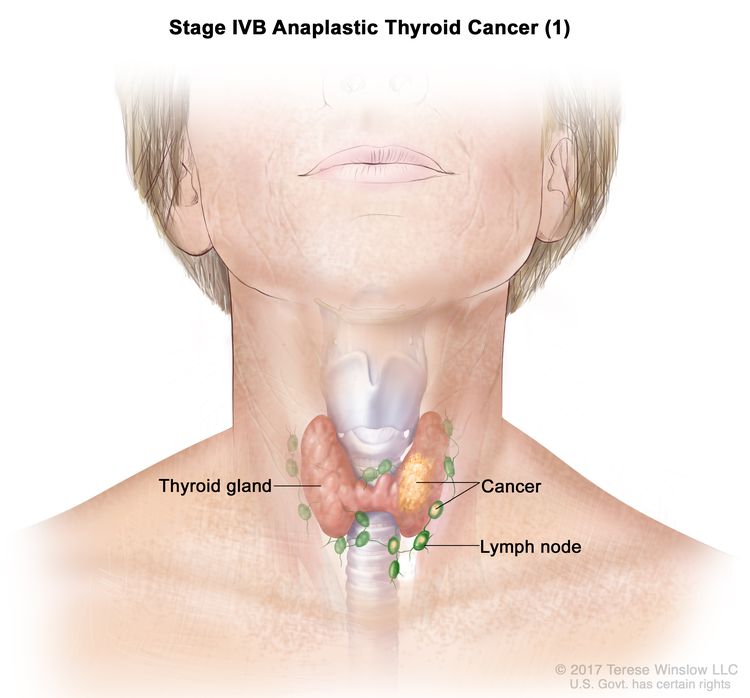 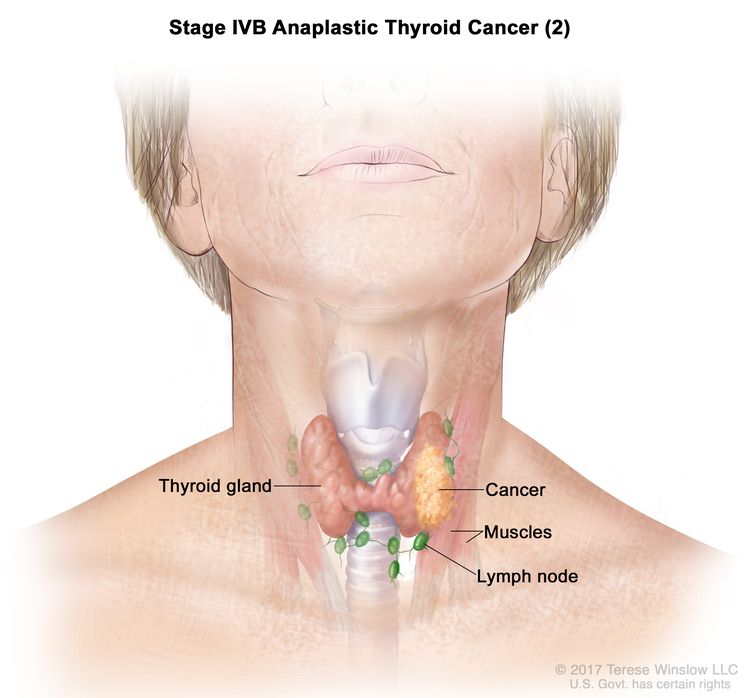 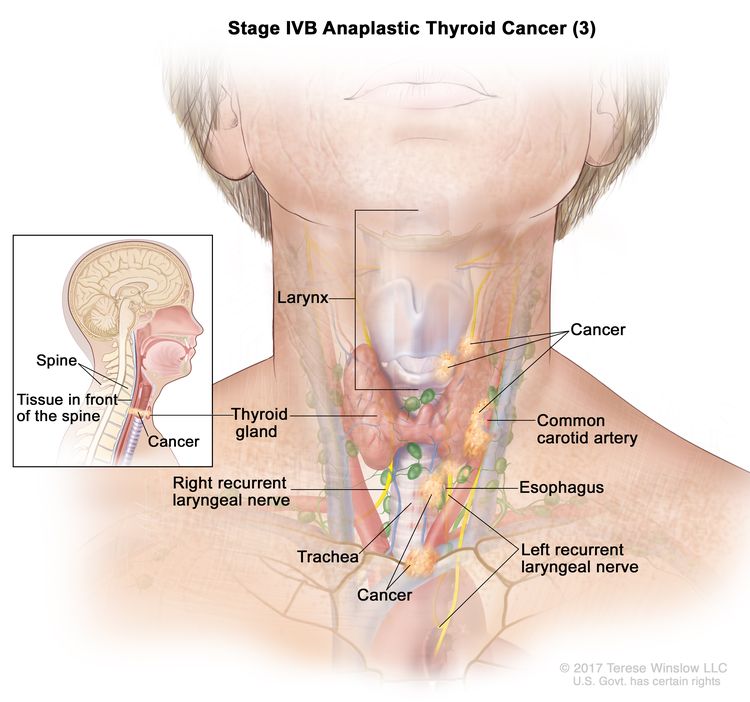 |
| –T1a = Tumor ≤1 cm in greatest dimension, limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension, limited to the thyroid. | |||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension, limited to the thyroid. | |||
| T3 = Tumor >4 cm limited to the thyroid, or gross extrathyroidal extension invading only strap muscles. | |||
| –T3a = Tumor >4 cm limited to the thyroid. | |||
| N1 = Metastasis to regional nodes. | |||
| –N1a = Metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes. This can be unilateral or bilateral disease. | |||
| –N1b = Metastasis to unilateral, bilateral, or contralateral cervical neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T3b, Any N, M0 | –T3b = Gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles) from a tumor of any size. | ||
| Any N = See descriptions in Table 2. | |||
| M0 = No distant metastasis. | |||
| T4, Any N, M0 | T4 = Includes gross extrathyroidal extension beyond the strap muscles. | ||
| –T4a = Gross extrathyroidal extension invading subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve from a tumor of any size. | |||
| –T4b = Gross extrathyroidal extension invading prevertebral fascia or encasing the carotid artery or mediastinal vessels from a tumor of any size. | |||
| Any N = See descriptions in Table 2. | |||
| M0 = No distant metastasis. | |||
| IVC | Any T, Any N, M1, | Any T = See descriptions in Table 2. |
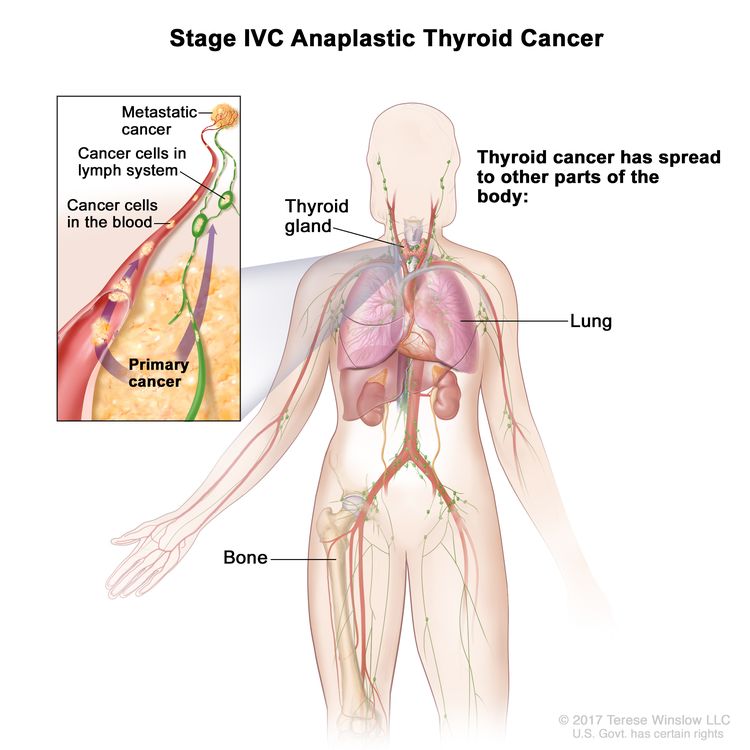 |
| Any N = See descriptions in Table 2. | |||
| M1 = Distant metastasis. | |||
Treatment Options for Papillary and Follicular Thyroid Cancer
Localized/regional papillary and follicular thyroid cancer
Surgery is the therapy of choice for all primary lesions. Surgical options include total thyroidectomy or lobectomy. The choice of procedure is influenced mainly by the age of the patient and the size of the nodule. For patients with early-stage disease, survival rates are similar when comparing the two procedures; however, the rates of surgical complications and local recurrences are different.[2-8]
Treatment options for localized/regional papillary and follicular thyroid cancer
Treatment options for localized/regional papillary and follicular thyroid cancer include:
Surgery
The objective of surgery is to completely remove the primary tumor, while minimizing treatment-related morbidity, and to guide postoperative treatment with RAI. The goal of RAI is to ablate the remnant thyroid tissue to improve the specificity of thyroglobulin assays, which allows the detection of persistent disease by follow-up whole-body scanning. For patients undergoing RAI, removal of all normal thyroid tissue is an important surgical objective. Additionally, for accurate long-term surveillance, RAI whole-body scanning and measurement of serum thyroglobulin are affected by residual, normal thyroid tissue. In these situations, near total or total thyroidectomy is required. This approach facilitates follow-up thyroid scanning.
Total thyroidectomy
Total thyroidectomy is often used because of the high incidence of multicentric involvement of both lobes of the gland and the possibility of dedifferentiation of any residual tumor to the anaplastic cell type.
Evidence (total thyroidectomy):
- An analysis of the National Cancer Data Base (NCDB) registry identified 52,173 patients who underwent surgery for papillary thyroid cancer. Of these individuals, 43,227 (82.9%) underwent total thyroidectomy, and 8,946 (17.1%) underwent lobectomy.[9][Level of evidence C1]
- For a papillary thyroid cancer tumor that measured less than 1 cm, the extent of surgery did not impact recurrence (P = .24) or survival (P = .83).
- For tumors that measured 1 cm or larger, lobectomy resulted in higher risk of recurrence (P = .04) and death (P = .009).
