Breast Cancer Treatment (PDQ®)–Health Professional Version
General Information About Breast Cancer
Incidence and Mortality
Estimated new cases and deaths from breast cancer (women only) in the United States in 2025:[1]
- New cases: 316,950.
- Deaths: 42,170.
Breast cancer is the most common noncutaneous cancer in U.S. women, with an estimated 59,080 cases of female breast ductal carcinoma in situ (DCIS) and 316,950 cases of invasive disease in 2025.[1] About 42,170 women diagnosed with breast cancer—fewer than one in eight—will die of the disease. By comparison, about 60,540 American women will die of lung cancer in 2025.[1] Men account for 1% of breast cancer cases and breast cancer deaths. For more information, see the Special Populations section in Breast Cancer Screening.
Widespread adoption of screening increases breast cancer incidence in a given population and changes the characteristics of cancers detected, with increased incidence of lower-risk cancers, premalignant lesions, and DCIS. For more information, see the Ductal carcinoma in situ (DCIS) section in Breast Cancer Screening. Population studies from the United States [2] and the United Kingdom [3] demonstrate an increase in DCIS and invasive breast cancer incidence since the 1970s, attributable to the widespread adoption of both postmenopausal hormone therapy and screening mammography. In the last decade, women have refrained from using postmenopausal hormones, and breast cancer incidence has declined, but not to the levels seen before the widespread use of screening mammography.[4]
Anatomy
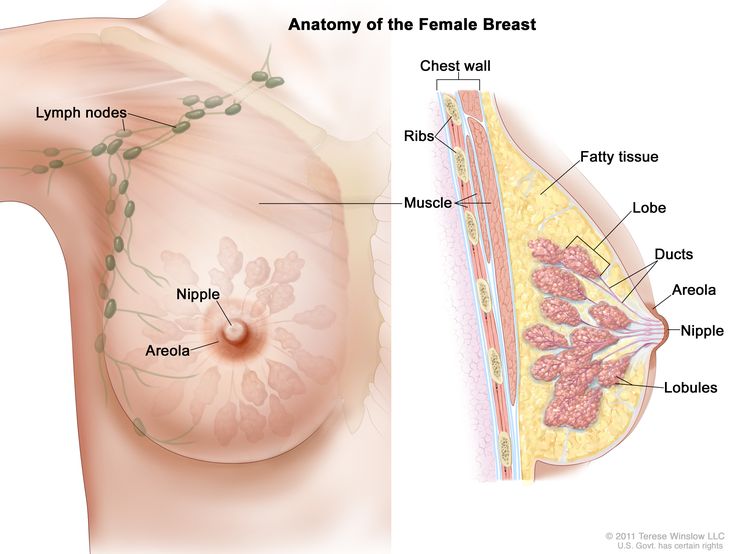
Risk Factors
Increasing age is the most important risk factor for most cancers. Other risk factors for breast cancer include:
- Family health history.[5]
- Major inheritance susceptibility.[6,7]
- Alcohol intake.
- Breast tissue density (mammographic).[10]
- Estrogen (endogenous).[11-13]
- Hormone therapy history.
- Combination estrogen plus progestin hormone replacement therapy.
- Obesity (postmenopausal).[16]
- Personal history of breast cancer.[17]
- Personal history of benign proliferative breast disease.[18-20]
- Radiation exposure to breast/chest.[21]
Age-specific risk estimates are available to help design screening strategies for women with and without a family history of breast cancer. The most commonly used tools include the Gail model and the IBIS/Tyrer-Cuzick model, version 8 (which incorporates family history to a greater extent than the Gail model, as well as breast density).[22]
Of all women with breast cancer, 5% to 10% may have a germline pathogenic variant in the BRCA1 and BRCA2 genes.[23] Specific BRCA1 and BRCA2 variants are more common in women of Jewish ancestry.[24] In women with BRCA1 and BRCA2 pathogenic variants, the estimated lifetime risk of developing breast cancer is 40% to 85%. BRCA1 and BRCA2 carriers with a history of breast cancer have increased risk of contralateral disease that may be as high as 5% per year.[25] Men with BRCA1 and BRCA2 pathogenic variants also have increased breast cancer risk.[26]
BRCA1 and BRCA2 pathogenic variants also increase risk of ovarian cancer [26,27] and other primary cancers.[26,27] Once a BRCA1 or BRCA2 variant has been identified, other family members can be referred for genetic counseling and testing.[28-31]
For more information, see Genetics of Breast and Gynecologic Cancers, Breast Cancer Prevention, and Breast Cancer Screening.
Protective Factors
The following protective factors and interventions reduce the risk of female breast cancer:
For more information about factors that decrease the risk of breast cancer, see Breast Cancer Prevention.
Screening
Clinical trials have established that screening asymptomatic women using mammography, with or without clinical breast examination, decreases breast cancer mortality. For more information, see Breast Cancer Screening.
Diagnosis
Patient evaluation
When breast cancer is suspected, patient management generally includes:
- Confirmation of the diagnosis.
- Evaluation of the stage of disease.
- Selection of therapy.
The following tests and procedures are used to diagnose breast cancer:
- Mammography.
- Ultrasonography.
- Breast magnetic resonance imaging (MRI), if clinically indicated.
- Biopsy.
Contralateral disease
Pathologically, breast cancer can be a multicentric and bilateral disease. Synchronous bilateral disease is somewhat more common in patients with infiltrating lobular carcinoma. At 10 years after diagnosis, the risk of a primary breast cancer in the contralateral breast ranges from 3% to 5%, although endocrine therapy decreases that risk.[50-53] The development of a contralateral breast cancer is associated with an increased risk of distant recurrence.[54] When patients with BRCA1 or BRCA2 pathogenic variants were diagnosed before age 40 years, the risk of a contralateral breast cancer reached nearly 50% in the ensuing 25 years.[55,56]
Patients who have breast cancer will undergo bilateral mammography at the time of diagnosis to rule out synchronous disease. To detect either recurrence in the ipsilateral breast in patients treated with breast-conserving surgery or a second primary cancer in the contralateral breast, patients will continue to have regular breast physical examinations and mammograms.
The role of MRI in screening the contralateral breast and monitoring women treated with breast-conserving therapy continues to evolve. Because an increased detection rate of mammographically occult disease has been demonstrated, the selective use of MRI for additional screening is occurring more frequently despite the absence of randomized, controlled data. Because only 25% of MRI-positive findings represent malignancy, pathological confirmation before treatment is recommended. Whether this increased detection rate will translate into improved treatment outcome is unknown.[57-59]
Prognostic and Predictive Factors
Breast cancer is commonly treated by various combinations of surgery, radiation therapy, chemotherapy, and hormone therapy. Prognosis and selection of therapy may be influenced by the following clinical and pathological features (based on conventional histology and immunohistochemistry):[60]
- Menopausal status of the patient.
- Stage of the disease.
- Grade of the primary tumor.
- Estrogen receptor (ER) and progesterone receptor (PR) status of the tumor.
- Human epidermal growth factor type 2 receptor (HER2) overexpression and/or amplification.
- Histological type. Breast cancer is classified into a variety of histological types, some of which have prognostic importance. Favorable histological types include mucinous, medullary, and tubular carcinomas.[61-63] Histological type can impact the treatment approach, including locoregional management decision-making. For more information about lobular carcinoma, see the Histopathological Classification of Breast Cancer section.
The use of molecular profiling in breast cancer includes:[64]
- ER and PR status testing.
- HER2 receptor status testing.
- Gene profile testing by microarray assay or reverse transcription-polymerase chain reaction (e.g., MammaPrint, Oncotype DX, Breast Cancer Index [BCI]).
On the basis of ER, PR, and HER2 results, breast cancer is classified as one of the following types:
- Hormone receptor positive.
- HER2 positive.
- Triple negative (ER, PR, and HER2 negative).
ER, PR, and HER2 status are important in determining prognosis and in predicting response to endocrine and HER2-directed therapy. The American Society of Clinical Oncology/College of American Pathologists consensus panel has published guidelines to help standardize the performance, interpretation, and reporting of assays used to assess the ER-PR status by immunohistochemistry and HER2 status by immunohistochemistry and in situ hybridization.[65,66]
Gene profile tests include:
- MammaPrint: The first gene profile test to be approved by the U.S. Food and Drug Administration was the MammaPrint gene signature. The 70-gene signature classifies tumors into high- and low-risk prognostic categories.[67-71] The aim of the MINDACT trial (NCT00433589) (see below) was to determine the clinical usefulness and patient benefit of adjuvant chemotherapy.
- Oncotype DX: The Oncotype DX 21 gene assay is the gene profile test with the most extensive clinical validation thus far and applies to HER2-negative hormone receptor–positive breast cancer. A 21-gene recurrence score is generated based on the level of expression of each of the 21 genes. This recurrence score informs prognosis and treatment decision-making.
In the node-negative population, the designated risk groups are as follows:
- Recurrence score ≤11: low risk. Chemotherapy is not indicated for this group.
- Recurrence score >11 and ≤25: intermediate risk. Chemotherapy decision-making is complex and personalized for this group. Patient age ( ≤50 vs. >50 years), clinicopathological features, patient preference, and more are incorporated into this decision.
- Recurrence score >25: high risk. Chemotherapy is indicated for this group.
In the postmenopausal node-positive population, the designated risk groups are as follows:
- Recurrence score ≤25: low risk. Chemotherapy is not indicated for this group.
- Recurrence score >25: high risk. Chemotherapy is indicated for this group.
- BCI: The BCI is a combination of two profiles, the HOXB13/IL17BR expression ratio (H/I ratio) and the Molecular Grade Index. It has been both prognostic and predictive in patients with hormone receptor–positive breast cancer.
The following trials describe the prognostic and predictive value of multigene assays in early breast cancer:
- The prognostic ability of the Oncotype DX 21-gene assay was assessed in two randomized trials.
- The National Surgical Adjuvant Breast and Bowel Project (NSABP B-14) trial randomly assigned patients to receive tamoxifen or placebo; the results favoring tamoxifen changed clinical practice in the late 1980s.[72] Formalin-fixed, paraffin-embedded tissue was available for 668 patients. The 10-year distant recurrence risk for patients treated with tamoxifen was 7% for those with a low recurrence score (defined in this trial as <18), 14% for those with an intermediate recurrence score (defined in this trial as 18–30), and 31% for those with high recurrence score (defined in this trial as ≥31) (P < .001).[73]
- A community-based, case-control study examined the prognostic ability of the recurrence score to predict breast cancer deaths after 10 years in a group of tamoxifen-treated patients and observed a similar prognostic pattern to that seen in patients from NSABP B-14.[74]
- The use of Oncotype Dx to predict benefit from chemotherapy in patients with node-negative, ER-positive breast cancer was initially assessed in a prospective-retrospective way using the tamoxifen alone (n = 227) and the combination arms (n = 424) of the NSABP B-20 trial.[72] Patients in the NSABP B-20 trial were randomly assigned to receive tamoxifen alone or tamoxifen concurrently with methotrexate and fluorouracil (MF) or cyclophosphamide with MF.[75]
- The 10-year distant disease-free survival (DFS) improved from 60% to 88% by adding chemotherapy to tamoxifen in the high-risk group (defined in this trial as ≥31), while no benefit was observed in the low recurrence score group.[76]
- Similar findings were reported in the prospective-retrospective evaluation of the SWOG-8814 trial (NCT00929591) in hormone receptor–positive, lymph node–positive, postmenopausal patients treated with tamoxifen with or without cyclophosphamide, doxorubicin, and fluorouracil.[77] However, the sample size in this analysis was small, follow-up was only 5 years, and the prognostic impact of having positive nodes needs to be taken into consideration.
- Of note, both analyses (NSABP B-20 and S8814) were underpowered for any conclusive predictive analysis among patients identified as having an intermediate recurrence score.
- Results from the prospective, randomized TAILORx trial (NCT00310180) indicate that chemotherapy is unlikely to provide substantial benefit to patients older than 50 years with ER-PR–positive and node-negative disease and a recurrence score of 11 to 25.[78] In this study, a low-risk score was defined as less than 11, an intermediate score was 11 to 25, and a high-risk score was greater than 25. These cut points differ from those described above.
Patients in this study with a low-risk score were found to have very low rates of recurrence at 5 years with endocrine therapy.[79]
- The invasive DFS (IDFS) rate was 93.8% at 5 years and 84.0% at 9 years.
- The rate of freedom from recurrence of breast cancer at a distant site was 99.3% at 5 years and 96.8% at 9 years.
- The rate of freedom from recurrence of breast cancer at a distant or local-regional site was 98.7% at 5 years and 95.0% at 9 years.
- The overall survival (OS) rate was 98.0% at 5 years and 93.7% at 9 years.
