Skin Cancer Screening (PDQ®)–Patient Version
What Is Screening?
Screening is looking for cancer before a person has any symptoms. This can help find cancer at an early stage. When abnormal tissue or cancer is found early, it may be easier to treat. By the time symptoms appear, cancer may have begun to spread.
Scientists are trying to better understand which people are more likely to get certain types of cancer. They also study the things we do and the things around us to see if they cause cancer. This information helps doctors recommend who should be screened for cancer, which screening tests should be used, and how often the tests should be done.
It is important to remember that your doctor does not necessarily think you have cancer if he or she suggests a screening test. Screening tests are given when you have no cancer symptoms.
If a screening test result is abnormal, you may need to have more tests done to find out if you have cancer. These are called diagnostic tests.
General Information About Skin Cancer
Key Points
- Skin cancer is a disease in which malignant (cancer) cells form in the tissues of the skin.
- Skin cancer is the most common cancer in the United States.
- Different factors increase or decrease the risk of skin cancer.
Skin cancer is a disease in which malignant (cancer) cells form in the tissues of the skin.
The skin is the body's largest organ. It protects against heat, sunlight, injury, and infection. Skin also helps control body temperature and stores water, fat, and vitamin D. The skin has several layers, but the two main layers are the epidermis (top or outer layer) and the dermis (lower or inner layer). Skin cancer begins in the epidermis, which is made up of three kinds of cells:
- Squamous cells: Thin, flat cells that form the top layer of the epidermis. Cancer that forms in the skin's squamous cells is called squamous cell carcinoma of the skin.
- Basal cells: Round cells under the squamous cells. Cancer that forms in basal cells is called basal cell carcinoma.
- Melanocytes: Found in the lower part of the epidermis, these cells make melanin, the pigment that gives skin its natural color. When skin is exposed to the sun, melanocytes make more pigment and cause the skin to tan, or darken. Cancer that forms in melanocytes is called melanoma.
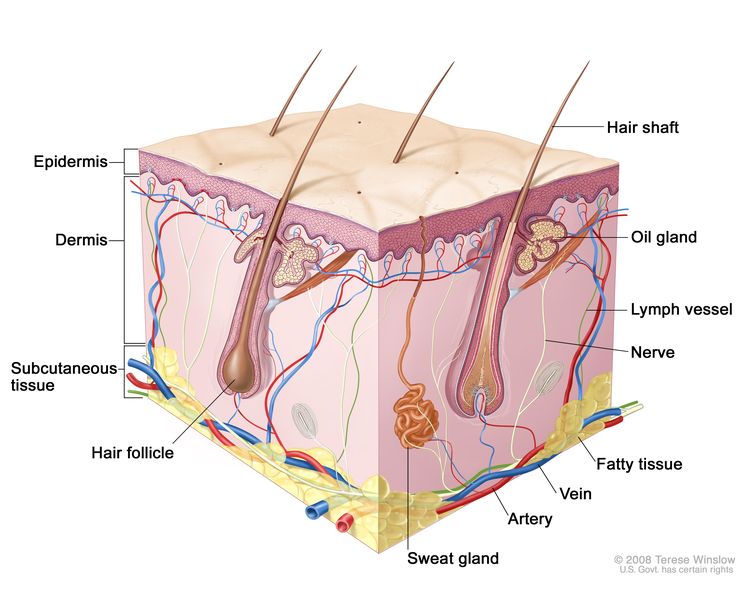
Skin cancer is the most common cancer in the United States.
There are two main types of skin cancer:
- nonmelanoma or keratinocyte carcinoma, which includes:
- squamous cell carcinoma (SCC)
- basal cell carcinoma
- melanoma
Basal cell carcinoma and squamous cell carcinoma of the skin, also called nonmelanoma skin cancer or keratinocyte carcinoma, are the most common forms of skin cancer. Most basal cell and squamous cell skin cancers can be cured.
Melanoma is more likely to spread to nearby tissues and other parts of the body and can be harder to cure. Melanoma is easier to cure if the tumor is found before it spreads to the dermis (inner layer of skin). Melanoma is less likely to cause death when it is found and treated early.
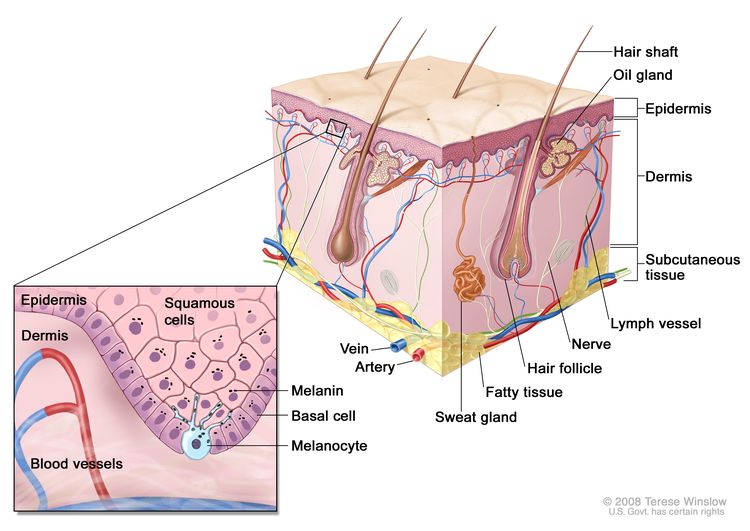
In the United States, about 3 million people are diagnosed with nonmelanoma skin cancer each year. Rates of nonmelanoma skin cancer have been increasing, possibly because greater public awareness has led to higher rates of screening exams, self-exams, and detection of these skin cancers.