- To minimize the influence of larger tumors, 1-cm to 2-cm lesions were examined separately. Lobectomy resulted in a higher risk of recurrence (P = .04) and death (P = .04).
- For patients with papillary thyroid cancer tumors that measured 1 cm or larger, total thyroidectomy resulted in lower recurrence rates and improved survival compared with lobectomy.
- In a pattern-of-care study that used the NCDB registry from 1985 to 2003, 57,243 patients with papillary thyroid cancer whose tumors measured 1 cm or larger underwent total thyroidectomy or lobectomy. Trends in the extent of surgery were examined for patients with papillary thyroid cancer over two decades. Logistic regression was used to identify factors that predicted the use of total thyroidectomy compared with lobectomy.[10][Level of evidence C1]
- Use of total thyroidectomy increased from 70.8% in 1985 to 90.4% in 2003 (P < .0001).
- Patients treated at high-volume medical facilities or academic centers were more likely to undergo total thyroidectomy than were patients examined at low-volume medical facilities or community hospitals (P < .0001).
Lobectomy
Thyroid lobectomy alone may be sufficient treatment for small (<1 cm), low-risk, unifocal, intrathyroidal papillary carcinomas in the absence of previous head and neck irradiation or radiologically or clinically involved cervical nodal metastases. This procedure is associated with a lower incidence of complications, but approximately 5% to 10% of patients will have a recurrence in the thyroid after a lobectomy.[11]
Abnormal regional lymph nodes are biopsied at the time of surgery. Recognized involved nodes are removed at initial surgery, but selective node removal can be performed, and radical neck dissection is usually not required. This results in a decreased recurrence rate but has not been shown to improve survival. Follicular thyroid cancer commonly metastasizes to lungs and bone. When a remnant lobe remains, use of iodine I 131 as ablative therapy is compromised because the radioiodine will be preferentially taken up by the remnant normal tissue rather than by the tumor.
Radioactive iodine (RAI) therapy
Studies have shown that a postoperative course of therapeutic (ablative) doses of radioiodine 131I results in a decreased recurrence rate among high-risk patients with papillary and follicular carcinomas.[5] RAI may be given in addition to exogenous thyroid hormone but is not considered routine.[12] RAI treatment is optimal after total thyroidectomy with minimal thyroid remnant. With a large thyroid remnant, a low thyroglobulin level cannot be achieved, which increases the chance of requiring multiple doses of RAI.
Consideration of RAI for remnant ablation is based on the following pathological risk features:
- The size of the primary tumor.
- The presence of lymphovascular invasion.
- Capsule invasion.
- The number of involved lymph nodes.
RAI may be given with one of two methods of thyroid-stimulating hormone (TSH/thyrotropin) stimulation:
- Withdrawal of thyroid hormone.
- Administration of recombinant human thyrotropin (rhTSH).
Administered rhTSH maintains quality of life and reduces the radiation dose delivered to the body compared with thyroid hormone withdrawal.[13] Patients presenting with papillary thyroid microcarcinomas (tumors <10 mm), which are considered to be very low risk, have an excellent prognosis when treated surgically. Additional therapy with 131I would not be expected to improve the prognosis.[14]
The role of RAI in low-risk patients is not clear because disease-free survival (DFS) or overall survival (OS) benefits have not been demonstrated.
Evidence (surgery with or without RAI):
- One study reviewed 1,298 patients with low-risk disease from the French Thyroid Cancer Registry.[15] Patients were identified as having low-risk papillary or follicular cancer as defined by the following American Thyroid Association and European Thyroid Association criteria:
- Complete tumor resection.
- Multifocal tumors 1 cm or smaller confined to the thyroid.
- Tumors larger than 1 cm but no larger than 4 cm confined to the thyroid.
- No lymph node involvement or distant metastatic disease.
Of the 1,298 patients, 911 patients received RAI after surgery, and 387 patients did not receive RAI after surgery. The follow-up period was 10.3 years.
- In multivariate analyses, there were no differences in OS (P = .243) or DFS (P = .2659), according to RAI use.[15]
Long-term complications of RAI with 131I include:
- Second malignancies.
- Sialadenitis.
- Lacrimal and salivary gland dysfunction.
Options for decreasing radiation exposure in patients with low-risk thyroid cancer have been explored. These options have included reducing the amount of RAI in each dose and giving RAI in combination with rhTSH injections.
Evidence (thyroid hormone withdrawal or use of rhTSH with 131I):
- A phase III, randomized, noninferiority study included patients with low-risk thyroid cancer. The study compared two thyrotropin-stimulation methods (thyroid hormone withdrawal or use of rhTSH) and two 131I doses (1.1 GBq [30 mCi] and 3.7 GBq [100 mCi]), using a 2 × 2 factorial design.[16][Level of evidence C1]
- Thyroid ablation rates were equivalent between high- and low-dose 131I at 6 to 10 months after administration of 131I.
- Patients with more advanced T stage (T1–T3, N0–1) and with less than a total thyroidectomy were included with a lower overall ablation rate of 85%.
- Another phase III, randomized, noninferiority study of patients with low-risk thyroid cancer compared two thyrotropin-stimulation methods (thyroid hormone withdrawal or use of rhTSH) and two 131I doses (1.1 GBq [30 mCi] and 3.7 GBq [100 mCi]), using a 2 × 2 factorial design. The inclusion criteria consisted of a low-risk, homogeneous cohort. All of the patients underwent total thyroidectomy and had pathological TNM stage pT1 (≤1 cm) and N1 or NX, pT1 (>1–2 cm) and any N, or pT2, N0 without thyroid capsule extension/distant metastases.[17][Level of evidence C1]
- Thyroid ablation rates were equivalent between high- and low-dose 131I at 6 to 10 months after administration of 131I.
- Complete thyroid ablation rate was 92%.
- Patients who underwent thyroid hormone withdrawal had more symptoms of hypothyroidism associated with deterioration in quality of life than the patients who received rhTSH.
Neither study assessed the effect of low-dose RAI on long-term recurrences or survival. The studies also did not address whether RAI could be safely omitted in specific low-risk groups.
Thyroid-suppression therapy
After thyroid surgery, all patients, except those undergoing lobectomies, require thyroid hormone replacement therapy. For patients who have undergone thyroidectomy, supratherapeutic doses of thyroid hormone are routinely given to suppress TSH levels. The degree of TSH suppression recommended depends on the risk of recurrence and the comorbidities of the patient. Studies have suggested that TSH suppression improves progression-free survival (PFS), but there is no definitive evidence that it improves OS.[18,19]
External-beam radiation therapy (EBRT)
EBRT is typically reserved for palliative treatment of unresectable or metastatic papillary or follicular thyroid cancer. In some cases, it may be appropriate to give EBRT in the adjuvant setting if there is confirmed or suspected microscopic residual disease that is confirmed or suspected to be refractory to RAI. There are no randomized trials to guide the selection of patients who might benefit from treatment with EBRT. The decision to use EBRT is based on retrospective data and clinical judgment.[20]
Metastatic papillary and follicular thyroid cancer
Total thyroidectomy is still recommended as the initial treatment for metastatic papillary or follicular thyroid cancer. RAI is the second treatment and is given to ablate the remnant thyroid and treat the metastatic disease. If a thyroidectomy is not done, then RAI is not a treatment option for the patient. The most common sites of distant metastases are the lungs and bones. Treatment of distant metastases is usually not curative but may produce significant palliation. Genomic testing to identify actionable variants like RET and NTRK gene fusions should be considered for patients with advanced progressive disease. Treatment options for iodine-sensitive and iodine-resistant metastatic papillary and follicular thyroid cancer are described below.
Treatment options for iodine-sensitive thyroid cancer
Treatment options for iodine-sensitive thyroid cancer include:
- RAI therapy. Metastases that demonstrate uptake of this isotope may be ablated by therapeutic doses of 131I.
- Thyroid-suppression therapy.
Treatment options for iodine-resistant thyroid cancer
Treatment options for iodine-resistant thyroid cancer include:
- Thyroid-suppression therapy.
- Targeted therapy.
- Surgery.
- EBRT.
- Chemotherapy (under clinical evaluation).
- Clinical trials.
Thyroid-suppression therapy
TSH suppression with thyroxine is effective in many lesions that are not sensitive to 131I.
Targeted therapy
Tyrosine kinase inhibitors
Sorafenib
Sorafenib is an orally active multitargeted tyrosine kinase inhibitor.
Evidence (sorafenib):
- A phase III, randomized, double-blind, placebo-controlled study (DECISION [NCT00984282]) evaluated the activity of sorafenib in patients with progressive, iodine-refractory, differentiated thyroid cancer.[21] The trial included 417 patients with locally advanced or metastatic RAI−refractory thyroid cancer (papillary, follicular [including Hürthle cell], and poorly differentiated, not anaplastic tumors, defined as beyond well-differentiated tumors or those well-differentiated tumors that become resistant to radioiodine treatment) whose disease had progressed within the past 14 months. Patients were randomly assigned to receive either sorafenib (400 mg twice a day) or placebo. Patients who received previous chemotherapy, thalidomide, or targeted therapy were excluded.[21][Level of evidence B1]
- The median PFS was 10.8 months in the sorafenib group and 5.8 months in the placebo group (hazard ratio [HR] 0.59; 95% confidence interval [CI], 0.45–0.76; P < .0001).
- OS was not significantly improved (HR, 0.80; 95% CI, 0.54–1.19; one-sided P = .14), but the median OS had not been reached at the time of primary analysis data cutoff, and crossover was allowed.
- The objective response rate (all partial responses) was 12.2% in the sorafenib group and 0.5% in the placebo group.
- Median time-to-progression was 11.1 months in the sorafenib group and 5.7 months in the placebo group (HR, 0.56; 95% CI, 0.43–0.72; P < .001).
- Adverse events occurred in 98.6% of patients treated with sorafenib and 87.6% of patients treated with placebo. The most common adverse events in the sorafenib group were hand-foot skin reactions (76.3%), diarrhea (68.6%), alopecia (67.1%), and rash or desquamation (50.2%). Most events were grade 1 or 2. Seven squamous cell carcinomas of the skin occurred in the sorafenib group.
Lenvatinib
Lenvatinib is an orally active, multitargeted tyrosine kinase inhibitor.