In the middle-risk group in the TAILORx study (recurrence score, 11–25), 6,907 women were randomly assigned to endocrine therapy alone or endocrine therapy plus chemotherapy.[78] Of these, 3,399 women on the endocrine therapy-alone arm and 3,312 women on the endocrine therapy-plus-chemotherapy arm were available for an analysis according to the randomized treatment assignments. After a median follow-up of 90 months, the difference in IDFS, the main study end point, met the prespecified noninferiority criterion (P > .10 for a test of no difference after 835 events had occurred) suggesting the noninferiority of endocrine therapy compared with endocrine therapy plus chemotherapy.
- In this population, the 9-year IDFS rate was 83.3% for endocrine therapy alone and 84.3% for endocrine therapy plus chemotherapy (hazard ratio [HR], 1.08; 95% confidence interval [CI], 0.94–1.24; P = .26).[78][Level of evidence B1]
- One hundred eighty-five patients in the endocrine-only arm received chemotherapy, and 608 patients in the endocrine therapy-plus-chemotherapy arm did not receive their assigned chemotherapy. In an analysis based on the actual treatment received, the HR for IDFS was 1.14 (95% CI, 0.99–1.31; P = .06).
- Outcomes for the other end points examined (freedom of distant breast cancer recurrence, freedom from local and distant recurrence, and OS) were similar between the two treatment arms and none were significant at P < .10.
- There was a significant interaction between treatment assignment and age (P = .03) with respect to IDFS, suggesting that chemotherapy might be beneficial in women younger than 50 years with recurrence scores ranging from 11 to 25.
- A secondary analysis of TAILORx demonstrated that integration of clinical risk (assessed by tumor size and grade) adds prognostic information to the recurrence score in women with a recurrence score of at least 11; however, clinical risk was not predictive of a chemotherapy benefit.[80] This secondary analysis further explored the interaction between age and chemotherapy benefit. Among women aged 50 years or younger, rates of distant recurrence were lower with chemotherapy for patients with recurrence scores of 16 to 20 and high clinical risk. Rates were also lower for patients with recurrence scores of 21 to 25, regardless of clinical risk.
- Most women received tamoxifen as their endocrine therapy. It is not certain if any of the observed benefits of chemotherapy are attributable to ovarian function suppression and if they could be achieved through endocrine therapy.
- The MINDACT trial (NCT00433589) tested whether adding MammaPrint genomic risk to a clinical-risk classification (modified from Adjuvant! Online) might guide more appropriate choices of chemotherapy in women with node negative- or 1-to-3 node-positive disease.[81][Level of evidence C2] Unlike the TAILORx study, which only had hormone receptor–positive patients, this trial included hormone receptor–negative patients. In this prospective study, women with both genomic and clinical high-risk classification received chemotherapy, while those with both genomic and clinical low-risk classification did not receive chemotherapy. Participants with discordant results (clinical high-risk- with genomic low-risk classification, or clinical low-risk- with genomic high-risk classification) were randomly assigned to receive or not receive chemotherapy. A total of 1,550 women with high clinical risk and low genomic risk, and 592 women with low clinical risk and high genomic risk, were randomly assigned to receive or not receive chemotherapy. The primary goal of the study was to determine whether patients with high clinical risk, but low genomic risk, who did not receive chemotherapy had a 5-year survival rate without distant metastases (primary study end point) of 92% or lower (a noninferiority design).
- This end point was met because the observed rate in the group was 94.7% (95% CI, 92.5%–96.2%). However, among patients with high clinical risk but low genomic risk, the rate of 5-year survival without distant metastases was 1.5% higher in the arm that did receive chemotherapy than in the arm that did not receive chemotherapy, although the study was not powered to detect a difference between these arms (HR chemotherapy vs. no chemotherapy, 0.78; 95% CI, 0.50–1.21; P = .27)
- Patients in the low clinical risk group with high genomic risk did well, and there was little evidence of benefit from chemotherapy in this group (5-year survival without distant metastases, 95.8% with chemotherapy vs. 95.0% without; HR, 1.17; 95% CI, 0.59–2.28; P = .66).
- The RxPONDER trial (NCT01272037) included 3,350 postmenopausal and 1,665 premenopausal women with HER2-negative hormone receptor–positive breast cancer who had a recurrence score of 25 or less. Patients were randomly assigned to receive either endocrine therapy alone or endocrine therapy plus chemotherapy. Results have been reported in abstract form; the primary study end point was IDFS. Because a prespecified test for interaction between treatment assignment and menopausal status was significant (P = .004), the premenopausal and postmenopausal groups were analyzed separately.[82]
- In postmenopausal patients, there was no evidence of a benefit with the addition of chemotherapy (HR for endocrine therapy plus chemotherapy vs. endocrine therapy, 0.97; 95% CI, 0.78–1.22; 5-year IDFS rate, 91.6% vs. 91.9%; P = .82).[82][Level of evidence B1]
- In premenopausal patients, however, there was evidence of a benefit from the addition of chemotherapy to endocrine therapy (HR, 0.54; 95% CI, 0.38–0.76; 5-year IDFS rate, 94.2% vs. 89.0%; P = .0004). OS was also significantly improved in patients who received endocrine therapy plus chemotherapy (HR, 0.47; 95% CI, 0.24–0.94; P = .032).[Level of evidence A1]
- The West German Study Group Plan B trial (NCT01049425) compared two chemotherapy regimens in patients with node-positive (pN1) or high-risk node-negative disease. Chemotherapy was not offered to patients with recurrence scores below 12, but they were followed. For a full description of the chemotherapy regimens, see Postoperative systemic therapy for HER2-negative hormone receptor–positive breast cancer.
- The 5-year DFS rates were very high in the 348 patients who did not receive chemotherapy and did not differ between node-negative patients (94.5%) and pN1 patients (94.9%).[83][Level of evidence C1]
- The prognostic ability of the BCI has been described in multiple trials.[84-86]
- The H/I expression ratio has been shown to predict DFS in patients with tamoxifen-treated, hormone receptor–positive, node-negative breast cancer.[84]
- The prognostic ability of the H/I ratio regarding late recurrence and treatment benefit was evaluated in the MA.17 trial. A high H/I ratio was statistically significantly associated with a decrease in late recurrence in patients who received extended letrozole therapy compared with those who did not (odds ratio, 0.35; 95% CI, 0.16–0.75; P = .007).[85]
- In a secondary analysis of the ATAC trial, the BCI was prognostic in patients with node-negative breast cancer for both early (years 0–5) and late (years 5–10) distant recurrence. For patients with stage I HER2-negative hormone receptor–positive tumors, a high H/I ratio predicted significant rates of late distant recurrence.[86]
- The BCI has also been evaluated for predictive capability.[87]
- The BCI H/I ratio was evaluated for its ability to predict benefit from extended endocrine therapy in patients who participated in the aTTom trial (NCT00003678). A BCI H/I-high designation was predictive of endocrine response. A subset of patients with hormone receptor–positive, node-positive disease had significant benefit from 10 years (versus 5 years) of tamoxifen therapy. Patients with a BCI H/I-low designation showed no significant benefit from extended endocrine therapy.[87]
- The BCI H/I ratio was evaluated as a predictive biomarker of extended endocrine therapy benefit in patients from the IDEAL trial. Tumor specimens from 908 patients randomly assigned to receive 2.5 years versus 5 years of extended letrozole were evaluated using the BCI. A BCI H/I-high designation significantly predicted benefit from extended aromatase inhibitor therapy, whereas patients with a BCI H/I-low designation did not derive significant benefit.[88]
Many other gene-based assays may guide treatment decisions in patients with early breast cancer (e.g., Predictor Analysis of Microarray 50 [PAM50] Risk of Recurrence score, EndoPredict).
Although certain rare inherited variants (like BRCA1 and BRCA2 variants) predispose women to breast cancer, prognostic data on BRCA1/BRCA2 carriers who developed breast cancer are conflicting. These women are at greater risk of developing contralateral breast cancer. For more information, see the Female Breast Cancer Risks section in BRCA1 and BRCA2: Cancer Risks and Management.
Posttherapy Considerations
Hormone replacement therapy
After careful consideration, certain patients with severe symptoms may be treated with hormone replacement therapy. For more information, see the Hormone Replacement Therapy section in Hot Flashes and Night Sweats and Breast Cancer Prevention.
References
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Altekruse SF, Kosary CL, Krapcho M, et al.: SEER Cancer Statistics Review, 1975-2007. National Cancer Institute, 2010. Also available online. Last accessed April 24, 2025.
- Johnson A, Shekhdar J: Breast cancer incidence: what do the figures mean? J Eval Clin Pract 11 (1): 27-31, 2005. [PUBMED Abstract]
- Haas JS, Kaplan CP, Gerstenberger EP, et al.: Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 140 (3): 184-8, 2004. [PUBMED Abstract]
- Colditz GA, Kaphingst KA, Hankinson SE, et al.: Family history and risk of breast cancer: nurses' health study. Breast Cancer Res Treat 133 (3): 1097-104, 2012. [PUBMED Abstract]
- Malone KE, Daling JR, Doody DR, et al.: Family history of breast cancer in relation to tumor characteristics and mortality in a population-based study of young women with invasive breast cancer. Cancer Epidemiol Biomarkers Prev 20 (12): 2560-71, 2011. [PUBMED Abstract]
- Cybulski C, Wokołorczyk D, Jakubowska A, et al.: Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol 29 (28): 3747-52, 2011. [PUBMED Abstract]
- Goodwin PJ, Phillips KA, West DW, et al.: Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol 30 (1): 19-26, 2012. [PUBMED Abstract]
- Mavaddat N, Barrowdale D, Andrulis IL, et al.: Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 21 (1): 134-47, 2012. [PUBMED Abstract]
- Razzaghi H, Troester MA, Gierach GL, et al.: Mammographic density and breast cancer risk in White and African American Women. Breast Cancer Res Treat 135 (2): 571-80, 2012. [PUBMED Abstract]
- Key TJ, Appleby PN, Reeves GK, et al.: Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer 105 (5): 709-22, 2011. [PUBMED Abstract]
- Kaaks R, Rinaldi S, Key TJ, et al.: Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer 12 (4): 1071-82, 2005. [PUBMED Abstract]
- Kaaks R, Berrino F, Key T, et al.: Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 97 (10): 755-65, 2005. [PUBMED Abstract]
- Collaborative Group on Hormonal Factors in Breast Cancer: Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 13 (11): 1141-51, 2012. [PUBMED Abstract]
- Ritte R, Lukanova A, Tjønneland A, et al.: Height, age at menarche and risk of hormone receptor-positive and -negative breast cancer: a cohort study. Int J Cancer 132 (11): 2619-29, 2013. [PUBMED Abstract]
- Wolin KY, Carson K, Colditz GA: Obesity and cancer. Oncologist 15 (6): 556-65, 2010. [PUBMED Abstract]
- Kotsopoulos J, Chen WY, Gates MA, et al.: Risk factors for ductal and lobular breast cancer: results from the nurses' health study. Breast Cancer Res 12 (6): R106, 2010. [PUBMED Abstract]
- Goldacre MJ, Abisgold JD, Yeates DG, et al.: Benign breast disease and subsequent breast cancer: English record linkage studies. J Public Health (Oxf) 32 (4): 565-71, 2010. [PUBMED Abstract]
- Kabat GC, Jones JG, Olson N, et al.: A multi-center prospective cohort study of benign breast disease and risk of subsequent breast cancer. Cancer Causes Control 21 (6): 821-8, 2010. [PUBMED Abstract]
- Worsham MJ, Raju U, Lu M, et al.: Risk factors for breast cancer from benign breast disease in a diverse population. Breast Cancer Res Treat 118 (1): 1-7, 2009. [PUBMED Abstract]
- Travis LB, Hill DA, Dores GM, et al.: Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA 290 (4): 465-75, 2003. [PUBMED Abstract]