Since the early 2000s, the rate of melanoma cases in adults younger than 50 years has held steady in women, but decreased by about 1% per year in men. In adults aged 50 years and older, the rate of melanoma cases has held steady in men, but increased by about 3% per year in women in recent years. From 2013 to 2022, the number of deaths from melanoma decreased by about 3% per year in men and 4% per year in women.
The rate of melanoma cases in children and adolescents increased until 2001. However, between 2001 and 2022, the yearly rates of melanoma in these age groups decreased slightly.
Other PDQ summaries containing information related to skin cancer include:
Different factors increase or decrease the risk of skin cancer.
Anything that increases your chance of getting a disease is called a risk factor. Anything that decreases your chance of getting a disease is called a protective factor.
For information about risk and protective factors for skin cancer, visit Skin Cancer Prevention.
Skin Cancer Screening
Key Points
- Tests are used to screen for different types of cancer when a person does not have symptoms.
- Screening for skin cancer may include examination by both the patient and the health care provider.
- Screening tests for skin cancer are being studied in clinical trials.
Tests are used to screen for different types of cancer when a person does not have symptoms.
Scientists study screening tests to find those with the fewest harms and most benefits. Cancer screening trials also are meant to show whether early detection (finding cancer before it causes symptoms) helps a person live longer or decreases a person's chance of dying from the disease. For some types of cancer, the chance of recovery is better if the disease is found and treated at an early stage. There is not enough evidence to know if screening the population for skin cancer lowers the rates of death from the disease.
Screening for skin cancer may include examination by both the patient and the health care provider.
A visual self-exam by the patient and a clinical examination by the health care provider may be used to screen for skin cancer.
During a skin exam a doctor or nurse checks the skin for moles, birthmarks, or other pigmented areas that look abnormal in color, size, shape, or texture. Skin exams to screen for skin cancer have not been shown to decrease the number of deaths from the disease.
Regular skin checks by a doctor are important for people who have already had skin cancer. If you are checking your skin and find a worrisome change, you should report it to your doctor.
If an area on the skin looks abnormal, a biopsy is usually done. The doctor will remove as much of the suspicious tissue as possible with a local excision. A pathologist then looks at the tissue under a microscope to check for cancer cells. Because it is sometimes difficult to tell if a skin growth is benign (not cancer) or malignant (cancer), you may want to have the biopsy sample checked by a second pathologist.
Most melanomas in the skin can be seen by the naked eye. Usually, melanoma grows for a long time under the top layer of skin (the epidermis) but does not grow into the deeper layer (the dermis). This allows time for skin cancer to be found early. Melanoma is easier to cure if it is found before it spreads.
Mobile apps that evaluate skin lesions to detect skin cancer and malignant melanoma have been developed. However, these apps require further study in large-scale testing programs to find out if they are accurate and useful for skin cancer screening.
Screening tests for skin cancer are being studied in clinical trials.
Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Risks of Skin Cancer Screening
Key Points
- Screening tests have risks.
- The risks of skin cancer screening tests include:
- Finding skin cancer does not always improve health or help you live longer.
- False-negative test results can occur.
- False-positive test results can occur.
- A biopsy may cause scarring.
Screening tests have risks.
Decisions about screening tests can be difficult. Not all screening tests are helpful and most have risks. Before having any screening test, you may want to discuss the test with your doctor. It is important to know the risks of the test and whether it has been proven to reduce the risk of dying from cancer.
The risks of skin cancer screening tests include:
Finding skin cancer does not always improve health or help you live longer.
Screening may not improve your health or help you live longer if you have advanced skin cancer.
Some cancers never cause symptoms or become life-threatening, but if found by a screening test, the cancer may be treated. Treatments for cancer may have serious side effects.
False-negative test results can occur.
Screening test results may appear to be normal even though cancer is present. A person who receives a false-negative test result (one that shows there is no cancer when there really is) may delay getting medical care even if there are symptoms.
False-positive test results can occur.
Screening test results may appear to be abnormal even though no cancer is present. A false-positive test result (one that shows there is cancer when there really isn't) can cause anxiety and is usually followed by more tests (such as a biopsy), which also have risks.
A biopsy may cause scarring.
When a skin biopsy is done, the doctor will try to leave the smallest scar possible, but there is a risk of scarring and infection.
Talk to your doctor about your risk for skin cancer and your need for screening tests.
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about skin cancer screening. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Screening and Prevention Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Skin Cancer Screening. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/skin/patient/skin-screening-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389182]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.