Evidence (lenvatinib):
- A phase III, randomized, double-blind, placebo-controlled study (SELECT [NCT01321554]) evaluated the activity of lenvatinib in patients with progressive, iodine-refractory, differentiated thyroid cancer.[22] In this trial, 261 patients were randomly assigned to receive lenvatinib (24 mg every day), and 131 patients were randomly assigned to receive placebo. Eligible patients had differentiated thyroid cancer (including papillary, follicular, poorly differentiated, and Hürthle cell carcinomas), RAI-refractory disease, and evidence of radiological progression within the previous 13 months. At disease progression, patients in the placebo group could receive open-label lenvatinib. The primary end point was PFS, while the secondary end points were OS, response rate, and safety.[22][Level of evidence B1]
- The median PFS was 18.3 months in the lenvatinib group and 3.6 months in the placebo group (HRprogression or death, 0.21; 99% CI, 0.14–0.31; P < .001). A PFS difference was observed in patients with all histological types of thyroid cancer enrolled in this trial.
- There was no significant difference in OS between the two groups (HR death, 0.73; 95% CI, 0.50–1.07; P = .10), even with the crossover design of the study.
- The objective response rate was 64.8% in the lenvatinib group versus 1.5% in the placebo group (odds ratio [OR], 28.87; 95% CI, 12.46–66.86; P < .001).
- Treatment-related adverse events (all grades) occurred in 97.3% of patients in the lenvatinib group and 59.5% of patients in the placebo group.
- Grade 3 or higher adverse events were observed in 75.9% of patients who received lenvatinib and 9.9% of patients who received placebo.
- The most common adverse events in the lenvatinib group were hypertension (67.8%), diarrhea (59.4%), fatigue (59%), decreased appetite (50.2%), decreased weight (46.4%), and nausea (41%).
- Adverse events led to discontinuation of the study drug in 14.2% of patients who received lenvatinib and 2.3% of patients who received placebo.
- In the lenvatinib group, 6 of 20 deaths occurring during the treatment period were considered drug related.
Surgery
Resection of limited metastases, especially symptomatic metastases, should be considered when the tumor has no uptake of 131I.
EBRT
EBRT is considered for patients with localized lesions that are unresponsive to 131I.[23]
Chemotherapy (under clinical evaluation)
Chemotherapy has produced occasional complete responses of long duration.[24-26]
Clinical trials
Patients with radioiodine-refractory papillary or follicular thyroid cancer can consider participating in clinical trials that evaluate new treatment approaches. Oral inhibitors targeting specific single nucleotide variants are under clinical evaluation, as are new immunotherapy approaches.[27-29][Level of evidence B4]
Recurrent papillary and follicular thyroid cancer
Approximately 10% to 30% of patients thought to be disease free after initial treatment develop recurrence and/or metastases. Of these patients, approximately 80% develop recurrence with disease in the neck alone, and 20% develop recurrence with distant metastases. The lungs are the most common site of distant metastasis. In a single series of 289 patients who developed recurrences after initial surgery, 16% died of cancer at a median time of 5 years after recurrence.[5]
The prognosis for patients with clinically detectable recurrences is generally poor, regardless of cell type.[30] Patients with local or regional tumor recurrence detected only by 131I scan have a better prognosis.[31]
The selection of further treatment depends on many factors, including:
- Cell type.
- Uptake of 131I.
- Previous treatment.
- Site of recurrence.
- Individual patient considerations.
- Presence of an activating RET or NTRK gene fusion.
Patients treated for differentiated thyroid cancer are monitored carefully with physical examinations, serum quantitative thyroglobulin levels, and radiological studies based on individual risk for recurrent disease.[32]
Treatment options for recurrent papillary and follicular thyroid cancer
Treatment options for recurrent papillary and follicular thyroid cancer include:
Surgery with or without postoperative RAI therapy
Surgery with or without 131I ablation can be useful in controlling local recurrences, regional node metastases, or occasionally, metastases at other localized sites.[33] Approximately 50% of the patients who undergo surgery for recurrent tumors can be rendered free of disease with a second operation.[30] Local and regional recurrences detected by 131I scan and not clinically apparent can be treated with 131I ablation and have an excellent prognosis.[34]
Up to 25% of recurrences and metastases from well-differentiated thyroid cancer may not show 131I uptake. For these patients, other standard imaging techniques such as ultrasonography, computed tomography, magnetic resonance imaging, and positron emission tomography scans may detect recurrent or metastatic disease.
Patients unresponsive to 131I should consider enrolling in clinical trials testing new approaches to treating this disease.
Targeted therapy
Tyrosine kinase inhibitors
Sorafenib
Sorafenib is an orally active, multitargeted tyrosine kinase inhibitor. The U.S. Food and Drug Administration (FDA) approved sorafenib as a treatment option when recurrent disease does not concentrate 131I or disease recurs after 131I ablation.
Evidence (sorafenib):
- A phase III, randomized, double-blind, placebo-controlled study (DECISION [NCT00984282]) evaluated sorafenib in patients with progressive, iodine-refractory, differentiated thyroid cancer.[21] This trial included 417 patients with locally advanced or metastatic RAI-refractory thyroid cancer (papillary, follicular [including Hürthle cell], and poorly differentiated tumors) whose disease had progressed within the past 14 months. Patients were randomly assigned to receive either sorafenib (400 mg twice a day) or placebo. Patients who received previous chemotherapy, thalidomide, or targeted therapy were excluded.[21][Level of evidence B1]
- The median PFS was 10.8 months in the sorafenib group and 5.8 months in the placebo group (HR, 0.59; 95% CI, 0.45–0.76; P < .001).
- OS was not significantly improved (HR, 0.80; 95% CI, 0.54–1.19; one-sided P = .14), but the median OS had not been reached at the time of primary analysis data cutoff, and crossover was allowed.
- Objective response rates (all partial responses) were 12.2% in the sorafenib group and 0.5% in the placebo group.
- Median time-to-progression was 11.1 months in the sorafenib group and 5.7 months in the placebo group (HR, 0.56; 95% CI, 0.43–0.72; P < .001).
- Adverse events occurred in 98.6% of patients treated with sorafenib and 87.6% of patients treated with placebo. The most common adverse events in the sorafenib group were hand-foot skin reactions (76.3%), diarrhea (68.6%), alopecia (67.1%), and rash or desquamation (50.2%). Most events were grade 1 or 2. Seven squamous cell carcinomas of the skin occurred in the sorafenib group.
Lenvatinib
Lenvatinib is an orally active, multitargeted tyrosine kinase inhibitor.
Evidence (lenvatinib):
- A phase III, randomized, double-blind, placebo-controlled study (SELECT [NCT01321554]) evaluated the activity of lenvatinib in patients with progressive, iodine-refractory, differentiated thyroid cancer.[22] In this trial, 261 patients were randomly assigned to receive lenvatinib (24 mg every day), and 131 patients were randomly assigned to receive placebo. Eligible patients had differentiated thyroid cancer (including papillary, follicular, poorly differentiated, and Hürthle cell carcinomas), RAI-refractory disease, and evidence of radiological progression within the previous 13 months. At disease progression, patients in the placebo group could receive open-label lenvatinib. The primary end point was PFS, while the secondary end points were OS, response rate, and safety.[22][Level of evidence B1]
- The median PFS in the lenvatinib group was 18.3 months versus 3.6 months in the placebo group (HRprogression or death, 0.21; 99% CI, 0.14–0.31; P < .001). A PFS difference was observed in patients with all histological types of thyroid cancer enrolled in this trial.
- There was no significant difference in OS between the two groups (HRdeath, 0.73; 95% CI, 0.50–1.07; P = .10), even with the crossover design of the study.
- The objective response rate was 64.8% in the lenvatinib group and 1.5% in the placebo group (OR, 28.87; 95% CI, 12.46–66.86; P < .001).
- Treatment-related adverse events (all grades) occurred in 97.3% of patients in the lenvatinib group and 59.5% of patients in the placebo group.
- Grade 3 or higher adverse events were observed in 75.9% of patients who received lenvatinib and 9.9% of patients who received placebo.
- The most frequent adverse events in the lenvatinib group were hypertension (67.8%), diarrhea (59.4%), fatigue (59%), decreased appetite (50.2%), decreased weight (46.4%), and nausea (41%).
- Adverse events led to discontinuation of the study drug in 14.2% of patients who received lenvatinib and 2.3% of patients who received placebo.
- In the lenvatinib group, 6 of 20 deaths occurring during the treatment period were considered drug related.
Cabozantinib
Cabozantinib is an orally bioavailable tyrosine kinase inhibitor of c-MET and vascular endothelial growth factor receptor-2 (VEGFR-2).
Evidence (cabozantinib):
- A phase III, randomized, double blind, placebo-controlled study (COSMIC-311 [NCT03690388]) was conducted to evaluate the efficacy of cabozantinib in patients aged 16 years and older with RAI-refractory differentiated thyroid cancer. Objective response rate was the primary end point in the first 100 randomly assigned patients (the objective response rate intention-to-treat [OITT] population), and PFS was the primary end point in all randomly assigned patients (the intention-to-treat [ITT] population).[35]
- Of 187 patients, 63% had received previous therapy with lenvatinib, 60% had received previous therapy with sorafenib, and 24% had received previous therapy with both lenvatinib and sorafenib. Most patients (76%) had experienced disease progression while receiving sorafenib or lenvatinib.
- In the OITT population, 15% of patients (99% CI, 5.8%–29.3%) in the cabozantinib group had a confirmed partial response, and median duration of response had not been reached at the data cutoff.
- In the ITT population, after a median follow-up of 6.2 months, patients who received cabozantinib showed significant improvement in PFS compared with patients who received placebo (median not reached [96% CI, 5.7–not estimable (NE)] vs. 1.9 months [1.8–3.6]) (HR, 0.22; 96% CI, 0.13–0.36; P < .0001). Median OS was not reached in either ITT group (95% CI, NE–NE) (HR, 0.54; 95% CI, 0.27–1.11). The 6-month OS rate was 85% (95% CI, 75%–91%) in the cabozantinib group and 73% (58.4%–83.7%) in the placebo group.