- Tyrer J, Duffy SW, Cuzick J: A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23 (7): 1111-30, 2004. [PUBMED Abstract]
- Blackwood MA, Weber BL: BRCA1 and BRCA2: from molecular genetics to clinical medicine. J Clin Oncol 16 (5): 1969-77, 1998. [PUBMED Abstract]
- Offit K, Gilewski T, McGuire P, et al.: Germline BRCA1 185delAG mutations in Jewish women with breast cancer. Lancet 347 (9016): 1643-5, 1996. [PUBMED Abstract]
- Frank TS, Manley SA, Olopade OI, et al.: Sequence analysis of BRCA1 and BRCA2: correlation of mutations with family history and ovarian cancer risk. J Clin Oncol 16 (7): 2417-25, 1998. [PUBMED Abstract]
- Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium. J Natl Cancer Inst 91 (15): 1310-6, 1999. [PUBMED Abstract]
- Ford D, Easton DF, Bishop DT, et al.: Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 343 (8899): 692-5, 1994. [PUBMED Abstract]
- Biesecker BB, Boehnke M, Calzone K, et al.: Genetic counseling for families with inherited susceptibility to breast and ovarian cancer. JAMA 269 (15): 1970-4, 1993. [PUBMED Abstract]
- Berry DA, Parmigiani G, Sanchez J, et al.: Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. J Natl Cancer Inst 89 (3): 227-38, 1997. [PUBMED Abstract]
- Hoskins KF, Stopfer JE, Calzone KA, et al.: Assessment and counseling for women with a family history of breast cancer. A guide for clinicians. JAMA 273 (7): 577-85, 1995. [PUBMED Abstract]
- Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility, Adopted on February 20, 1996. J Clin Oncol 14 (5): 1730-6; discussion 1737-40, 1996. [PUBMED Abstract]
- Anderson GL, Limacher M, Assaf AR, et al.: Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291 (14): 1701-12, 2004. [PUBMED Abstract]
- LaCroix AZ, Chlebowski RT, Manson JE, et al.: Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA 305 (13): 1305-14, 2011. [PUBMED Abstract]
- Anderson GL, Chlebowski RT, Aragaki AK, et al.: Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol 13 (5): 476-86, 2012. [PUBMED Abstract]
- Bernstein L, Henderson BE, Hanisch R, et al.: Physical exercise and reduced risk of breast cancer in young women. J Natl Cancer Inst 86 (18): 1403-8, 1994. [PUBMED Abstract]
- Thune I, Brenn T, Lund E, et al.: Physical activity and the risk of breast cancer. N Engl J Med 336 (18): 1269-75, 1997. [PUBMED Abstract]
- Adams-Campbell LL, Rosenberg L, Rao RS, et al.: Strenuous physical activity and breast cancer risk in African-American women. J Natl Med Assoc 93 (7-8): 267-75, 2001 Jul-Aug. [PUBMED Abstract]
- Kampert JB, Whittemore AS, Paffenbarger RS: Combined effect of childbearing, menstrual events, and body size on age-specific breast cancer risk. Am J Epidemiol 128 (5): 962-79, 1988. [PUBMED Abstract]
- Pike MC, Krailo MD, Henderson BE, et al.: 'Hormonal' risk factors, 'breast tissue age' and the age-incidence of breast cancer. Nature 303 (5920): 767-70, 1983. [PUBMED Abstract]
- Lambe M, Hsieh C, Trichopoulos D, et al.: Transient increase in the risk of breast cancer after giving birth. N Engl J Med 331 (1): 5-9, 1994. [PUBMED Abstract]
- Col: Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 360 (9328): 187-95, 2002. [PUBMED Abstract]
- Cuzick J, Sestak I, Bonanni B, et al.: Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 381 (9880): 1827-34, 2013. [PUBMED Abstract]
- Goss PE, Ingle JN, Alés-Martínez JE, et al.: Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364 (25): 2381-91, 2011. [PUBMED Abstract]
- Cuzick J, Sestak I, Forbes JF, et al.: Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 383 (9922): 1041-8, 2014. [PUBMED Abstract]
- Hartmann LC, Schaid DJ, Woods JE, et al.: Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 340 (2): 77-84, 1999. [PUBMED Abstract]
- Rebbeck TR, Levin AM, Eisen A, et al.: Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst 91 (17): 1475-9, 1999. [PUBMED Abstract]
- Kauff ND, Satagopan JM, Robson ME, et al.: Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 346 (21): 1609-15, 2002. [PUBMED Abstract]
- Rebbeck TR, Lynch HT, Neuhausen SL, et al.: Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 346 (21): 1616-22, 2002. [PUBMED Abstract]
- Kauff ND, Domchek SM, Friebel TM, et al.: Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol 26 (8): 1331-7, 2008. [PUBMED Abstract]
- Rosen PP, Groshen S, Kinne DW, et al.: Factors influencing prognosis in node-negative breast carcinoma: analysis of 767 T1N0M0/T2N0M0 patients with long-term follow-up. J Clin Oncol 11 (11): 2090-100, 1993. [PUBMED Abstract]
- Abbott A, Rueth N, Pappas-Varco S, et al.: Perceptions of contralateral breast cancer: an overestimation of risk. Ann Surg Oncol 18 (11): 3129-36, 2011. [PUBMED Abstract]
- Nichols HB, Berrington de González A, Lacey JV, et al.: Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol 29 (12): 1564-9, 2011. [PUBMED Abstract]
- Giannakeas V, Lim DW, Narod SA: The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer 125 (4): 601-610, 2021. [PUBMED Abstract]
- Heron DE, Komarnicky LT, Hyslop T, et al.: Bilateral breast carcinoma: risk factors and outcomes for patients with synchronous and metachronous disease. Cancer 88 (12): 2739-50, 2000. [PUBMED Abstract]
- Graeser MK, Engel C, Rhiem K, et al.: Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 27 (35): 5887-92, 2009. [PUBMED Abstract]
- Garber JE, Golshan M: Contralateral breast cancer in BRCA1/BRCA2 mutation carriers: the story of the other side. J Clin Oncol 27 (35): 5862-4, 2009. [PUBMED Abstract]
- Lehman CD, Gatsonis C, Kuhl CK, et al.: MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 356 (13): 1295-303, 2007. [PUBMED Abstract]
- Solin LJ, Orel SG, Hwang WT, et al.: Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol 26 (3): 386-91, 2008. [PUBMED Abstract]
- Morrow M: Magnetic resonance imaging in the breast cancer patient: curb your enthusiasm. J Clin Oncol 26 (3): 352-3, 2008. [PUBMED Abstract]
- Simpson JF, Gray R, Dressler LG, et al.: Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern Cooperative Oncology Group Companion Study, EST 4189. J Clin Oncol 18 (10): 2059-69, 2000. [PUBMED Abstract]
- Rosen PP, Groshen S, Kinne DW: Prognosis in T2N0M0 stage I breast carcinoma: a 20-year follow-up study. J Clin Oncol 9 (9): 1650-61, 1991. [PUBMED Abstract]
- Diab SG, Clark GM, Osborne CK, et al.: Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol 17 (5): 1442-8, 1999. [PUBMED Abstract]
- Rakha EA, Lee AH, Evans AJ, et al.: Tubular carcinoma of the breast: further evidence to support its excellent prognosis. J Clin Oncol 28 (1): 99-104, 2010. [PUBMED Abstract]
- Sørlie T, Perou CM, Tibshirani R, et al.: Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98 (19): 10869-74, 2001. [PUBMED Abstract]
- Wolff AC, Somerfield MR, Dowsett M, et al.: Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J Clin Oncol 41 (22): 3867-3872, 2023. [PUBMED Abstract]
- Allison KH, Hammond MEH, Dowsett M, et al.: Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol 38 (12): 1346-1366, 2020. [PUBMED Abstract]
- Buyse M, Loi S, van't Veer L, et al.: Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 98 (17): 1183-92, 2006. [PUBMED Abstract]
- Wittner BS, Sgroi DC, Ryan PD, et al.: Analysis of the MammaPrint breast cancer assay in a predominantly postmenopausal cohort. Clin Cancer Res 14 (10): 2988-93, 2008. [PUBMED Abstract]
- Mook S, Knauer M, Bueno-de-Mesquita JM, et al.: Metastatic potential of T1 breast cancer can be predicted by the 70-gene MammaPrint signature. Ann Surg Oncol 17 (5): 1406-13, 2010. [PUBMED Abstract]
- Ishitobi M, Goranova TE, Komoike Y, et al.: Clinical utility of the 70-gene MammaPrint profile in a Japanese population. Jpn J Clin Oncol 40 (6): 508-12, 2010. [PUBMED Abstract]
- Knauer M, Mook S, Rutgers EJ, et al.: The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 120 (3): 655-61, 2010. [PUBMED Abstract]
- Fisher B, Jeong JH, Bryant J, et al.: Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 364 (9437): 858-68, 2004. [PUBMED Abstract]
- Paik S, Shak S, Tang G, et al.: A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351 (27): 2817-26, 2004. [PUBMED Abstract]
- Habel LA, Shak S, Jacobs MK, et al.: A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 8 (3): R25, 2006. [PUBMED Abstract]
- Mamounas EP, Tang G, Fisher B, et al.: Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 28 (10): 1677-83, 2010. [PUBMED Abstract]
- Paik S, Tang G, Shak S, et al.: Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24 (23): 3726-34, 2006. [PUBMED Abstract]
- Albain KS, Barlow WE, Shak S, et al.: Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11 (1): 55-65, 2010. [PUBMED Abstract]
- Sparano JA, Gray RJ, Makower DF, et al.: Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 379 (2): 111-121, 2018. [PUBMED Abstract]
- Sparano JA, Gray RJ, Makower DF, et al.: Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 373 (21): 2005-14, 2015. [PUBMED Abstract]
- Sparano JA, Gray RJ, Ravdin PM, et al.: Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N Engl J Med 380 (25): 2395-2405, 2019. [PUBMED Abstract]
- Cardoso F, van't Veer LJ, Bogaerts J, et al.: 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med 375 (8): 717-29, 2016. [PUBMED Abstract]
- Kalinsky K, Barlow WE, Meric-Bernstam F, et al.: Abstract GS3-00: first results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET) +/- chemotherapy (CT) in patients (pts) with 1-3 positive nodes, hormone receptor-positive (HR+) and HER2-negative (HER2-) breast cancer (BC) with recurrence score (RS) < 25: SWOG S1007 (RxPonder). [Abstract] Cancer Res 81 (4): A-GS3-00, 2021. Also available online. Last accessed April 24, 2025.
- Nitz U, Gluz O, Clemens M, et al.: West German Study PlanB Trial: Adjuvant Four Cycles of Epirubicin and Cyclophosphamide Plus Docetaxel Versus Six Cycles of Docetaxel and Cyclophosphamide in HER2-Negative Early Breast Cancer. J Clin Oncol 37 (10): 799-808, 2019. [PUBMED Abstract]
- Ma XJ, Wang Z, Ryan PD, et al.: A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 5 (6): 607-16, 2004. [PUBMED Abstract]
- Sgroi DC, Carney E, Zarrella E, et al.: Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 105 (14): 1036-42, 2013. [PUBMED Abstract]
- Sgroi DC, Sestak I, Cuzick J, et al.: Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 14 (11): 1067-1076, 2013. [PUBMED Abstract]
- Bartlett JMS, Sgroi DC, Treuner K, et al.: Breast Cancer Index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the Adjuvant Tamoxifen-To Offer More? (aTTom) trial. Ann Oncol 30 (11): 1776-1783, 2019. [PUBMED Abstract]
- Noordhoek I, Treuner K, Putter H, et al.: Breast Cancer Index Predicts Extended Endocrine Benefit to Individualize Selection of Patients with HR+ Early-stage Breast Cancer for 10 Years of Endocrine Therapy. Clin Cancer Res 27 (1): 311-319, 2021. [PUBMED Abstract]
Histopathological Classification of Breast Cancer
Table 1 describes the histological classification of breast cancer based on tumor location.[1] Infiltrating or invasive ductal cancer is the most common breast cancer histological type and comprises 70% to 80% of all cases.
| Tumor Location | Histological Subtype |
|---|---|
| NOS = not otherwise specified. | |
| Carcinoma, NOS | |
| Ductal | Intraductal (in situ) |
| Invasive with predominant component | |
| Invasive, NOS | |
| Comedo | |
| Inflammatory | |
| Medullary with lymphocytic infiltrate | |
| Mucinous (colloid) | |
| Papillary | |
| Scirrhous | |
| Tubular | |
| Other | |
| Lobular | Invasive with predominant in situ component |
| Invasive [2,3] | |
| Nipple | Paget disease, NOS |
| Paget disease with intraductal carcinoma | |
| Paget disease with invasive ductal carcinoma | |
| Other | Undifferentiated carcinoma |
| Metaplastic | |
Lobular carcinoma is the second most common breast cancer histological type, comprising 10% to 15% of all cases. Lobular carcinoma has characteristics that define a natural history distinct from that of ductal carcinoma (see Figure 2).
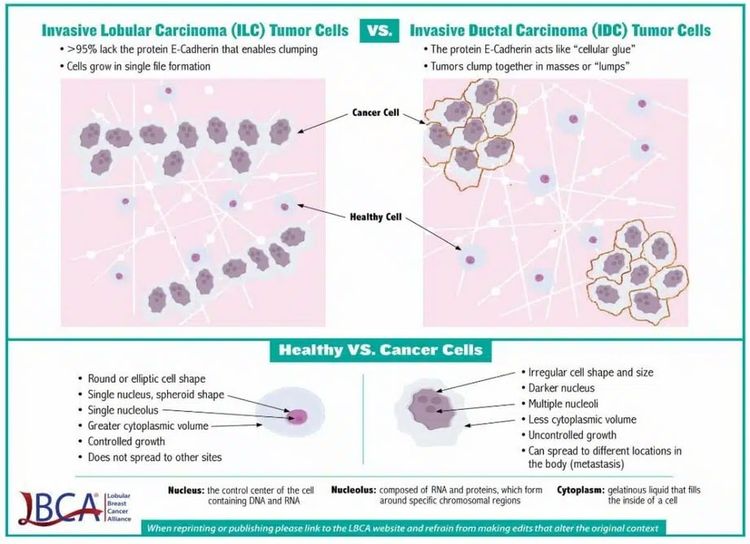
This cellular distinction leads to variation in imaging modality utility, pathological diagnostic criteria, metastatic pattern of spread, timing of metastatic presentation, and sensitivity to antineoplastic therapeutics. Lobular carcinoma characteristics include, but are not limited to, the following:[3]
- Absence of E-cadherin expression, which can lead to a more linear, rather than mass-like, growth pattern. This pattern can make mammography less sensitive and increases breast magnetic resonance imaging utility in assessing extent of disease in the breast.
- Less usual patterns of metastatic spread, including, but not limited to, pleural, gastrointestinal, genitourinary, and peritoneal metastatic involvement.
- Higher likelihood of estrogen-receptor expression.
- Lower sensitivity of positron emission tomography (PET) imaging for detection of disease. Compared with ductal carcinoma, lobular carcinoma has a lower level of fluorine F 18-fludeoxyglucose (18F-FDG) uptake on PET and is detected at a significantly lower sensitivity.[4-6]
- One series demonstrated a mean maximum standard uptake value of 18F-FDG in invasive lobular carcinoma (1.99 ± 1.72) that was significantly lower compared with invasive ductal carcinoma (3.91 ± 3.99) (P = .032).[4,5]
- In another series, the relative risk of PET-computed tomography revealing unsuspected distant metastases in patients with stage III invasive ductal carcinoma was 1.98 times (95% confidence interval, 0.98–3.98) that of patients with stage III invasive lobular carcinoma (P = .049).[6]
- More frequent diagnoses at later stages and a greater likelihood of lymph node involvement.
The following tumor subtypes occur in the breast but are not considered typical breast cancers:
References
- Breast. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. Springer, 2010, pp 347-76.
- Yeatman TJ, Cantor AB, Smith TJ, et al.: Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg 222 (4): 549-59; discussion 559-61, 1995. [PUBMED Abstract]
- Oesterreich S, Nasrazadani A, Zou J, et al.: Clinicopathological Features and Outcomes Comparing Patients With Invasive Ductal and Lobular Breast Cancer. J Natl Cancer Inst 114 (11): 1511-1522, 2022. [PUBMED Abstract]
- Fujii T, Yajima R, Kurozumi S, et al.: Clinical Significance of 18F-FDG-PET in Invasive Lobular Carcinoma. Anticancer Res 36 (10): 5481-5485, 2016. [PUBMED Abstract]
- Jung NY, Kim SH, Choi BB, et al.: Associations between the standardized uptake value of (18)F-FDG PET/CT and the prognostic factors of invasive lobular carcinoma: in comparison with invasive ductal carcinoma. World J Surg Oncol 13: 113, 2015. [PUBMED Abstract]
- Hogan MP, Goldman DA, Dashevsky B, et al.: Comparison of 18F-FDG PET/CT for Systemic Staging of Newly Diagnosed Invasive Lobular Carcinoma Versus Invasive Ductal Carcinoma. J Nucl Med 56 (11): 1674-80, 2015. [PUBMED Abstract]
- Chaney AW, Pollack A, McNeese MD, et al.: Primary treatment of cystosarcoma phyllodes of the breast. Cancer 89 (7): 1502-11, 2000. [PUBMED Abstract]
- Carter BA, Page DL: Phyllodes tumor of the breast: local recurrence versus metastatic capacity. Hum Pathol 35 (9): 1051-2, 2004. [PUBMED Abstract]
Stage Information for Breast Cancer
The American Joint Committee on Cancer (AJCC) staging system provides a strategy for grouping patients with respect to prognosis. Therapeutic decisions are formulated in part according to staging categories but also other clinical factors such as the following, some of which are included in the determination of stage:
- Tumor size.
- Lymph node status.
- Estrogen-receptor and progesterone-receptor levels in the tumor tissue.
- Human epidermal growth factor receptor 2 (HER2) status in the tumor.
- Tumor grade.
- Menopausal status.
- General health of the patient.
The standards used to define biomarker status are described as follows:
- Estrogen receptor (ER) expression: ER expression is measured primarily by immunohistochemistry (IHC). Any staining of 1% of cells or more is considered positive for ER.[1]
- Progesterone receptor (PR) expression: PR expression is measured primarily by IHC. Any staining of 1% of cells or more is considered positive for PR.
- HER2 expression: HER2 is measured primarily by either IHC to assess expression of the HER2 protein or by in situ hybridization (ISH) to assess gene copy number. The American Society of Clinical Oncology/College of American Pathologists consensus panel has published guidelines for cases when either IHC or ISH testing is equivocal.[2]
IHC:
- Negative: 0 or 1+ staining
- Equivocal: 2+ staining
- Positive: 3+ staining
ISH (dual probe):
- Possible negative results:
- HER2/chromosome enumeration probe (CEP17) ratio <2.0 AND HER2 copy number <4
- Possible equivocal results: (requires performing alternative ISH test to confirm equivocal or IHC if not previously performed)
- HER2/CEP17 ratio <2.0 AND HER2 copy number ≥4 but <6
- Possible positive results:
- HER2/CEP17 ratio ≥2.0 by ISH
- HER2 copy number ≥6 regardless of ratio by ISH
ISH (single probe):
- Negative: <4 HER2 copies
- Equivocal: ≥4 but <6 HER2 copies
- Positive: ≥6 HER2 copies
TNM Definitions
The AJCC has designated staging by TNM (tumor, node, metastasis) classification to define breast cancer.[3] The grade of the tumor is determined by its morphologic features, such as tubule formation, nuclear pleomorphism, and mitotic count.
| T Category | T Criteria |
|---|---|
| DCIS = ductal carcinoma in situ. | |
| aReprinted with permission from AJCC: Breast, revised version. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 4–96. | |
| bLobular carcinoma in situ is a benign entity and is removed from TNM staging in the AJCC Cancer Staging Manual, 8th ed. | |
| cRules for Classification - The anatomical TNM system is a method for coding extent of disease. This is done by assigning a category of extent of disease for the tumor (T), regional lymph nodes (N), and distant metastases (M). T, N, and M are assigned by clinical means and by adding surgical findings and pathological information to the clinical information. The documented prognostic impact of postneoadjuvant extent of disease and response to therapy warrant clear definitions of the use of the yp prefix and response to therapy. The use of neoadjuvant therapy does not change the clinical (pretreatment) stage. As per TNM rules, the anatomical component of clinical stage is identified with the prefix c (e.g., cT). In addition, clinical staging can include the use of fine-needle aspiration (FNA) or core-needle biopsy and sentinel lymph node biopsy before neoadjuvant therapy. These are denoted with the postscripts f and sn, respectively. Nodal metastases confirmed by FNA or core-needle biopsy are classified as macrometastases (cN1), regardless of the size of the tumor focus in the final pathological specimen. For example, if, prior to neoadjuvant systemic therapy, a patient with a 1 cm primary has no palpable nodes but has an ultrasound-guided FNA biopsy of an axillary lymph node that is positive, the patient will be categorized as cN1 (f) for clinical (pretreatment) staging and is assigned to Stage IIA. Likewise, if the patient has a positive axillary sentinel node identified before neoadjuvant systemic therapy, the tumor is categorized as cN1 (sn) (Stage IIA). As per TNM rules, in the absence of pathological T evaluation (removal of the primary tumor), which is identified with prefix p (e.g., pT), microscopic evaluation of nodes before neoadjuvant therapy, even by complete removal such as sentinel node biopsy, is still classified as clinical (cN). | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. |
| Tisb | DCIS. |
| Tis (Paget) | Paget disease of the nipple NOT associated with invasive carcinoma and/or DCIS in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget disease should still be noted. |
| T1 | Tumor ≤20 mm in greatest dimension. |
| –T1mi | Tumor ≤1 mm in greatest dimension. |
| –T1a | Tumor >1 mm but ≤5 mm in greatest dimension (round any measurement >1.0–1.9 mm to 2 mm). |
| –T1b | Tumor >5 mm but ≤10 mm in greatest dimension. |
| –T1c | Tumor >10 mm but ≤20 mm in greatest dimension. |
| T2 | Tumor >20 mm but ≤50 mm in greatest dimension. |
| T3 | Tumor >50 mm in greatest dimension. |
| T4 | Tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or macroscopic nodules); invasion of the dermis alone does not qualify as T4. |
| –T4a | Extension to the chest wall; invasion or adherence to pectoralis muscle in the absence of invasion of chest wall structures does not qualify as T4. |
| –T4b | Ulceration and/or ipsilateral macroscopic satellite nodules and/or edema (including peau d'orange) of the skin that does not meet the criteria for inflammatory carcinoma. |
| –T4c | Both T4a and T4b are present. |
| –T4d | Inflammatory carcinoma (see Rules for Classificationc). |
| cN Category | cN Criteria |
|---|---|
| aReprinted with permission from AJCC: Breast, revised version. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 4–96. | |
| b(sn) and (f) suffixes should be added to the N category to denote confirmation of metastasis by sentinel node biopsy or fine-needle aspiration/core needle biopsy, respectively. | |
| cThe cNX category is used sparingly in cases where regional lymph nodes have previously been surgically removed or where there is no documentation of physical examination of the axilla. | |
| dcN1mi is rarely used but may be appropriate in cases where sentinel node biopsy is performed before tumor resection, most likely to occur in cases treated with neoadjuvant therapy. | |
| cNXc | Regional lymph nodes cannot be assessed (e.g., previously removed). |
| cN0 | No regional lymph node metastases (by imaging or clinical examination). |
| cN1 | Metastases to movable ipsilateral Level I, II axillary lymph nodes(s). |
| –cN1mid | Micrometastases (approximately 200 cells, >0.2 mm, but ≤2.0 mm). |
| cN2 | Metastases in ipsilateral Level I, II axillary lymph nodes that are clinically fixed or matted; |
| or in ipsilateral internal mammary nodes in the absence of axillary lymph node metastases. | |
| –cN2a | Metastases in ipsilateral Level I, II axillary lymph nodes fixed to one another (matted) or to other structures. |
| –cN2b | Metastases only in ipsilateral internal mammary nodes in the absence of axillary lymph node metastases. |
| cN3 | Metastases in ipsilateral infraclavicular (Level Ill axillary) lymph node(s) with or without Level l, II axillary lymph node involvement; or in ipsilateral internal mammary lymph node(s) with Level l, II axillary lymph node metastases; or metastases in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement. |
| –cN3a | Metastases in ipsilateral infraclavicular lymph node(s). |
| –cN3b | Metastases in ipsilateral internal mammary lymph node(s) and axillary lymph node(s). |
| –cN3c | Metastases in ipsilateral supraclavicular lymph node(s). |
| pN Category | pN Criteria |
|---|---|
| ITCs = isolated tumor cells; RT-PCR = reverse transcriptase-polymerase chain reaction. | |
| aReprinted with permission from AJCC: Breast, revised version. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 4–96. | |
| b(sn) and (f) suffixes should be added to the N category to denote confirmation of metastasis by sentinel node biopsy or fine-needle aspiration/core needle biopsy, respectively, with NO further resection of nodes. | |
| pNX | Regional lymph nodes cannot be assessed (e.g., not removed for pathological study or previously removed). |
| pN0 | No regional lymph node metastasis identified or ITCs only. |
| –pN0(I+) | ITCs only (malignant cell clusters ≤0.2 mm) in regional lymph node(s). |
| –pN0(mol+) | Positive molecular findings by RT-PCR; no ITCs detected. |
| pN1 | Micrometastases; or metastases in 1–3 axillary lymph nodes; and/or clinically negative internal mammary nodes with micrometastases or macrometastases by sentinel lymph node biopsy. |
| –pN1mi | Micrometastases (~200 cells, >0.2 mm, but ≤2.0 mm). |
| –pN1a | Metastases in 1–3 axillary lymph nodes, at least one metastasis >2.0 mm. |
| –pN1b | Metastases in ipsilateral internal mammary sentinel nodes, excluding ITCs. |
| –pN1c | pN1a and pN1b combined. |
| pN2 | Metastases in 4–9 axillary lymph nodes; or positive ipsilateral internal mammary lymph nodes by imaging in the absence of axillary lymph node metastases. |
| –pN2a | Metastases in 4–9 axillary lymph nodes (at least 1 tumor deposit >2.0 mm). |
| –pN2b | Metastases in clinically detected internal mammary lymph nodes with or without microscopic confirmation; with pathologically negative axillary nodes. |
| pN3 | Metastases in ≥10 axillary lymph nodes; or in infraclavicular (Level Ill axillary) lymph nodes; or positive ipsilateral internal mammary lymph nodes by imaging in the presence of one or more positive Level l, II axillary lymph nodes; or in >3 axillary lymph nodes and micrometastases or macrometastases by sentinel lymph node biopsy in clinically negative ipsilateral internal mammary lymph nodes; or in ipsilateral supraclavicular lymph nodes. |
| –pN3a | Metastases in ≥10 axillary lymph nodes (at least 1 tumor deposit >2.0 mm); or metastases to the infraclavicular (Level III axillary lymph) nodes. |
| –pN3b | pN1a or pN2a in the presence of cN2b (positive internal mammary nodes by imaging); |
| or pN2a in the presence of pN1b. | |
| –pN3c | Metastases in ipsilateral supraclavicular lymph nodes. |
| M Category | M Criteria |
|---|---|
| aReprinted with permission from AJCC: Breast, revised version. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 4–96. | |
| bNote that imaging studies are not required to assign the cM0 category. | |
| M0 | No clinical or radiographic evidence of distant metastases.b |
| cM0(I+) | No clinical or radiographic evidence of distant metastases in the presence of tumor cells or deposits ≤0.2 mm detected microscopically or by molecular techniques in circulating blood, bone marrow, or other nonregional nodal tissue in a patient without symptoms or signs of metastases. |
| cM1 | Distant metastases detected by clinical and radiographic means. |
| pM1 | Any histologically proven metastases in distant organs; or if in nonregional nodes, metastases >0.2 mm. |
| G | G Definition |
|---|---|
| SBR = Scarff-Bloom-Richardson grading system, Nottingham Modification. | |
| aReprinted with permission from AJCC: Breast, revised version. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 4–96. | |
| GX | Grade cannot be assessed. |
| G1 | Low combined histological grade (favorable), SBR score of 3–5 points. |
| G2 | Intermediate combined histological grade (moderately favorable); SBR score of 6–7 points. |
| G3 | High combined histological grade (unfavorable); SBR score of 8–9 points. |
| G | G Definition |
|---|---|
| aReprinted with permission from AJCC: Breast, revised version. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 4–96. | |
| GX | Grade cannot be assessed. |
| G1 | Low nuclear grade. |
| G2 | Intermediate nuclear grade. |
| G3 | High nuclear grade. |
AJCC Anatomical and Prognostic Stage Groups
There are three stage group tables for invasive cancer:[3]
- Anatomical Stage Group. The Anatomical Stage Group table is used in regions of the world where tumor grading and/or biomarker testing for ER, PR, and HER2 are not routinely available. (See Table 8.)