- Grade 3 to 4 treatment-related adverse events occurred in 57% of patients who received cabozantinib and 26% of patients who received placebo, and included palmar-plantar erythrodysesthesia (10%), hypertension (11%), fatigue (8%), diarrhea (7%), and hypocalcemia (7%). Serious treatment-related adverse events occurred in 20 of 125 patients (16%) in the cabozantinib group and 1 of 62 patients (2%) in the placebo group. There were no treatment-related deaths.
- In 2021, the FDA reviewed a new drug application for the use of cabozantinib in patients aged 12 years and older with differentiated thyroid cancer because there are no standard-of-care treatments for this population after anti-VEGFR therapy, such as lenvatinib or sorafenib.
RET kinase inhibitors
Selpercatinib
Selpercatinib is an orally active, highly selective, small-molecule RET kinase inhibitor.
Evidence (selpercatinib):
- A phase I/II trial (LIBRETTO-001 [NCT03157128]) evaluated the safety and efficacy of selpercatinib in adolescent and adult patients with any solid tumor with an activating RET pathogenic variant. The primary end point was an objective response (complete response or partial response). Patients with RET fusion–positive thyroid cancer had radioiodine-refractory disease and received at least one previous systemic therapy other than radioiodine. Nineteen patients with RET fusion–positive thyroid cancer were enrolled; 13 patients had papillary thyroid cancer, 3 had poorly differentiated thyroid cancer, 1 had anaplastic thyroid cancer, and 1 had Hürthle cell thyroid cancer. Ninety-five percent of patients with RET fusion–positive thyroid cancer received the phase II dose of 160 mg twice daily.[36]
- The objective response rate was 79% (95% CI, 54%−94%).[36][Level of evidence C2]
- At 1 year, 71% of responses were ongoing (95% CI, 39%−88%) and 64% of patients were progression-free.
- Treatment-related adverse events (any grade) occurred in 94% of patients.
- Grade 3 adverse events were observed in 28% of patients, and grade 4 adverse events were observed in 2% of patients.
- The most frequent adverse events were dry mouth (46%), hypertension (43%), diarrhea (38%), fatigue (38%), increased aspartate aminotransferase (AST) level (35%), nausea (35%), and constipation (35%).
- Thirty percent of patients had dose reductions because of treatment-related adverse events, and 2% of patients discontinued the drug because of adverse events.
- A phase III randomized trial (NCT04211337) compared first-line selpercatinib with physician’s choice of cabozantinib or vandetanib (control group). Eligible patients had progressive disease within the 14 months before enrollment. The primary end point in the protocol-specified interim efficacy analysis was PFS, assessed by blinded independent central review. Patients in the control group with disease progression were permitted to cross over to the selpercatinib group. Treatment failure–free survival, assessed by blinded independent central review, was a secondary, alpha-controlled end point that was to be tested only if PFS was significant. Other secondary end points included overall response and safety.[37]
- At a median follow-up of 12 months, the median PFS was not reached in the selpercatinib group and was 16.8 months in the control group (95% CI, 12.2–25.1) (HRdisease progression or death, 0.28; 95% CI, 0.16–0.48; P < .001).[37][Level of evidence B1]
- The 12-month PFS rate was 86.8% (95% CI, 79.8%–91.6%) in the selpercatinib group and 65.7% (95% CI, 51.9%–76.4%) in the control group.
- The overall response rate was 69.4% (95% CI, 62.4%–75.8%) in the selpercatinib group and 38.8% (95% CI, 29.1%–49.2%) in the control group.
- Adverse events led to a dose reduction in 38.9% of patients in the selpercatinib group and 77.3% of patients in the control group. Adverse events led to treatment discontinuation in 4.7% of patients in the selpercatinib group and 26.8% of patients in the control group.
Pralsetinib
Pralsetinib is an oral RET-targeted therapy. In December 2020, the FDA approved pralsetinib for patients aged 12 years and older and for adults with advanced or metastatic RET-altered medullary thyroid cancer (MTC) or RET fusion–positive RAI-refractory differentiated thyroid cancer.
Evidence (pralsetinib):
- A phase I/II, multicenter, open-label study (ARROW [NCT03037385]) evaluated the efficacy and safety of pralsetinib.[38]
- At data cutoff, 147 patients had RET-altered MTC and 22 patients had RET fusion–positive differentiated thyroid cancer. A total of 142 patients with RET-altered MTC or RET fusion–positive differentiated thyroid cancer initiated the phase II dose of pralsetinib at 400 mg once daily.
- There were 61 patients with RET-altered MTC who had previously received cabozantinib, vandetanib, or both, and 23 patients were treatment-naïve.
- In patients with RET fusion–positive differentiated thyroid cancer, the overall response rate was 89% (95% CI, 52%–100%), all of which were partial responses. Duration of response was not reached after a median follow-up of 9.5 months. Median PFS was not reached (95% CI, NE), with an estimated 1-year PFS rate of 81% after a median follow-up of 12.9 months. Median OS was also not reached, with an estimated 1-year OS rate of 91% (95% CI, 74%–100%) after a median follow-up of 15.8 months.
- The most frequent all-cause adverse events were anemia (45%), musculoskeletal pain (45%), constipation (44%), increased AST (42%), and hypertension (40%). Grade 3 to 4 treatment-related adverse events included hypertension (17%), neutropenia (13%), lymphopenia (12%), and anemia (10%). Serious adverse events were reported in 15% of patients; the most frequently reported serious adverse event was pneumonitis (4%).
- Of note, in patients with RET-altered MTC previously treated with cabozantinib, vandetanib, or both, the overall response rate was 60% (95% CI, 46%–73%). One patient (2%) had a complete response. However, in 2023 pralsetinib was withdrawn from FDA approval for this indication.[39]
NTRK inhibitors
Larotrectinib
Larotrectinib is a potent and highly selective small-molecule inhibitor of the TRKA, TRKB, and TRKC proteins.
Evidence (larotrectinib):
- An expanded pooled efficacy analysis included 159 patients with TRK fusion−positive cancers treated with larotrectinib. The safety analysis included 260 patients treated with larotrectinib, regardless of TRK gene fusion status.[40]
- For the 24 patients with thyroid cancer, the overall response rate was 79% (95% CI, 58%−93%). The median duration of response was NE (14.8 months−NE).[40][Level of evidence C2]
- Of the 260 patients in the safety analysis, 13% had grade 3 treatment-related adverse events and less than 1% had grade 4 treatment-related adverse events.
- The most common grade 3 or 4 treatment-related adverse events were increased alanine aminotransferase (3%), anemia (2%), and decreased neutrophil count (2%).
- Doses were reduced in 8% of patients with TRK fusion–positive cancer because of adverse events. The drug was discontinued in 2% of patients because of treatment-related adverse events.
Entrectinib
Entrectinib is a potent small-molecule inhibitor of the TRKA, TRKB, TRKC, ROS1, and ALK proteins.
Evidence (entrectinib):
- A pooled analysis of three phase I/II trials evaluated the efficacy and safety of entrectinib in patients with metastatic or locally advanced or unresectable NTRK fusion−positive solid tumors. The efficacy analysis included 54 adult patients with advanced or metastatic NTRK fusion−positive solid tumors.[41]
- The objective response rate was 57% (95% CI, 34.2%−70.8%). Seven percent of patients had a complete response, and 50% of patients had a partial response.[41][Level of evidence C2]
- The median duration of response was 10 months (95% CI, 7.1−NE), and the median PFS was 11 months (95% CI, 8−14.9).
- For the five patients with thyroid cancer, the overall response rate was 20% (95% CI, 1%−72%).
- In the overall safety population (n = 355), the most frequently reported treatment-related adverse events were dysgeusia (41%), fatigue (28%), dizziness (26%), constipation (23%), and diarrhea (22%).
- The most common grade 3 or 4 treatment-related adverse events were increased weight (5%) and anemia (5%).
EBRT
EBRT or intraoperative radiation therapy can be useful in controlling symptoms related to local tumor recurrences.[42]
Chemotherapy
Systemic chemotherapy can be considered. Chemotherapy has been reported to produce occasional objective responses, usually of short duration.[26,31]
Clinical trials
Patients with recurrent papillary or follicular thyroid cancer should consider enrolling in clinical trials evaluating new treatment approaches to this disease. Oral inhibitors targeting specific single nucleotide variants are under clinical evaluation, as are new immunotherapy approaches.[27-29][Level of evidence B4]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Haigh PI, Urbach DR: The treatment and prognosis of Hürthle cell follicular thyroid carcinoma compared with its non-Hürthle cell counterpart. Surgery 138 (6): 1152-7; discussion 1157-8, 2005. [PUBMED Abstract]
- Carling T, Udelsman R: Thyroid tumors. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 1457-72.