- Clinical Prognostic Stage Group. The Clinical Prognostic Stage Group table is used for all patients in the United States. Patients who have neoadjuvant therapy as their initial treatment should have the clinical prognostic stage and the observed degree of response to treatment recorded, but these patients are not assigned a pathological prognostic stage. (See Table 9.)
- Pathological Prognostic Stage Group. The Pathological Prognostic Stage Group table is used for all patients in the United States who have surgery as initial treatment and have pathological T and N information reported. (See Table 10.)
In the United States, cancer registries and clinicians must use the Clinical and Pathological Prognostic Stage Group tables for reporting. It is expected that testing is performed for grade, HER2, ER, and PR status and that results are reported for all cases of invasive cancer in the United States.
AJCC Anatomical Stage Groups
| Stage | TNM |
|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |
| aAdapted with permission from AJCC: Breast, revised version. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 4–96. | |
| Notes: | |
| 1. T1 includes T1mi. | |
| 2. T0 and T1 tumors with nodal micrometastases (N1mi) are staged as Stage IB. | |
| 3. T2, T3, and T4 tumors with nodal micrometastases (N1mi) are staged using the N1 category. | |
| 4. M0 includes M0(I+). | |
| 5. The designation pM0 is not valid; any M0 is clinical. | |
| 6. If a patient presents with M1 disease before receiving neoadjuvant systemic therapy, the stage is Stage IV and remains Stage IV regardless of response to neoadjuvant therapy. | |
| 7. Stage designation may be changed if postsurgical imaging studies reveal the presence of distant metastases, provided the studies are performed within 4 months of diagnosis in the absence of disease progression, and provided the patient has not received neoadjuvant therapy. | |
| 8. Staging following neoadjuvant therapy is denoted with a yc or ypn prefix to the T and N classification. There is no anatomical stage group assigned if there is a complete pathological response (pCR) to neoadjuvant therapy, for example, ypT0, ypN0, cM0. | |
| 0 | Tis, N0, M0 |
| IA | T1, N0, M0 |
| IB | T0, N1mi, M0 |
| T1, N1mi, M0 | |
| IIA | T0, N1, M0 |
| T1, N1, M0 | |
| T2, N0, M0 | |
| IIB | T2, N1, M0 |
| T3, N0, M0 | |
| IIIA | T0, N2, M0 |
| T1, N2, M0 | |
| T2, N2, M0 | |
| T3, N1, M0 | |
| T3, N2, M0 | |
| IIIB | T4, N0, M0 |
| T4, N1, M0 | |
| T4, N2, M0 | |
| IIIC | Any T (Tis, T1, T0, T2, T3, T4), N3, M0 |
| IV | Any T (Tis, T1, T0, T2, T3, T4), Any N (N0, N1mi, N1, N2, N3), M1 |
AJCC Prognostic Stage Groups
The Clinical Prognostic Stage is used for clinical classification and staging of patients in the United States with invasive breast cancer. It uses TNM information based on the patient’s history, physical examination, imaging results (not required for clinical staging), and biopsies.
| TNM | Grade | HER2 Status | ER Status | PR Status | Stage Group |
|---|---|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||||
| aAdapted with permission from AJCC: Breast, revised version. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 4–96. | |||||
| bT1 includes T1mi. | |||||
| cN1 does not include N1mi. T1, N1mi, M0, and T0, N1mi, M0 cancers are included for prognostic staging with T1, N0, M0 cancers of the same prognostic factor status. | |||||
| dN1 includes N1mi. T2, T3, and T4 cancers and N1mi are included for prognostic staging with T2, N1; T3, N1; and T4, N1, respectively. | |||||
| Notes: | |||||
| 1. Because N1mi categorization requires evaluation of the entire node, and cannot be assigned on the basis of a fine-needle aspiration or core biopsy, N1mi can only be used with Clinical Prognostic Staging when clinical staging is based on a resected lymph node in the absence of resection of the primary cancer, such as in the situation where sentinel node biopsy is performed before receiving neoadjuvant chemotherapy or endocrine therapy. | |||||
| 2. For cases with lymph node involvement with no evidence of primary tumor (e.g., T0, N1, etc.) or with breast ductal carcinoma in situ (e.g.,Tis, N1, etc.), the grade, human epidermal growth factor receptor 2 (HER2), estrogen receptor, and progesterone receptor information from the tumor in the lymph node should be used for assigning stage group. | |||||
| 3. For cases where HER2 is determined to be equivocal by in situ hybridization (fluorescence in situ hybridization or chromogenic in situ hybridization) testing under the 2013 American Society of Clinical Oncologists/College of American Pathologists HER2 testing guidelines, the HER2-negative category should be used for staging in the Pathological Prognostic Stage Group table.[4,5] | |||||
| 4. The prognostic value of these Prognostic Stage Groups is based on populations of persons with breast cancer that have been offered and mostly treated with appropriate endocrine and/or systemic chemotherapy (including anti–HER2 therapy). | |||||
| Tis, N0, M0 | Any (see Table 6 and Table 7) | Any | Any | Any | 0 |
| T1b, N0, M0 | G1 | Positive | Positive | Positive | IA |
| Negative | IA | ||||
| T0, N1mi, M0 | Negative | Positive | IA | ||
| Negative | IA | ||||
| T1b, N1mi, M0 | Negative | Positive | Positive | IA | |
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IB | ||||
| G2 | Positive | Positive | Positive | IA | |
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IA | ||||
| Negative | Positive | Positive | IA | ||
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IB | ||||
| G3 | Positive | Positive | Positive | IA | |
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IA | ||||
| Negative | Positive | Positive | IA | ||
| Negative | IB | ||||
| Negative | Positive | IB | |||
| Negative | IB | ||||
| T0, N1c, M0; T1b, N1c, M0; T2, N0, M0 | G1 | Positive | Positive | Positive | IB |
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIA | ||||
| Negative | Positive | Positive | IB | ||
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIA | ||||
| G2 | Positive | Positive | Positive | IB | |
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIA | ||||
| Negative | Positive | Positive | IB | ||
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIB | ||||
| G3 | Positive | Positive | Positive | IB | |
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIA | ||||
| Negative | Positive | Positive | IIA | ||
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIB | ||||
| T2, N1d, M0; T3, N0, M0 | G1 | Positive | Positive | Positive | IB |
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIB | ||||
| Negative | Positive | Positive | IIA | ||
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIB | ||||
| G2 | Positive | Positive | Positive | IB | |
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIB | ||||
| Negative | Positive | Positive | IIA | ||
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIIB | ||||
| G3 | Positive | Positive | Positive | IB | |
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIB | ||||
| Negative | Positive | Positive | IIB | ||
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIB | ||||
| T0, N2, M0; T1b, N2, M0; T2, N2, M0; T3, N1d, M0; T3, N2, M0 | G1 | Positive | Positive | Positive | IIA |
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIA | ||||
| Negative | Positive | Positive | IIA | ||
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIB | ||||
| G2 | Positive | Positive | Positive | IIA | |
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIA | ||||
| Negative | Positive | Positive | IIA | ||
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIB | ||||
| G3 | Positive | Positive | Positive | IIB | |
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIA | ||||
| Negative | Positive | Positive | IIIA | ||
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIC | ||||
| T4, N0, M0; T4, N1d, M0; T4, N2, M0; Any T, N3, M0 | G1 | Positive | Positive | Positive | IIIA |
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIB | ||||
| Negative | Positive | Positive | IIIB | ||
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIC | ||||
| G2 | Positive | Positive | Positive | IIIA | |
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIB | ||||
| Negative | Positive | Positive | IIIB | ||
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIC | ||||
| G3 | Positive | Positive | Positive | IIIB | |
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIB | ||||
| Negative | Positive | Positive | IIIB | ||
| Negative | IIIC | ||||
| Negative | Positive | IIIC | |||
| Negative | IIIC | ||||
| Any T, Any N, M1 | Any (see Table 6 and Table 7) | Any | Any | Any | IV |
AJCC Pathological Prognostic Stage Groups
The Pathological Prognostic Stage applies to patients with invasive breast cancer initially treated with surgery. It includes all information used for clinical staging, surgical findings, and pathological findings following surgery to remove the tumor. Pathological Prognostic Stage is not used for patients treated with neoadjuvant therapy before surgery to remove the tumor.[3]
| TNM | Grade | HER2 Status | ER Status | PR Status | Stage Group |
|---|---|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||||
| aAdapted with permission from AJCC: Breast, revised version. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 4–96. | |||||
| bT1 includes T1mi. | |||||
| cN1 does not include N1mi. T1, N1mi, M0 and T0, N1mi, M0 cancers are included for prognostic staging with T1, N0, M0 cancers of the same prognostic factor status. | |||||
| dN1 includes N1mi. T2, T3, and T4 cancers and N1mi are included for prognostic staging with T2, N1; T3, N1; and T4, N1, respectively. | |||||
| Notes: | |||||
| 1. For cases with lymph node involvement with no evidence of primary tumor (e.g., T0, N1, etc.) or with breast ductal carcinoma in situ (e.g.,Tis, N1, etc.), the grade, human epidermal growth factor receptor 2 (HER2), estrogen receptor, and progesterone receptor information from the tumor in the lymph node should be used for assigning stage group. | |||||
| 2. For cases where HER2 is determined to be equivocal by in situ hybridization (fluorescence in situ hybridization or chromogenic in situ hybridization) testing under the 2013 American Society of Clinical Oncologists/College of American Pathologists HER2 testing guidelines, the HER2-negative category should be used for staging in the Pathological Prognostic Stage Group table.[4,5] | |||||
| 3. The prognostic value of these Prognostic Stage Groups is based on populations of persons with breast cancer that have been offered and mostly treated with appropriate endocrine and/or systemic chemotherapy (including anti–HER2 therapy). | |||||
| Tis, N0, M0 | Any (see Table 6 and Table 7) | Any | Any | Any | 0 |
| T1b, N0, M0; T0, N1mi, M0; T1b, N1mi, M0 | G1 | Positive | Positive | Positive | IA |
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IA | ||||
| Negative | Positive | Positive | IA | ||
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IA | ||||
| G2 | Positive | Positive | Positive | IA | |
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IA | ||||
| Negative | Positive | Positive | IA | ||
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IB | ||||
| G3 | Positive | Positive | Positive | IA | |
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IA | ||||
| Negative | Positive | Positive | IA | ||
| Negative | IA | ||||
| Negative | Positive | IA | |||
| Negative | IB | ||||
| T0, N1c , M0; T1b, N1c, M0; T2, N0, M0 | G1 | Positive | Positive | Positive | IA |
| Negative | IB | ||||
| Negative | Positive | IB | |||
| Negative | IIA | ||||
| Negative | Positive | Positive | IA | ||
| Negative | IB | ||||
| Negative | Positive | IB | |||
| Negative | IIA | ||||
| G2 | Positive | Positive | Positive | IA | |
| Negative | IB | ||||
| Negative | Positive | IB | |||
| Negative | IIA | ||||
| Negative | Positive | Positive | IA | ||
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIA | ||||
| G3 | Positive | Positive | Positive | IA | |
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIA | ||||
| Negative | Positive | Positive | IB | ||
| Negative | IIA | ||||
| Negative | Positive | IIA | |||
| Negative | IIA | ||||
| T2, N1c, M0; T3, N0, M0 | G1 | Positive | Positive | Positive | IA |
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIB | ||||
| Negative | Positive | Positive | IA | ||
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIB | ||||
| G2 | Positive | Positive | Positive | IB | |
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIB | ||||
| Negative | Positive | Positive | IB | ||
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIB | ||||
| G3 | Positive | Positive | Positive | IB | |
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIB | ||||
| Negative | Positive | Positive | IIA | ||
| Negative | IIB | ||||
| Negative | Positive | IIB | |||
| Negative | IIIA | ||||
| T0, N2, M0; T1b, N2, M0; T2, N2, M0, T3, N1d, M0; T3, N2, M0 | G1 | Positive | Positive | Positive | IB |
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIA | ||||
| Negative | Positive | Positive | IB | ||
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIA | ||||
| G2 | Positive | Positive | Positive | IB | |
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIA | ||||
| Negative | Positive | Positive | IB | ||
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIB | ||||
| G3 | Positive | Positive | Positive | IIA | |
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIA | ||||
| Negative | Positive | Positive | IIB | ||
| Negative | IIIA | ||||
| Negative | Positive | IIIA | |||
| Negative | IIIC | ||||
| T4, N0, M0; T4, N1d, M0; T4, N2, M0; Any T, N3, M0 | G1 | Positive | Positive | Positive | IIIA |
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIB | ||||
| Negative | Positive | Positive | IIIA | ||
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIB | ||||
| G2 | Positive | Positive | Positive | IIIA | |
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIB | ||||
| Negative | Positive | Positive | IIIA | ||
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIC | ||||
| G3 | Positive | Positive | Positive | IIIB | |
| Negative | IIIB | ||||
| Negative | Positive | IIIB | |||
| Negative | IIIB | ||||
| Negative | Positive | Positive | IIIB | ||
| Negative | IIIC | ||||
| Negative | Positive | IIIC | |||
| Negative | IIIC | ||||
| Any T, Any N, M1 | Any (see Table 6 and Table 7) | Any | Any | Any | IV |
References
- Barnes DM, Harris WH, Smith P, et al.: Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer 74 (9): 1445-51, 1996. [PUBMED Abstract]
- Wolff AC, Somerfield MR, Dowsett M, et al.: Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J Clin Oncol 41 (22): 3867-3872, 2023. [PUBMED Abstract]
- Breast. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 589–628.