- Grant CS, Hay ID, Gough IR, et al.: Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery 104 (6): 954-62, 1988. [PUBMED Abstract]
- Cady B, Rossi R: An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104 (6): 947-53, 1988. [PUBMED Abstract]
- Mazzaferri EL, Jhiang SM: Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97 (5): 418-28, 1994. [PUBMED Abstract]
- Staunton MD: Thyroid cancer: a multivariate analysis on influence of treatment on long-term survival. Eur J Surg Oncol 20 (6): 613-21, 1994. [PUBMED Abstract]
- Tollefsen HR, Shah JP, Huvos AG: Follicular carcinoma of the thyroid. Am J Surg 126 (4): 523-8, 1973. [PUBMED Abstract]
- Edis AJ: Surgical treatment for thyroid cancer. Surg Clin North Am 57 (3): 533-42, 1977. [PUBMED Abstract]
- Bilimoria KY, Bentrem DJ, Ko CY, et al.: Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 246 (3): 375-81; discussion 381-4, 2007. [PUBMED Abstract]
- Bilimoria KY, Bentrem DJ, Linn JG, et al.: Utilization of total thyroidectomy for papillary thyroid cancer in the United States. Surgery 142 (6): 906-13; discussion 913.e1-2, 2007. [PUBMED Abstract]
- Hay ID, Grant CS, Bergstralh EJ, et al.: Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery 124 (6): 958-64; discussion 964-6, 1998. [PUBMED Abstract]
- Beierwaltes WH, Rabbani R, Dmuchowski C, et al.: An analysis of "ablation of thyroid remnants" with I-131 in 511 patients from 1947-1984: experience at University of Michigan. J Nucl Med 25 (12): 1287-93, 1984. [PUBMED Abstract]
- Hänscheid H, Lassmann M, Luster M, et al.: Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med 47 (4): 648-54, 2006. [PUBMED Abstract]
- Hay ID, Grant CS, van Heerden JA, et al.: Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery 112 (6): 1139-46; discussion 1146-7, 1992. [PUBMED Abstract]
- Schvartz C, Bonnetain F, Dabakuyo S, et al.: Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab 97 (5): 1526-35, 2012. [PUBMED Abstract]
- Schlumberger M, Catargi B, Borget I, et al.: Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med 366 (18): 1663-73, 2012. [PUBMED Abstract]
- Mallick U, Harmer C, Yap B, et al.: Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med 366 (18): 1674-85, 2012. [PUBMED Abstract]
- Pujol P, Daures JP, Nsakala N, et al.: Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J Clin Endocrinol Metab 81 (12): 4318-23, 1996. [PUBMED Abstract]
- Cooper DS, Specker B, Ho M, et al.: Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 8 (9): 737-44, 1998. [PUBMED Abstract]
- Giuliani M, Brierley J: Indications for the use of external beam radiation in thyroid cancer. Curr Opin Oncol 26 (1): 45-50, 2014. [PUBMED Abstract]
- Brose MS, Nutting CM, Jarzab B, et al.: Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384 (9940): 319-28, 2014. [PUBMED Abstract]
- Schlumberger M, Tahara M, Wirth LJ, et al.: Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372 (7): 621-30, 2015. [PUBMED Abstract]
- Simpson WJ, Carruthers JS: The role of external radiation in the management of papillary and follicular thyroid cancer. Am J Surg 136 (4): 457-60, 1978. [PUBMED Abstract]
- Gottlieb JA, Hill CS, Ibanez ML, et al.: Chemotherapy of thyroid cancer. An evaluation of experience with 37 patients. Cancer 30 (3): 848-53, 1972. [PUBMED Abstract]
- Harada T, Nishikawa Y, Suzuki T, et al.: Bleomycin treatment for cancer of the thyroid. Am J Surg 122 (1): 53-7, 1971. [PUBMED Abstract]
- Shimaoka K, Schoenfeld DA, DeWys WD, et al.: A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 56 (9): 2155-60, 1985. [PUBMED Abstract]
- Sherman SI, Wirth LJ, Droz JP, et al.: Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 359 (1): 31-42, 2008. [PUBMED Abstract]
- Jin Y, Van Nostrand D, Cheng L, et al.: Radioiodine refractory differentiated thyroid cancer. Crit Rev Oncol Hematol 125: 111-120, 2018. [PUBMED Abstract]
- Naoum GE, Morkos M, Kim B, et al.: Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol Cancer 17 (1): 51, 2018. [PUBMED Abstract]
- Goretzki PE, Simon D, Frilling A, et al.: Surgical reintervention for differentiated thyroid cancer. Br J Surg 80 (8): 1009-12, 1993. [PUBMED Abstract]
- De Besi P, Busnardo B, Toso S, et al.: Combined chemotherapy with bleomycin, adriamycin, and platinum in advanced thyroid cancer. J Endocrinol Invest 14 (6): 475-80, 1991. [PUBMED Abstract]
- Ross DS: Long-term management of differentiated thyroid cancer. Endocrinol Metab Clin North Am 19 (3): 719-39, 1990. [PUBMED Abstract]
- Pak H, Gourgiotis L, Chang WI, et al.: Role of metastasectomy in the management of thyroid carcinoma: the NIH experience. J Surg Oncol 82 (1): 10-8, 2003. [PUBMED Abstract]
- Coburn M, Teates D, Wanebo HJ: Recurrent thyroid cancer. Role of surgery versus radioactive iodine (I131) Ann Surg 219 (6): 587-93; discussion 593-5, 1994. [PUBMED Abstract]
- Brose MS, Robinson B, Sherman SI, et al.: Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 22 (8): 1126-1138, 2021. [PUBMED Abstract]
- Wirth LJ, Sherman E, Robinson B, et al.: Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med 383 (9): 825-835, 2020. [PUBMED Abstract]
- Hadoux J, Elisei R, Brose MS, et al.: Phase 3 Trial of Selpercatinib in Advanced RET-Mutant Medullary Thyroid Cancer. N Engl J Med 389 (20): 1851-1861, 2023. [PUBMED Abstract]
- Subbiah V, Hu MI, Wirth LJ, et al.: Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 9 (8): 491-501, 2021. [PUBMED Abstract]
- GAVRETO Full prescribing information. Rigel, 2024. Available online. Last accessed April 2, 2025.
- Hong DS, DuBois SG, Kummar S, et al.: Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21 (4): 531-540, 2020. [PUBMED Abstract]
- Doebele RC, Drilon A, Paz-Ares L, et al.: Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 21 (2): 271-282, 2020. [PUBMED Abstract]
- Vikram B, Strong EW, Shah JP, et al.: Intraoperative radiotherapy in patients with recurrent head and neck cancer. Am J Surg 150 (4): 485-7, 1985. [PUBMED Abstract]
Medullary Thyroid Cancer
Sporadic and Hereditary Medullary Thyroid Cancer (MTC)
MTC occurs in two forms, sporadic and hereditary. In sporadic MTC, the tumor is usually unilateral. In the hereditary form, the tumor is almost always bilateral. In addition, hereditary MTC may be associated with benign or malignant tumors of other endocrine organs, commonly referred to as the multiple endocrine neoplasia (MEN) syndromes types 2A and 2B (MEN2A or MEN2B). These syndromes are associated with pheochromocytoma of the adrenal gland and parathyroid hyperplasia.
Approximately 25% of reported MTC cases are hereditary. Hereditary MTC syndromes include MEN2A, which is the most common, MEN2B, and hereditary non-MEN syndromes. Any patient with a hereditary variant is screened for other associated endocrine tumors, particularly parathyroid hyperplasia and pheochromocytoma. Medullary carcinoma usually secretes calcitonin, a hormonal marker for the tumor, and may be detectable in blood even when the tumor is clinically occult. Measuring calcitonin levels is useful for diagnostic purposes and for following the results of treatment. For more information, see Multiple Endocrine Neoplasia Type 2 (MEN2).
Patients with MTC (whether hereditary or sporadic) are tested for RET pathogenic variants, and if RET variants are detected, family members will also be tested. Family members may be screened for calcitonin elevation and the RET proto-oncogene variant. These tests can identify others at risk of developing hereditary MTC. Because a modest elevation of calcitonin may lead to a false-positive diagnosis of MTC, genetic testing for the RET pathogenic variant is the optimal approach. Family members who carry the RET pathogenic variant may choose to undergo prophylactic thyroidectomy at an early age.[1,2]
Clinical Features and Prognosis
MTC accounts for 3% to 4% of all thyroid cancers. These tumors usually present as a hard mass in the neck or thyroid, often associated with lymphadenopathy.[3] MTC can also be diagnosed by fine-needle aspiration biopsy. Cytology typically reveals hypercellular tumors with spindle-shaped cells and poor adhesion.[4] Metastases to regional lymph nodes are found in about 50% of the cases.
The overall survival (OS) rate of patients with MTC is 86% at 5 years and 65% at 10 years.
Prognosis depends on the following factors:[5]
- Extent of disease at presentation.
- Presence or absence of regional lymph node metastases.
- Completeness of the surgical resection.
Poor prognostic factors include:[4,6,7]
- Advanced age.
- Advanced stage.
- Previous neck surgery.
- Associated MEN2B.
Stage Information for MTC
Several staging systems have been employed to correlate extent of disease with long-term survival in MTC. The clinical staging system of the American Joint Committee on Cancer correlates survival to size of the primary tumor (T), presence or absence of lymph node involvement (N), and presence or absence of distant metastasis (M). Patients who are diagnosed with hereditary MTC after positive genetic testing for a RET pathogenic variant have the best prognoses.[8]
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; MTC = medullary thyroid cancer. | |||
| aReprinted with permission from AJCC: Thyroid--Medullary. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 891–901. | |||
| I | T1, N0, M0 | T1 = Tumor ≤2 cm in greatest dimension limited to the thyroid. |
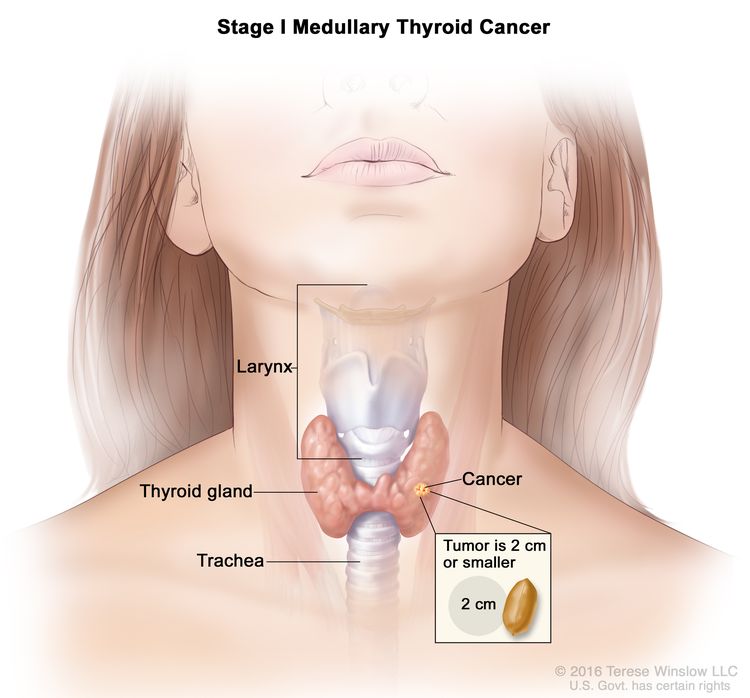 |
| –T1a = Tumor ≤1 cm in greatest dimension limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension limited to the thyroid. | |||
| N0 = No evidence of locoregional lymph node metastasis. | |||
| –N0a = One or more cytologically or histologically confirmed benign lymph nodes. | |||
| –N0b = No radiological or clinical evidence of locoregional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; MTC = medullary thyroid cancer. | |||
| aReprinted with permission from AJCC: Thyroid--Medullary. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 891–901. | |||
| II | T2, N0, M0 | T2 = Tumor >2 cm but ≤4 cm in greatest dimension limited to the thyroid. |
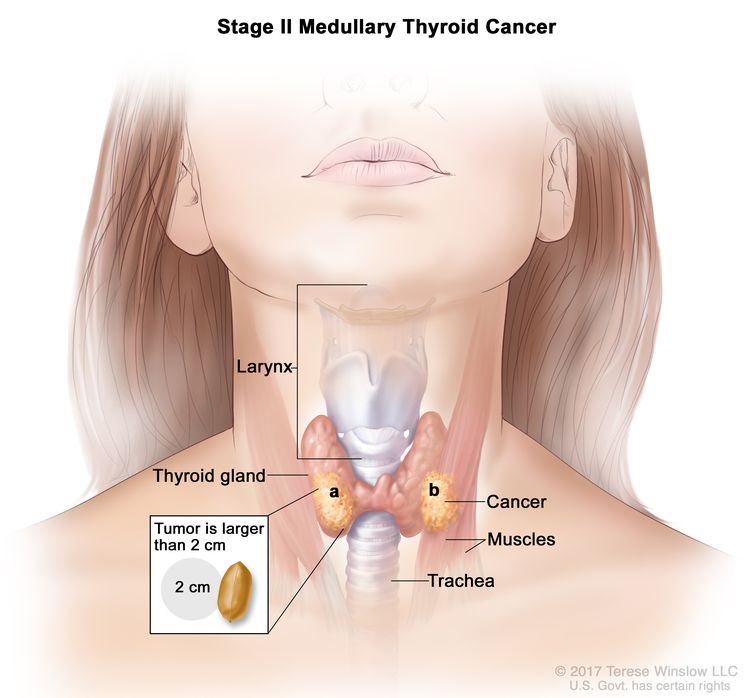 |
| N0 = No evidence of locoregional lymph node metastasis. | |||
| –N0a = One or more cytologically or histologically confirmed benign lymph nodes. | |||
| –N0b = No radiological or clinical evidence of locoregional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| T3, N0, M0 | T3 = Tumor >4 cm or with extrathyroidal extension. | ||
| –T3a = Tumor >4 cm in greatest dimension limited to the thyroid. | |||
| –T3b = Tumor of any size with gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles). | |||
| N0 = No evidence of locoregional lymph node metastasis. | |||
| –N0a = One or more cytologically of histologically confirmed benign lymph nodes. | |||
| –N0b = No radiological or clinical evidence of locoregional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; MTC = medullary thyroid cancer. | |||
| aReprinted with permission from AJCC: Thyroid--Medullary. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 891–901. | |||
| III | T1–3, N1a, M0 | T1 = Tumor ≤2 cm in greatest dimension limited to the thyroid. |
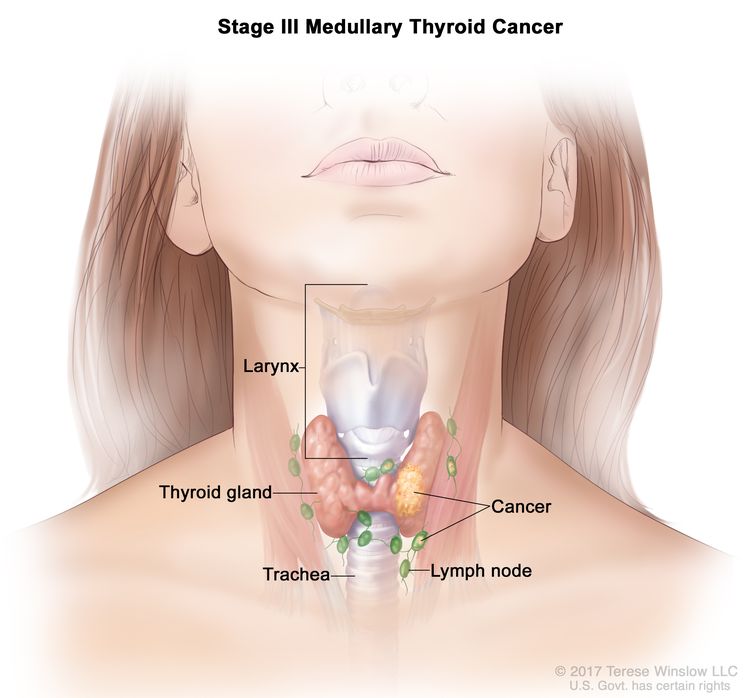 |
| –T1a = Tumor ≤1 cm in greatest dimension limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension limited to the thyroid. | |||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension limited to the thyroid. | |||
| T3 = Tumor >4 cm or with extrathyroidal extension. | |||
| –T3a = Tumor >4 cm in greatest dimension limited to the thyroid. | |||
| –T3b = Tumor of any size with gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles). | |||
| N1a = Metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes. This can be unilateral or bilateral disease. | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; MTC = medullary thyroid cancer. | |||
| aReprinted with permission from AJCC: Thyroid--Medullary. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 891–901. | |||
| IVA | T4a, Any N, M0 | –T4a = Moderately advanced disease; tumor of any size with gross extrathyroidal extension into the nearby tissues of the neck, including subcutaneous soft tissue, larynx, trachea, esophagus, or recurrent laryngeal nerve. |
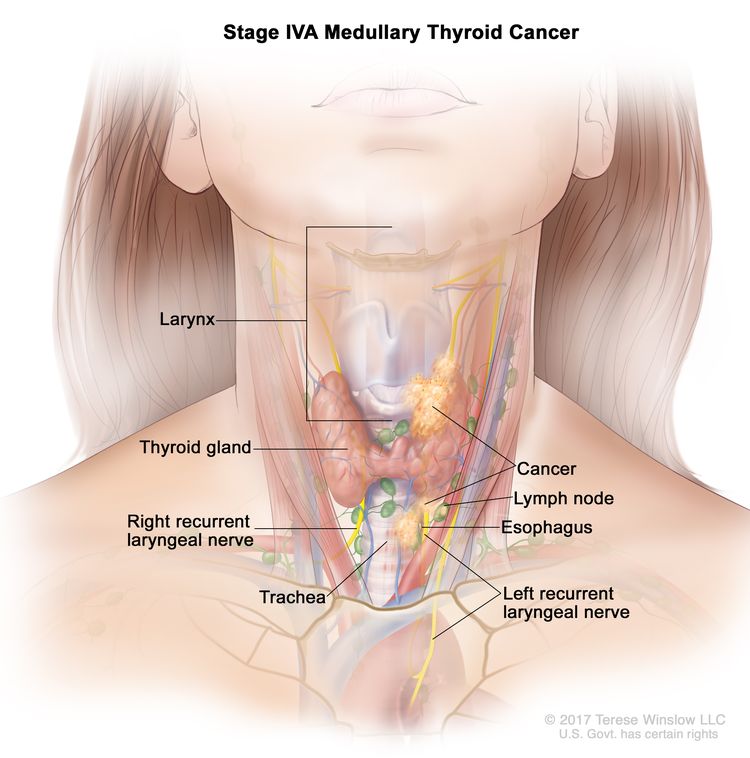 |
| NX = Regional lymph nodes cannot be assessed. | |||
| N0 = No evidence of locoregional lymph node metastasis. | |||
| –N0a = One or more cytologically or histologically confirmed benign lymph nodes. | |||
| –N0b = No radiological or clinical evidence of locoregional lymph node metastasis. | |||
| N1 = Metastasis to regional nodes. | |||
| –N1a = Metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes. This can be unilateral or bilateral disease. | |||
| –N1b = Metastasis to unilateral, bilateral, or contralateral cervical neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T1–3, N1b, M0 | T1 = Tumor ≤2 cm in greatest dimension limited to the thyroid. | ||
| –T1a = Tumor ≤1 cm in greatest dimension limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension limited to the thyroid. | |||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension limited to the thyroid. | |||
| T3 = Tumor >4 cm or with extrathyroidal extension. | |||
| –T3a = Tumor >4 cm in greatest dimension limited to the thyroid. | |||
| –T3b = Tumor of any size with gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles). | |||
| –N1b = Metastasis to unilateral, bilateral, or contralateral cervical neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes. | |||
| M0 = No distant metastasis. | |||
| IVB | T4b, Any N, M0 | T4b = Very advanced disease; tumor of any size with extension toward the spine or into nearby large blood vessels, gross extrathyroidal extension invading the prevertebral fascia, or encasing the carotid artery or mediastinal vessels. |
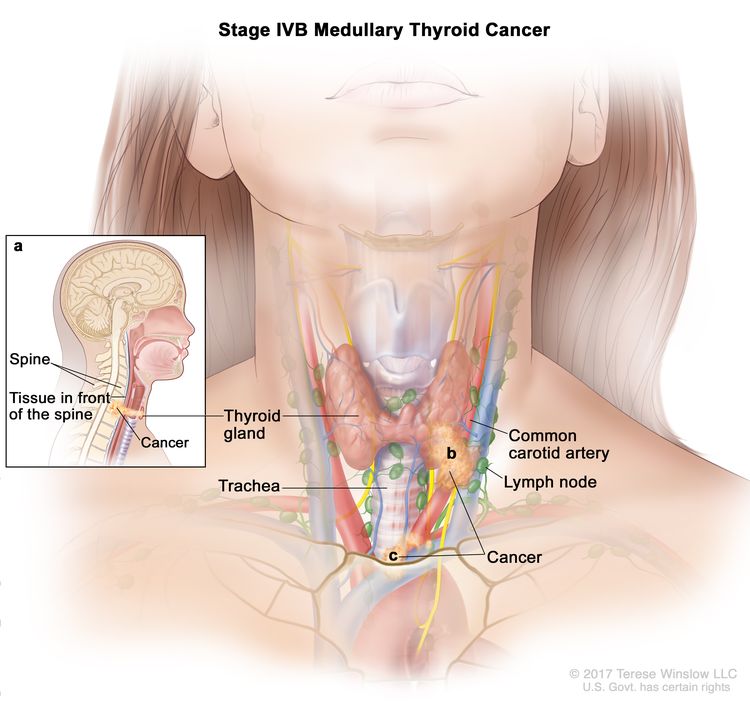 |
| Any N = See descriptions in this table, Stage IVA. | |||
| M0 = No distant metastasis. | |||
| IVC | Any T, Any N, M1 | TX = Primary tumor cannot be assessed. |
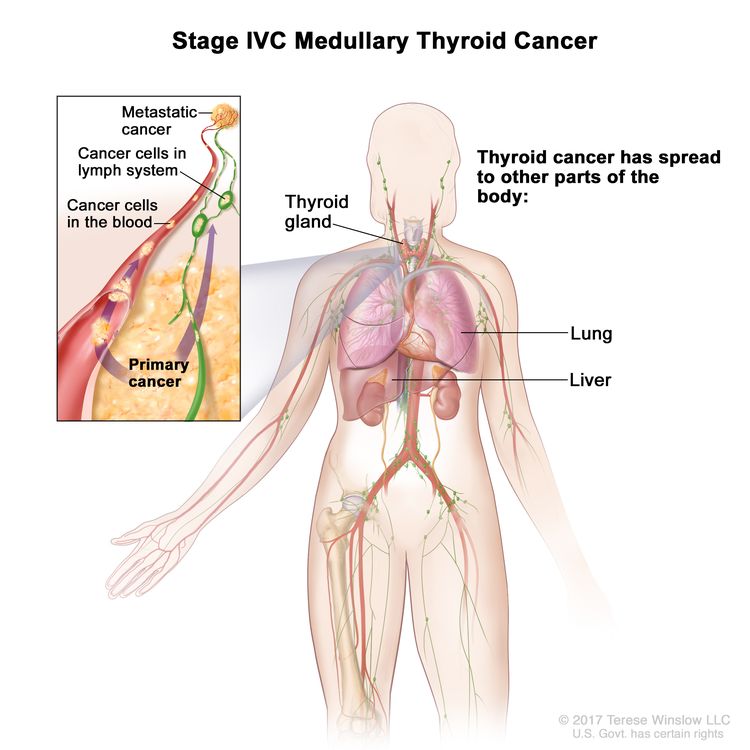 |
| T0 = No evidence of primary tumor. | |||
| T1 = Tumor ≤2 cm in greatest dimension limited to the thyroid. | |||
| –T1a = Tumor ≤1 cm in greatest dimension limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension limited to the thyroid. | |||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension limited to the thyroid. | |||
| T3 = Tumor >4 cm or with extrathyroidal extension. | |||
| –T3a = Tumor >4 cm in greatest dimension limited to the thyroid. | |||
| –T3b = Tumor of any size with gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles). | |||
| T4 = Advanced disease. | |||
| T4a = Moderately advanced disease; tumor of any size with gross extrathyroidal extension into the nearby tissues of the neck, including subcutaneous soft tissue, larynx, trachea, esophagus, or recurrent laryngeal nerve. | |||
| T4b = Very advanced disease; tumor of any size with extension toward the spine or into nearby large blood vessels, gross extrathyroidal extension invading the prevertebral fascia, or encasing the carotid artery or mediastinal vessels. | |||
| Any N = See descriptions in this table, Stage IVA, T4a, Any N, M0. | |||
| M1 = Distant metastasis. | |||
Treatment Options for MTC
Localized disease
Treatment options for localized MTC include:
Radioactive iodine is not used in the treatment of patients with MTC.
Total thyroidectomy