- Wolff AC, Hammond ME, Hicks DG, et al.: Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31 (31): 3997-4013, 2013. [PUBMED Abstract]
- Wolff AC, Hammond ME, Hicks DG, et al.: Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 138 (2): 241-56, 2014. [PUBMED Abstract]
Surgical Treatment for Breast Cancer
Operable breast cancer requires a multimodal approach to treatment. After the presence of a malignancy is confirmed by biopsy, the following surgical treatment options can be discussed with the patient before a therapeutic procedure is selected:
- Breast-conserving surgery.
- Modified radical mastectomy (removal of the entire breast with axillary dissection of levels I and II) with or without breast reconstruction.
To guide the selection of neoadjuvant or adjuvant therapy, many factors including stage, grade, and molecular status of the tumor (e.g., estrogen receptor [ER], progesterone receptor [PR], human epidermal growth factor type 2 receptor [HER2], or triple-negative status) are considered.[1-5]
Surgical Staging of the Primary Tumor
Selection of a local therapeutic approach depends on the following factors:[6]
- Location and size of the lesion.
- Analysis of the mammogram and/or magnetic resonance imaging or additional imaging.
- Breast size.
- Patient’s desire to preserve the breast.
Options for surgical management of the primary tumor include:
- Breast-conserving surgery (with consideration of radiation therapy). All histological types of invasive breast cancer may be treated with breast-conserving surgery plus radiation therapy.[7] However, the presence of inflammatory breast cancer, regardless of histological subtype, is a contraindication to breast-conserving therapy. The presence of multifocal disease in the breast and a history of collagen vascular disease are relative contraindications to breast-conserving therapy. Prior radiation to the breast was previously considered a contraindication to breast-conserving surgery. However, research has increasingly shown that repeat radiation therapy may be feasible and safe in select patient populations.[8]
- Mastectomy with or without breast reconstruction.
Survival is equivalent with any of these options, as documented in the EORTC-10801 trial [9] and other prospective randomized trials.[10-16] Also, a retrospective study of 753 patients who were divided into three groups based on hormone receptor status (ER positive or PR positive; ER negative and PR negative but HER2 positive; and triple negative) found no differences in disease control within the breast in patients treated with standard breast-conserving surgery; however, there are not yet substantive data to support this finding.[17]
The rate of local recurrence in the breast after conservative treatment is low and varies slightly with the surgical technique used (e.g., lumpectomy, quadrantectomy, segmental mastectomy, and others). Whether completely clear microscopic margins are necessary has been debated.[18-20] However, a multidisciplinary consensus panel recently used margin width and ipsilateral breast tumor recurrence from a meta-analysis of 33 studies (N = 28,162 patients) as the primary evidence base for a new consensus regarding margins in patients with stage I and stage II breast cancer treated with breast-conserving surgery plus radiation therapy. Results of the meta-analysis include the following:[21]
- Positive margins (ink on invasive carcinoma or ductal carcinoma in situ) were associated with a twofold increase in the risk of ipsilateral breast tumor recurrence compared with negative margins.
- More widely clear margins were not found to significantly decrease the rate of ipsilateral breast tumor recurrence compared with no ink on tumor. Thus, it was recommended that the use of no ink on tumor be the new standard for an adequate margin in invasive cancer.
- There was no evidence that more widely clear margins reduced ipsilateral breast tumor recurrence for young patients or for those with unfavorable biology, lobular cancers, or cancers with an extensive intraductal component.
For patients undergoing partial mastectomy, margins may be positive after primary surgery, often leading to re-excision. A clinical trial of 235 patients with stage 0 to III breast cancer who underwent partial mastectomy, with or without resection of selective margins, randomly assigned patients to have additional cavity shave margins resected (shave group) or not (no-shave group).[22] Patients in the shave group had a significantly lower rate of positive margins than those in the no-shave group (19% vs. 34%, P = .01) and a lower rate of second surgery for clearing margins (10% vs. 21%, P = .02).[22][Level of evidence B3]
Axillary Lymph Node Management
Axillary node status remains the most important predictor of outcome in patients with breast cancer. The axillary lymph nodes are staged to aid in determining prognosis and therapy.
Sentinel lymph node (SLN) biopsy is the initial standard axillary staging procedure performed in women with invasive breast cancer. The SLN is defined as any node that receives drainage directly from the primary tumor, allowing for more than one SLN, which is often the case. Studies have shown that the injection of technetium Tc 99m-labeled sulfur colloid, vital blue dye, or both around the tumor or biopsy cavity, or in the subareolar area, and subsequent drainage of these compounds to the axilla results in the identification of the SLN in 92% to 98% of patients.[23,24] These reports demonstrate a 97.5% to 100% concordance between SLN biopsy and complete axillary lymph node dissection (ALND). SLN biopsy alone is associated with less morbidity than axillary lymphadenectomy.[25-28]
Evidence (SLN biopsy):
- ALMANAC, a randomized trial of 1,031 women compared SLN biopsy followed by ALND when the SLN was positive with ALND in all patients.[29][Level of evidence A3]
- Quality of life at 1 year (as assessed by the frequency of patients experiencing a clinically significant deterioration in the Trial Outcome Index of the Functional Assessment of Cancer Therapy-Breast scale) was superior in the SLN biopsy group (23% deteriorating in the SLN biopsy group vs. 35% in the ALND group; P = .001). Arm function was also better in the SLN group.
- The National Surgical Adjuvant Breast and Bowel Project’s (NSABP-B-32 [NCT00003830]) multicenter, phase III trial randomly assigned women (N = 5,611) to undergo either SLN plus ALND or SLN resection alone. ALND was only performed if the SLNs were positive.[30][Level of evidence A1]
- The study showed no detectable difference in overall survival (OS), disease-free survival (DFS), and regional control. The OS rate was 91.8% for SLN plus ALND versus 90.3% for SLN resection alone (P = .12).
Because of the following trial results, ALND is unnecessary after a positive SLN biopsy in patients with limited SLN-positive breast cancer treated with breast conservation or mastectomy, radiation therapy, and systemic therapy.
Evidence (ALND after a positive SLN biopsy in patients with limited SLN-positive breast cancer):
- ACOSOG Z0011 (Alliance, NCT00003855), a phase III, noninferiority, multicenter, randomized clinical trial, evaluated whether ALND is required after a positive SLN biopsy. Women were randomly assigned to undergo ALND or no further axillary treatment. Patients had clinical T1 or T2 invasive breast cancer without palpable adenopathy and one to two SLNs containing metastases identified by frozen section. All patients underwent lumpectomy, tangential whole-breast radiation therapy, and appropriate systemic therapy. OS was the primary end point, and DFS was the secondary end point. Because of enrollment challenges, a total of 891 women out of a target enrollment of 1,900 women were randomly assigned to one of the two treatment arms.[Level of evidence A1]
- At a median follow-up of 6.3 years, the 5-year OS rate was 91.8% (95% confidence interval [CI], 89.1%–94.5%) with ALND and 92.5% (95% CI, 90.0%–95.1%) with SLN biopsy alone.
- Detailed analysis of the radiation field design found that 15% of patients also received treatment to the supraclavicular region (in addition to standard tangents). Of those with detailed radiation records available for review, 43 patients (18.9%) received regional nodal radiation therapy.[31]
- The 5-year DFS rate was 82.2% (95% CI, 78.3%–86.3%) with ALND and 83.9% (95% CI, 80.2%–87.9%) with SLN biopsy alone.
- In a similarly designed trial (IBCSG 23-01), 929 women with breast tumors smaller than 5 cm and SLN involvement smaller than 2 mm were randomly assigned to ALND or no ALND.[32][Level of evidence A1]
- Patients without axillary dissection had fewer DFS events (hazard ratio [HR], 0.78; 95% CI, 0.55–1.11).
- No difference in OS was observed.
- The AMAROS trial (NCT00014612) studied ALND and axillary radiation therapy after identification of a positive SLN.[33][Level of evidence A1]
- ALND and axillary radiation therapy provided excellent and comparable axillary control for patients with T1 or T2 primary breast cancer and no palpable lymphadenopathy who underwent breast-conserving therapy or mastectomy.
- The use of axillary radiation therapy was also associated with significantly less morbidity.
For patients who require an ALND, the standard evaluation usually involves only a level I and II dissection, thereby removing a satisfactory number of nodes for evaluation (i.e., at least 6–10), while reducing morbidity from the procedure.
Although SLN biopsy has been the standard for the axillary staging of patients with invasive breast cancer and a clinically negative axilla, two randomized controlled trials have identified populations for which SLN biopsy could be omitted.
Evidence (omission of SLN biopsy):
- The SOUND trial (NCT02167490), a multicenter, noninferiority, randomized clinical trial, evaluated omitting SLN biopsy in women with invasive breast cancer. Patients were of any age, had tumors smaller than 2 cm, had negative preoperative axillary ultrasonography, and planned to receive breast-conserving surgery and adjuvant radiation therapy. A total of 1,493 women were randomly assigned to undergo SLN biopsy or no axillary surgery. The median follow-up was 5.7 years.[34][Level of evidence A1]
- The 5-year distant DFS rate was 97.7% in the SLN biopsy group and 98.0% in the no-surgery group (log-rank P = .67; HR, 0.84; 90% CI, 0.45–1.54; noninferiority P = .02).
- A total of 12 locoregional relapses (1.7%), 13 distant metastases (1.8%), and 21 deaths (3.0%) were observed in the SLN biopsy group, compared with 11 locoregional relapses (1.6%), 14 distant metastases (2.0%), and 18 deaths (2.6%) in the no-surgery group.