Patients with MTC are treated with total thyroidectomy unless there is evidence of distant metastasis. In patients with clinically palpable MTC, the incidence of microscopically positive nodes is more than 75%. Routine central and bilateral modified neck dissections are generally done.[9] When cancer is confined to the thyroid gland, the prognosis is excellent.
EBRT
EBRT has been used for palliation of locally recurrent tumors without evidence that it provides any survival advantage.[10]
Locally advanced and metastatic disease
Treatment options for locally advanced and metastatic MTC include:
Targeted therapy
Tyrosine kinase inhibitors
Vandetanib
Vandetanib is an oral inhibitor of rearranged during transfection (RET) receptor kinase, vascular endothelial growth factor receptor (VEGFR), and epidermal growth-factor receptor.
Evidence (vandetanib):
- A placebo-controlled prospective trial (NCT00410761) evaluated vandetanib in 331 patients with locally advanced or metastatic disease. Patients were randomly assigned in a 2:1 ratio to receive vandetanib or placebo.[11][Level of evidence B1]
- With a median follow-up of 24 months, progression-free survival (PFS) favored vandetanib (hazard ratio [HR], 0.46; 95% confidence interval [CI], 0.31–0.69; P < .001), with a median PFS estimated at 30.5 months for vandetanib versus 19.3 months for placebo.
- OS was not different at 24 months. Longer follow-up will be required because all but 47 patients were alive at the time of analysis, and there was a crossover to the study drug on progression from placebo, making analysis of OS problematic.
- Vandetanib treatment produces significant side effects, including diarrhea, rash, hypertension, and QT prolongation. Quality of life was not formally assessed in this trial.
Cabozantinib
Cabozantinib is an oral tyrosine kinase inhibitor of RET receptor kinase, hepatocyte growth factor receptor MET, and VEGFR-2.
Evidence (cabozantinib):
- Cabozantinib was evaluated in a randomized, double-blind, placebo-controlled, phase III trial (EXAM [NCT00704730]). The study included 330 patients with metastatic MTC and radiographic progression of disease. Patients were randomly assigned in a 2:1 ratio to receive cabozantinib (140 mg per day) or placebo. Patients who received placebo were not permitted to cross
over to cabozantinib. The primary end point was PFS. Additional outcomes included response rate, OS, and safety.[12][Level of evidence B1]
- The estimated median PFS was 11.2 months in the cabozantinib group and 4.0 months in the placebo group (HR, 0.28; 95% CI, 0.19‒0.40; P < .001). A difference in PFS was observed across all subgroups and was independent of previous tyrosine kinase inhibitor treatment and RET pathogenic variant status.
- During a planned interim analysis of OS, no statistically significant difference was observed between the two groups (HR, 0.98; 95% CI, 0.63‒1.52).
- The objective response rate was 28% in the cabozantinib group (all partial responses) and 0% in the placebo group (P < .001). The median estimated duration of response was 14.6 months (95% CI, 11.1‒17.5 months). Responses were observed regardless of RET pathogenic variant status.
- The most common cabozantinib-associated adverse events (all grades) included diarrhea (63.1%), palmar-plantar erythrodysesthesia (50%), decreased weight (47.7%), decreased appetite (45.8%), nausea (43%), and fatigue (40.7%)
- Grade 3 or 4 adverse events were reported in 69% of patients in the cabozantinib group and 33% of patients in the placebo group.
- Adverse events resulted in treatment discontinuation in 16% of patients who received cabozantinib and in 8% of patients who received placebo.
RET kinase inhibitors
Selpercatinib
Selpercatinib is an orally active, highly selective, small-molecule RET kinase inhibitor.
Evidence (selpercatinib):
- A phase I/II trial (LIBRETTO-001 [NCT03157128]) evaluated the safety and efficacy of selpercatinib in adolescent and adult patients with any solid tumor type harboring an activating RET alteration. The primary end point was an objective response (complete response or partial response). The trial enrolled 55 patients with RET-altered MTC who were previously treated with vandetanib, cabozantinib, or both; and 88 patients with RET-altered MTC who were not previously treated with vandetanib or cabozantinib. Eighty two percent of previously treated patients received the recommended phase II dose of 160 mg twice daily, and 98% of previously untreated patients received 160 mg twice daily.[13]
- For patients with RET-altered MTC who were previously treated with vandetanib or cabozantinib:
- The objective response rate was 69% (95% CI, 55%−81%); 9% of patients had a complete response, and 60% of patients had a partial response.
- At 1 year, 86% of responses were ongoing (95% CI, 67%−95%), and 100% of patients were progression-free.
- For patients with RET-altered MTC who were not previously treated with vandetanib or cabozantinib:
- The objective response rate was 73% (95% CI, 62%−82%); 11% of patients had a complete response, and 61% of patients had a partial response.[13][Level of evidence C2]
- At 1 year, 91% of responses were ongoing (95% CI, 72%−97%), and 92% of patients were progression-free.
- Treatment-related adverse events (any grade) occurred in 94% of patients.
- Grade 3 adverse events were observed in 28% of patients, and grade 4 adverse events were observed in 2% of patients.
- The most frequent adverse events were dry mouth (46%), hypertension (43%), diarrhea (38%), fatigue (38%), increased aspartate aminotransferase level (35%), nausea (35%), and constipation (35%).
- Thirty percent of patients had dose reductions because of treatment-related adverse events, and 2% of patients discontinued the drug because of adverse events.
- For patients with RET-altered MTC who were previously treated with vandetanib or cabozantinib:
Palliative chemotherapy
Palliative chemotherapy has been reported to produce occasional responses in patients with metastatic disease.[14-17] No single drug regimen can be considered standard. Some patients with distant metastases will experience prolonged survival and can be managed expectantly until they become symptomatic.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Lips CJ, Landsvater RM, Höppener JW, et al.: Clinical screening as compared with DNA analysis in families with multiple endocrine neoplasia type 2A. N Engl J Med 331 (13): 828-35, 1994. [PUBMED Abstract]
- Decker RA, Peacock ML, Borst MJ, et al.: Progress in genetic screening of multiple endocrine neoplasia type 2A: is calcitonin testing obsolete? Surgery 118 (2): 257-63; discussion 263-4, 1995. [PUBMED Abstract]
- Soh EY, Clark OH: Surgical considerations and approach to thyroid cancer. Endocrinol Metab Clin North Am 25 (1): 115-39, 1996. [PUBMED Abstract]
- Giuffrida D, Gharib H: Current diagnosis and management of medullary thyroid carcinoma. Ann Oncol 9 (7): 695-701, 1998. [PUBMED Abstract]
- Carling T, Udelsman R: Thyroid tumors. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 1457-72.
- Saad MF, Ordonez NG, Rashid RK, et al.: Medullary carcinoma of the thyroid. A study of the clinical features and prognostic factors in 161 patients. Medicine (Baltimore) 63 (6): 319-42, 1984. [PUBMED Abstract]
- Bergholm U, Bergström R, Ekbom A: Long-term follow-up of patients with medullary carcinoma of the thyroid. Cancer 79 (1): 132-8, 1997. [PUBMED Abstract]
- Colson YL, Carty SE: Medullary thyroid carcinoma. Am J Otolaryngol 14 (2): 73-81, 1993 Mar-Apr. [PUBMED Abstract]
- Moley JF, DeBenedetti MK: Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg 229 (6): 880-7; discussion 887-8, 1999. [PUBMED Abstract]
- Brierley JD, Tsang RW: External radiation therapy in the treatment of thyroid malignancy. Endocrinol Metab Clin North Am 25 (1): 141-57, 1996. [PUBMED Abstract]
- Wells SA, Robinson BG, Gagel RF, et al.: Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30 (2): 134-41, 2012. [PUBMED Abstract]
- Elisei R, Schlumberger MJ, Müller SP, et al.: Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31 (29): 3639-46, 2013. [PUBMED Abstract]
- Wirth LJ, Sherman E, Robinson B, et al.: Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med 383 (9): 825-835, 2020. [PUBMED Abstract]
- Shimaoka K, Schoenfeld DA, DeWys WD, et al.: A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 56 (9): 2155-60, 1985. [PUBMED Abstract]
- De Besi P, Busnardo B, Toso S, et al.: Combined chemotherapy with bleomycin, adriamycin, and platinum in advanced thyroid cancer. J Endocrinol Invest 14 (6): 475-80, 1991. [PUBMED Abstract]
- Wu LT, Averbuch SD, Ball DW, et al.: Treatment of advanced medullary thyroid carcinoma with a combination of cyclophosphamide, vincristine, and dacarbazine. Cancer 73 (2): 432-6, 1994. [PUBMED Abstract]
- Orlandi F, Caraci P, Berruti A, et al.: Chemotherapy with dacarbazine and 5-fluorouracil in advanced medullary thyroid cancer. Ann Oncol 5 (8): 763-5, 1994. [PUBMED Abstract]
Anaplastic Thyroid Cancer
Clinical Features and Prognosis
Undifferentiated (anaplastic) carcinoma is a highly malignant type of thyroid cancer. It grows rapidly and extends to structures beyond the thyroid. It typically presents as a hard, ill-defined mass, often with extension into the structures surrounding the thyroid. Anaplastic thyroid cancer must be carefully distinguished from lymphoma, which can have a similar presentation. This tumor usually occurs in an older age group and is characterized by extensive local invasion and rapid progression.[1]
Patients with this tumor have poor 5-year survival rates. Death is usually from uncontrolled local cancer in the neck, often within months of diagnosis.
Stage Information for Anaplastic Thyroid Cancer
All patients with anaplastic thyroid cancer are considered to have stage IV disease.
| Stage | TbNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Thyroid--Differentiated and Anaplastic Carcinoma. In: Amin, MB, Edge Sb, Greene FL et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 873–90. | |||
| bAll categories may be subdivided: (s) solitary tumor and (m) multifocal tumor (the largest determines the classification). | |||
| IVA | T1–T3a, N0/NX, M0 | T1 = Tumor ≤2 cm in greatest dimension, limited to the thyroid. |
 |
| –T1a = Tumor ≤1 cm in greatest dimension, limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension, limited to the thyroid. | |||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension, limited to the thyroid. | |||
| T3 = Tumor >4 cm limited to the thyroid, or gross extrathyroidal extension invading only strap muscles. | |||
| –T3a = Tumor >4 cm limited to the thyroid. | |||
| NX = Regional lymph nodes cannot be assessed. | |||
| N0 = No evidence of locoregional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| IVB | T1–T3a, N1, M0 | T1 = Tumor ≤2 cm in greatest dimension, limited to the thyroid. |
 |
| –T1a = Tumor ≤1 cm in greatest dimension, limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension, limited to the thyroid. | |||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension, limited to the thyroid. | |||
| T3 = Tumor >4 cm limited to the thyroid, or gross extrathyroidal extension invading only strap muscles. | |||
| –T3a = Tumor >4 cm limited to the thyroid. | |||
| N1 = Metastasis to regional nodes. | |||
| –N1a = Metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes. This can be unilateral or bilateral disease. | |||
| –N1b = Metastasis to unilateral, bilateral, or contralateral lateral neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T3b, Any N, M0 | –T3b = Gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles) from a tumor of any size. | ||
| NX = regional lymph nodes cannot be assessed. | |||
| N0 = No evidence of locoregional lymph node metastasis. | |||
| –N0a = One or more cytologically or histologically confirmed benign lymph nodes. | |||
| –N0b = No radiological or clinical evidence of locoregional lymph node metastasis. | |||
| N1 = Metastasis to regional nodes. | |||
| –N1a = Metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes. This can be unilateral or bilateral disease. | |||
| –N1b = Metastasis to unilateral, bilateral, or contralateral lateral neck lymph nodes (levels I, II, III, IV, or V) or retropharyngeal lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T4, Any N, M0 | T4 = Includes gross extrathyroidal extension beyond the strap muscles. | ||
| –T4a = Gross extrathyroidal extension invading subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve from a tumor of any size. | |||
| –T4b = Gross extrathyroidal extension invading prevertebral fascia or encasing the carotid artery or mediastinal vessels from a tumor of any size. | |||
| Any N = See descriptions in this table, Stage IVB, T3b, Any N, M0. | |||
| M0 = No distant metastasis. | |||
| IVC | Any T, Any N, M1 | TX = Primary tumor cannot be assessed. |
 |
| T0 = No evidence of primary tumor. | |||
| T1 = Tumor ≤2 cm in greatest dimension, limited to the thyroid. | |||
| –T1a = Tumor ≤1 cm in greatest dimension, limited to the thyroid. | |||
| –T1b = Tumor >1 cm but ≤2 cm in greatest dimension, limited to the thyroid. | |||
| T2 = Tumor >2 cm but ≤4 cm in greatest dimension, limited to the thyroid. | |||
| T3 = Tumor >4 cm limited to the thyroid, or gross extrathyroidal extension invading only strap muscles. | |||
| –T3a = Tumor >4 cm limited to the thyroid. | |||
| –T3b = Gross extrathyroidal extension invading only strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles) from a tumor of any size. | |||
| T4 = Includes gross extrathyroidal extension beyond the strap muscles. | |||
| –T4a = Gross extrathyroidal extension invading subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve from a tumor of any size. | |||
| –T4b = Gross extrathyroidal extension invading prevertebral fascia or encasing the carotid artery or mediastinal vessels from a tumor of any size. | |||
| Any N = See descriptions in this table, Stage IVB, T3b, Any N, M0. | |||
| M1 = Distant metastasis. | |||
Treatment Options for Anaplastic Thyroid Cancer
Treatment options for anaplastic thyroid cancer include:
Surgery
If the disease is localized, which is rare, total thyroidectomy is warranted to reduce symptoms caused by the tumor mass.[2,3] Tracheostomy is frequently necessary.
EBRT
EBRT may be used in patients who are not surgical candidates or whose tumor cannot be surgically excised.
Systemic Therapy
Anaplastic thyroid cancer is not responsive to iodine I 131 therapy. Treatment with individual anticancer drugs has been reported to produce partial remissions in some patients. Approximately 30% of patients achieve a partial remission with doxorubicin.[4] The combination of doxorubicin plus cisplatin appears to be more active than doxorubicin alone and has produced more complete responses.[5]
The combination of chemotherapy plus radiation therapy after complete resection may provide prolonged survival in some patients, but this treatment combination has not been compared with any one modality alone.[6,7]
An estimated 25% of patients with anaplastic thyroid cancers have an activating BRAF V600E variant.[8]
Evidence (dabrafenib and trametinib):
- A phase II trial of BRAF and MEK inhibitors, dabrafenib and trametinib, included 16 patients with anaplastic thyroid cancer and a BRAF V600E variant.[9][Level of evidence C2]
- The confirmed overall response rate was 69%. The median duration of response, progression-free survival (PFS), and overall survival (OS) were not reached.
- Twelve-month estimates were 90% for duration of response, 79% for PFS, and 80% for OS.
Based on this data, the U.S. Food and Drug Administration approved the combination of dabrafenib and trametinib for the treatment of unresectable or metastatic anaplastic thyroid cancer with a BRAF V600E variant. Molecular testing for the BRAF V600E variant is advised for patients with anaplastic thyroid cancer.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Neff RL, Farrar WB, Kloos RT, et al.: Anaplastic thyroid cancer. Endocrinol Metab Clin North Am 37 (2): 525-38, xi, 2008. [PUBMED Abstract]
- Goldman JM, Goren EN, Cohen MH, et al.: Anaplastic thyroid carcinoma: long-term survival after radical surgery. J Surg Oncol 14 (4): 389-94, 1980. [PUBMED Abstract]
- Aldinger KA, Samaan NA, Ibanez M, et al.: Anaplastic carcinoma of the thyroid: a review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer 41 (6): 2267-75, 1978. [PUBMED Abstract]
- Carling T, Udelsman R: Thyroid tumors. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 1457-72.
- Shimaoka K, Schoenfeld DA, DeWys WD, et al.: A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 56 (9): 2155-60, 1985. [PUBMED Abstract]
- Haigh PI, Ituarte PH, Wu HS, et al.: Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer 91 (12): 2335-42, 2001. [PUBMED Abstract]
- De Crevoisier R, Baudin E, Bachelot A, et al.: Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated external radiotherapy. Int J Radiat Oncol Biol Phys 60 (4): 1137-43, 2004. [PUBMED Abstract]
- Forbes SA, Beare D, Boutselakis H, et al.: COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res 45 (D1): D777-D783, 2017. [PUBMED Abstract]
- Subbiah V, Kreitman RJ, Wainberg ZA, et al.: Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol 36 (1): 7-13, 2018. [PUBMED Abstract]
Latest Updates to This Summary (05/09/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult thyroid cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Thyroid Cancer Treatment are:
- Andrea Bonetti, MD (Pederzoli Hospital)
- Minh Tam Truong, MD (Boston University Medical Center)
- Jaydira del Rivero, MD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Thyroid Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/thyroid/hp/thyroid-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389164]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.