- The multicenter, noninferiority, randomized INSEMA trial (NCT02466737) evaluated omitting SLN biopsy in 5,502 women with clinically node-negative invasive breast cancer. Tumors had to be smaller than 5 cm, and most patients had T1 disease and ER-positive tumors. Patients were scheduled to undergo breast-conserving surgery and whole-breast radiation therapy. Patients were randomly assigned in a 1:4 ratio to undergo no axillary surgery or SLN biopsy. The median follow-up was 6 years.[35][Level of evidence A1]
- The 5-year invasive DFS rate was 91.9% in the no-surgery group and 91.7% in the SLN biopsy group (HR, 0.91; 95% CI, 0.73–1.14), which was below the prespecified noninferiority margin.
- Patients in the no-surgery group had a lower incidence of lymphedema, greater arm mobility, and less pain with movement of the arm or shoulder than patients who underwent SLN biopsy.
Breast Reconstruction
For patients who opt for a total mastectomy, reconstructive surgery may be performed at the time of the mastectomy (immediate reconstruction) or at some subsequent time (delayed reconstruction).[36-39] Breast contour can be restored by the following procedures:
- Mastopexy.
- Submuscular insertion of an artificial implant (silicone or saline filled). If an immediate implant cannot technically be performed, a tissue expander can be inserted beneath the pectoral muscle. Saline is injected into the expander to stretch the tissues for a period of weeks or months until the desired volume is obtained. The tissue expander is then replaced by a permanent implant. For more information on breast implants, see the U.S. Food and Drug Administration.
- Rectus muscle or other flap. Muscle flaps require a considerably more complicated and prolonged operative procedure, and blood transfusions may be required.
After breast reconstruction, radiation therapy can be delivered to the chest wall and regional nodes if indicated in either the adjuvant or local recurrent disease setting. Radiation therapy after reconstruction with either a breast prosthesis or a tissue flap may affect cosmesis and symmetry. The incidence of capsular fibrosis, pain, or the need for implant removal may also be increased.[40]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Fisher B, Fisher ER, Redmond C, et al.: Tumor nuclear grade, estrogen receptor, and progesterone receptor: their value alone or in combination as indicators of outcome following adjuvant therapy for breast cancer. Breast Cancer Res Treat 7 (3): 147-60, 1986. [PUBMED Abstract]
- Thor AD, Berry DA, Budman DR, et al.: erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer Inst 90 (18): 1346-60, 1998. [PUBMED Abstract]
- Paik S, Bryant J, Park C, et al.: erbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst 90 (18): 1361-70, 1998. [PUBMED Abstract]
- Simpson JF, Gray R, Dressler LG, et al.: Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern Cooperative Oncology Group Companion Study, EST 4189. J Clin Oncol 18 (10): 2059-69, 2000. [PUBMED Abstract]
- Hutchins LF, Green SJ, Ravdin PM, et al.: Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol 23 (33): 8313-21, 2005. [PUBMED Abstract]
- Abrams JS, Phillips PH, Friedman MA: Meeting highlights: a reappraisal of research results for the local treatment of early stage breast cancer. J Natl Cancer Inst 87 (24): 1837-45, 1995. [PUBMED Abstract]
- Weiss MC, Fowble BL, Solin LJ, et al.: Outcome of conservative therapy for invasive breast cancer by histologic subtype. Int J Radiat Oncol Biol Phys 23 (5): 941-7, 1992. [PUBMED Abstract]
- Arthur DW, Winter KA, Kuerer HM, et al.: Effectiveness of Breast-Conserving Surgery and 3-Dimensional Conformal Partial Breast Reirradiation for Recurrence of Breast Cancer in the Ipsilateral Breast: The NRG Oncology/RTOG 1014 Phase 2 Clinical Trial. JAMA Oncol 6 (1): 75-82, 2020. [PUBMED Abstract]
- van Dongen JA, Voogd AC, Fentiman IS, et al.: Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 92 (14): 1143-50, 2000. [PUBMED Abstract]
- Fisher B, Anderson S, Bryant J, et al.: Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347 (16): 1233-41, 2002. [PUBMED Abstract]
- Blichert-Toft M, Rose C, Andersen JA, et al.: Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr (11): 19-25, 1992. [PUBMED Abstract]
- van Dongen JA, Bartelink H, Fentiman IS, et al.: Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Inst Monogr (11): 15-8, 1992. [PUBMED Abstract]
- Sarrazin D, Lê MG, Arriagada R, et al.: Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol 14 (3): 177-84, 1989. [PUBMED Abstract]
- Jacobson JA, Danforth DN, Cowan KH, et al.: Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 332 (14): 907-11, 1995. [PUBMED Abstract]
- Veronesi U, Cascinelli N, Mariani L, et al.: Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347 (16): 1227-32, 2002. [PUBMED Abstract]
- Veronesi U, Salvadori B, Luini A, et al.: Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer 31A (10): 1574-9, 1995. [PUBMED Abstract]
- Freedman GM, Anderson PR, Li T, et al.: Locoregional recurrence of triple-negative breast cancer after breast-conserving surgery and radiation. Cancer 115 (5): 946-51, 2009. [PUBMED Abstract]
- Schmidt-Ullrich R, Wazer DE, Tercilla O, et al.: Tumor margin assessment as a guide to optimal conservation surgery and irradiation in early stage breast carcinoma. Int J Radiat Oncol Biol Phys 17 (4): 733-8, 1989. [PUBMED Abstract]
- Solin LJ, Fowble BL, Schultz DJ, et al.: The significance of the pathology margins of the tumor excision on the outcome of patients treated with definitive irradiation for early stage breast cancer. Int J Radiat Oncol Biol Phys 21 (2): 279-87, 1991. [PUBMED Abstract]
- Wazer DE, Schmidt-Ullrich RK, Schmid CH, et al.: The value of breast lumpectomy margin assessment as a predictor of residual tumor burden. Int J Radiat Oncol Biol Phys 38 (2): 291-9, 1997. [PUBMED Abstract]
- Moran MS, Schnitt SJ, Giuliano AE, et al.: Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 32 (14): 1507-15, 2014. [PUBMED Abstract]
- Chagpar AB, Killelea BK, Tsangaris TN, et al.: A Randomized, Controlled Trial of Cavity Shave Margins in Breast Cancer. N Engl J Med 373 (6): 503-10, 2015. [PUBMED Abstract]
- Kern KA: Sentinel lymph node mapping in breast cancer using subareolar injection of blue dye. J Am Coll Surg 189 (6): 539-45, 1999. [PUBMED Abstract]
- Rubio IT, Korourian S, Cowan C, et al.: Sentinel lymph node biopsy for staging breast cancer. Am J Surg 176 (6): 532-7, 1998. [PUBMED Abstract]
- Veronesi U, Paganelli G, Galimberti V, et al.: Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 349 (9069): 1864-7, 1997. [PUBMED Abstract]
- Albertini JJ, Lyman GH, Cox C, et al.: Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 276 (22): 1818-22, 1996. [PUBMED Abstract]
- Krag D, Weaver D, Ashikaga T, et al.: The sentinel node in breast cancer--a multicenter validation study. N Engl J Med 339 (14): 941-6, 1998. [PUBMED Abstract]
- Veronesi U, Paganelli G, Viale G, et al.: Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst 91 (4): 368-73, 1999. [PUBMED Abstract]
- Mansel RE, Fallowfield L, Kissin M, et al.: Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 98 (9): 599-609, 2006. [PUBMED Abstract]
- Krag DN, Anderson SJ, Julian TB, et al.: Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11 (10): 927-33, 2010. [PUBMED Abstract]
- Jagsi R, Chadha M, Moni J, et al.: Radiation field design in the ACOSOG Z0011 (Alliance) Trial. J Clin Oncol 32 (32): 3600-6, 2014. [PUBMED Abstract]
- Galimberti V, Cole BF, Zurrida S, et al.: Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 14 (4): 297-305, 2013. [PUBMED Abstract]
- Donker M, van Tienhoven G, Straver ME, et al.: Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 15 (12): 1303-10, 2014. [PUBMED Abstract]
- Gentilini OD, Botteri E, Sangalli C, et al.: Sentinel Lymph Node Biopsy vs No Axillary Surgery in Patients With Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes: The SOUND Randomized Clinical Trial. JAMA Oncol 9 (11): 1557-1564, 2023. [PUBMED Abstract]
- Reimer T, Stachs A, Veselinovic K, et al.: Axillary Surgery in Breast Cancer - Primary Results of the INSEMA Trial. N Engl J Med 392 (11): 1051-1064, 2025. [PUBMED Abstract]
- Cunningham BL: Breast reconstruction following mastectomy. In: Najarian JS, Delaney JP, eds.: Advances in Breast and Endocrine Surgery. Year Book Medical Publishers, 1986, pp 213-226.
- Scanlon EF: The role of reconstruction in breast cancer. Cancer 68 (5 Suppl): 1144-7, 1991. [PUBMED Abstract]
- Hang-Fu L, Snyderman RK: State-of-the-art breast reconstruction. Cancer 68 (5 Suppl): 1148-56, 1991. [PUBMED Abstract]
- Feller WF, Holt R, Spear S, et al.: Modified radical mastectomy with immediate breast reconstruction. Am Surg 52 (3): 129-33, 1986. [PUBMED Abstract]
- Kuske RR, Schuster R, Klein E, et al.: Radiotherapy and breast reconstruction: clinical results and dosimetry. Int J Radiat Oncol Biol Phys 21 (2): 339-46, 1991. [PUBMED Abstract]
Radiation Therapy for Breast Cancer
Radiation therapy is standard after breast-conserving surgery as part of breast-conserving therapy. Radiation therapy is also considered for high-risk postmastectomy patients. The main goal of adjuvant radiation therapy is to eradicate residual disease, reducing local recurrence and increasing breast cancer–specific survival.[1]
Post–Breast-Conserving Surgery
For women who have breast-conserving surgery without radiation therapy, the risk of recurrence in the conserved breast is substantial (>20%) even in women with confirmed axillary lymph node–negative disease.[2] Although all trials assessing the role of radiation therapy in breast-conserving therapy have shown highly statistically significant reductions in local recurrence rate, no single trial has demonstrated a statistically significant reduction in mortality. However, a large meta-analysis demonstrated a significant reduction in risk of recurrence and breast cancer death.[3] Overall, evidence supports the use of whole-breast radiation therapy after breast-conserving surgery.
Evidence (breast-conserving surgery followed by radiation therapy):
- A 2011 meta-analysis of 17 clinical trials performed by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), which included over 10,000 women with early-stage breast cancer, supported whole-breast radiation therapy after breast-conserving surgery.[3][Level of evidence A1]
- Whole-breast radiation therapy resulted in a significant reduction in the 10-year risk of recurrence compared with breast-conserving surgery alone (19% for whole-breast radiation therapy vs. 35% for breast-conserving surgery alone; relative risk [RR], 0.52; 95% confidence interval [CI], 0.48–0.56) and a significant reduction in the 15-year risk of breast cancer death (21% for whole-breast radiation therapy vs. 25% for breast-conserving surgery alone; RR, 0.82; 95% CI, 0.75–0.90).
Regarding radiation dosing and schedule, the following has been noted:
- Whole-breast radiation dose. Conventional whole-breast radiation therapy is delivered to the whole breast (with or without regional lymph nodes) in 1.8 Gy to 2 Gy daily fractions over 5 to 6.5 weeks to a total dose of 45 Gy to 50 Gy.
- Radiation boost. A further radiation boost is commonly given to the tumor bed. Two randomized trials conducted in Europe have shown that boosts of 10 Gy to 16 Gy reduce the risk of local recurrence from 4.6% to 3.6% at 3 years (P = .044),[4][Level of evidence B1] and from 7.3% to 4.3% at 5 years (P < .001).[5][Level of evidence B1] Results were similar after a median follow-up of 17.2 years.[6][Level of evidence B1] If a boost is used, it can be delivered either by external-beam radiation therapy, generally with electrons, or by using an interstitial radioactive implant.[7] Administering a radiation boost may, however, be associated with unfavorable quality-of-life outcomes.[8]
- Radiation schedule. Some studies show that a shorter fractionation schedule of 42.5 Gy over 3 to 4 weeks is a reasonable alternative for some patients with breast cancer.
- A noninferiority trial of 1,234 randomly assigned patients with node-negative invasive breast cancer analyzed locoregional recurrence rates with conventional whole-breast radiation therapy versus a shorter fractionation schedule.[9] The 10-year locoregional relapse rate among women who received shorter fractionation was not inferior to conventional whole-breast radiation therapy (6.2% for a shorter fractionation schedule vs. 6.7% for whole-breast radiation therapy with absolute difference, 0.5 percentage points; 95% CI, −2.5 to 3.5).[9][Level of evidence B1
- Similarly, a combined analysis of the randomized United Kingdom Standardisation of Breast Radiotherapy trials (START), (START-A [ISRCTN59368779]) and START-B [ISRCTN59368779]) revealed no difference in a 10-year locoregional relapse rate. These trials collectively randomly assigned 4,451 women with completely excised invasive (pT1–3a, pN0–1, M0) early-stage breast cancer after breast-conserving surgery to receive conventional whole-breast radiation therapy dosing or shorter fractionation.[10][Level of evidence B1]
- A meta-analysis that included the three trials mentioned above plus six others confirmed that differences with respect to local recurrence or cosmesis between shorter and conventional fractionation schedules were neither statistically nor clinically significant.[11]
Additional studies are needed to determine whether shorter fractionation is appropriate for women with higher nodal disease burden.[10]
Omission of radiation therapy for favorable, early-stage breast cancer
The omission of radiation therapy after breast-conserving surgery has been tested in older patients with early-stage (T1 and small T2), hormone receptor–positive tumors after primary surgery. Two large trials found that radiation can be safely omitted without survival deficit, but with an increased risk of local recurrence of about 8% at 10 years, assuming that hormonal therapy is used.
- CALGB 9343 was a phase III randomized trial of radiation omission. A total of 636 women were enrolled; 317 women received tamoxifen plus radiation therapy, and 319 women received tamoxifen alone. Eligible women were aged 70 years or older and had clinical stage I estrogen receptor (ER)-positive breast cancer. Initial eligibility criteria included breast cancers measuring up to 4 cm regardless of ER status, but in August 1996 this was reduced to measuring up to 2 cm (T1) with ER-positive or indeterminate receptor status. Patients were required to have clinically negative axillae.[12][Level of evidence A1]
- At 10 years, 98% of patients in the tamoxifen-plus-radiation group (95% CI, 96%–99%) were free from local and regional recurrences compared with 90% in the tamoxifen-alone group (95% CI, 85%–93%).
- There were no significant differences in time-to-mastectomy, time-to-distant metastasis, breast cancer–specific survival, or survival between the two groups. The 10-year overall survival (OS) rate was 67% (95% CI, 62%–72%) in the tamoxifen-plus-radiation group, and 66% (95% CI, 61%–71%) in the tamoxifen-alone group.
- PRIME-II was a phase III trial of radiation omission. A total of 1,326 women were randomly assigned to receive either whole-breast irradiation (n = 658) or no irradiation (n = 668). Eligible women were aged 65 years or older and had hormone receptor–positive, node-negative, T1 or T2 primary breast cancer (with tumors measuring ≤3 cm in the largest dimension). Patients had previously undergone breast-conserving surgery with clear excision margins and adjuvant endocrine therapy.[13][Level of evidence A1]
- At a median follow-up of 10 years, the cumulative incidence of local breast cancer recurrence was 9.5% (95% CI, 6.8%–12.3%) in the no-radiation therapy group and 0.9% (95% CI, 0.1%–1.7%) in the radiation therapy group. The 10-year OS rate was almost identical in the two groups, at 80.8% (95% CI, 77.2%–84.3%) in the no-radiation therapy group and 80.7% (95% CI, 76.9%–84.3%) in the radiation therapy group.
Partial breast irradiation
Guidelines that address identifying appropriate candidates for partial breast irradiation have been published.[14]
Evidence (partial breast irradiation):
- The RAPID trial (NCT00282035) randomly assigned 2,135 women aged 40 years or older with ductal carcinoma in situ or node-negative breast cancer treated by breast-conserving surgery to receive either external-beam accelerated partial breast irradiation (APBI) (38.5 Gy in ten fractions delivered twice per day over 5–8 days) or whole-breast radiation therapy (42.5 Gy in 16 fractions delivered once per day over 21 days, or 50 Gy in 25 fractions once per day over 35 days).[15] Sixty-five ipsilateral breast tumor recurrences were observed, 37 in the APBI group, and 28 in the whole-breast irradiation group.
- In patients treated with APBI, the 5-year cumulative rate of ipsilateral breast tumor recurrence was 2.3% (95% CI, 1.4%–3.2%) and the 8-year cumulative rate was 3.0% (95% CI, 1.9%–4.0%).
- In patients treated with whole-breast radiation therapy, the 5-year cumulative rate of ipsilateral breast tumor recurrence was 1.7% (range, 0.9%–2.5%) and the 8-year cumulative rate was 2.8% (range, 1.8%–3.9%).
- The hazard ratio (HR) for APBI versus whole-breast radiation therapy was 1.27 (90% CI, 0.84–1.91).
- Thus, the upper bound of the estimated 90% CI did not exceed the noninferiority margin of 2.02. The APBI arm was associated with less short-term but more long-term toxicity.[15][Level of evidence B1]
- The NSABP B-39/RTOG 0413 trial (NCT00103181) randomly assigned 4,216 women to whole-breast radiation therapy or APBI.[16] Whole-breast radiation therapy was delivered in 25 daily fractions of 50 Gy over 5 weeks, with or without a supplemental boost to the tumor bed, and APBI was delivered as 34 Gy of brachytherapy or 38.5 Gy of external-bream radiation therapy in 10 fractions, over 5 treatment days within an 8-day period.
- At a median follow-up of 10.2 years (interquartile range, 7.5–11.5), 90 of 2,089 women (4%) eligible for the primary outcome in the APBI group and 71 of 2,036 women (3%) in the whole-breast radiation therapy group had an ipsilateral breast tumor recurrence (HR, 1.22; 90% CI, 0.94–1.58). The results did not meet the prespecified criterion for equivalence, an HR of 1.50 or less.
- Toxicity was not substantially different between the arms.[16][Level of evidence B1]
- The randomized, phase III, single-center APBI-IMRT-Florence trial (NCT02104895) evaluated differences in ipsilateral breast tumor recurrence (IBTR) among patients who received APBI using either intensity-modulated radiation therapy, an advanced radiation technique, (30 Gy in five once-daily fractions) or whole-breast radiation therapy with tangents (50 Gy in 25 fractions with a tumor bed boost). Patients had previously undergone breast-conserving surgery. A total of 520 patients were randomly assigned (whole-breast radiation therapy, n = 260; APBI, n = 260).[17]
- The 10-year cumulative incidence of IBTR was 2.5% in the whole-breast radiation therapy arm and 3.7% in the APBI arm (HR, 1.56; 95% CI, 0.55–4.37; P = .40).
- The 10-year OS rate was 91.9% in both arms (HR, 0.95; 95% CI, 0.50–1.79; P = .86). Breast cancer–specific survival at 10 years was 96.7% in the whole-breast radiation therapy arm and 97.8% in the APBI arm (HR, 0.65; 95% CI, 0.21–1.99; P = .45).
- There were fewer acute and late toxicities in the APBI arm (P = .0001 for both comparisons). The APBI arm had improved cosmetic outcomes as evaluated by both physicians and patients (P = .0001 for both comparisons).
Regional nodal irradiation
Regional nodal irradiation is routinely given postmastectomy to patients with involved lymph nodes; however, its role in patients who have breast-conserving surgery and whole-breast radiation therapy has been less clear. A randomized trial (NCT00005957) of 1,832 women showed that administering regional nodal irradiation after breast-conserving surgery and whole-breast radiation therapy reduced the risk of recurrence (10-year disease-free survival [DFS] rate, 82.0% vs. 77.0%; HR, 0.76; 95% CI, 0.61–0.94; P = .01) but did not affect survival (10-year OS rate, 82.8% vs. 81.8%; HR, 0.91; 95% CI, 0.72–1.13; P = .38).[18][Level of evidence A1]
Similar findings were reported from the EORTC trial (NCT00002851). Women with a centrally or medially located primary tumor with or without axillary node involvement, or an externally located tumor with axillary involvement, were randomly assigned to receive whole-breast or thoracic-wall radiation therapy in addition to regional nodal irradiation or not. Breast-conserving surgery was performed for 76.1% of the study population, and the remaining participants underwent mastectomy. No improvement in OS was seen at 10 years among patients who underwent regional nodal irradiation, compared with patients who did not undergo regional nodal radiation (82.3% vs. 80.7%, P = .06). Distant DFS was improved among patients who underwent regional nodal irradiation when compared with patients who did not undergo regional nodal irradiation (78% vs. 75%, P = .02).[19][Level of evidence A1]
A meta-analysis of individual patient data from all randomized trials of regional lymph node radiation therapy versus no regional lymph node radiation therapy in women with early breast cancer included 16 clinical trials involving 14,324 participants. It found that radiation therapy significantly reduced breast cancer mortality (RR, 0.87; 95% CI, 0.80–0.94; P = .0010), with no significant effect on non–breast cancer mortality (RR, 0.97; 0.84–1.11; P = .63), leading to significantly reduced all-cause mortality (RR, 0.90; 0.84–0.96; P = .0022). Estimated absolute reductions in 15-year breast cancer mortality were 1.6% for women with zero positive axillary nodes, 2.7% for those with one to three positive axillary nodes, and 4.5% for those with four or more positive axillary nodes.[20]
Postmastectomy
Postoperative chest wall and regional lymph node adjuvant radiation therapy has traditionally been given to selected patients considered at high risk of locoregional failure after mastectomy. Patients at highest risk of local recurrence meet one or more of the following criteria:[21-23]
- Four or more positive axillary nodes.
- Grossly evident extracapsular nodal extension.
- Lymphovascular space invasion.
- Large primary tumors.
- Very close or positive deep margins of resection of the primary tumor.
In this high-risk group, radiation therapy can decrease locoregional recurrence, even among patients who receive adjuvant chemotherapy.[24]
Patients with one to three involved nodes without any of the high-risk factors may be at a lower risk of local recurrence, and the value of routine use of adjuvant radiation therapy in this setting is an area of controversy.
Evidence (postoperative radiation therapy in patients with one to three involved lymph nodes):
- The 2005 EBCTCG meta-analysis of 42,000 women in 78 randomized treatment comparisons indicated that radiation therapy is beneficial, regardless of the number of lymph nodes involved.[1][Level of evidence A1]
- For women with node-positive disease postmastectomy and axillary clearance (removal of axillary lymph nodes and surrounding fat), radiation therapy reduced the 5-year local recurrence risk from 23% to 6% (absolute gain, 17%; 95% CI, 15.2%–18.8%). This translated into a significant reduction (P = .002) in breast cancer mortality, 54.7% versus 60.1%, with an absolute gain of 5.4% (95% CI, 2.9%–7.9%).
- In subgroup analyses, the 5-year local recurrence rate was reduced by 12% (95% CI, 8%–16%) for women with one to three involved lymph nodes and by 14% (95% CI, 10%–18%) for women with four or more involved lymph nodes. In an updated meta-analysis of 1,314 women with axillary dissection and one to three positive nodes, radiation therapy reduced locoregional recurrence (2-sided P < .00001), overall recurrence (RR, 0.68; 95% CI, 0.57–0.82; 2-sided P = .00006), and breast cancer mortality (RR, 0.80; 95% CI, 0.67–0.95; 2-sided P = .01).[25][Level of evidence A1]
- In contrast, for women at low risk of local recurrence with node-negative disease, the absolute reduction in 5-year local recurrence was only 4% (P = .002; 95% CI, 1.8%–6.2%), and there was not a statistically significant reduction in 15-year breast cancer mortality (absolute gain, 1.0%; P > .1; 95% CI, -0.8%–2.8%).
Further, an analysis of National Surgical Adjuvant Breast and Bowel Project (NSABP) trials showed that even in patients with large (>5 cm) primary tumors and negative axillary lymph nodes, the risk of isolated locoregional recurrence was low enough (7.1%) that routine locoregional radiation therapy was not warranted.[26]
Timing of Postoperative Radiation Therapy
The optimal sequence of adjuvant chemotherapy and radiation therapy after breast-conserving surgery has been studied. Based on studies, delaying radiation therapy for several months after breast-conserving surgery until the completion of adjuvant chemotherapy does not appear to have a negative impact on overall outcome. Additionally, initiating chemotherapy soon after breast-conserving surgery may be preferable for patients at high risk of distant dissemination.
Evidence (timing of postoperative radiation therapy):
- In a randomized trial, patients
received one of the following regimens:[27][Level of evidence A1]
- Chemotherapy first (n = 122), consisting of cyclophosphamide, methotrexate, fluorouracil (5-FU), and prednisone plus doxorubicin repeated every 21 days for four cycles, followed by breast radiation.
- Breast radiation first (n = 122), followed by the same chemotherapy.
The following results were observed:
- With a median follow-up of 5 years, OS was 73% for the radiation-first group and 81% for the chemotherapy-first group (P = .11).
- The 5-year crude rate of first recurrence by site was 5% in the radiation-first group and 14% in the chemotherapy-first group for local recurrence and 32% in the radiation-first group and 20% in the chemotherapy-first group for distant or regional recurrence or both. This difference in the pattern of recurrence was of borderline statistical significance (P = .07).
- Further analyses revealed that differences in recurrence patterns persisted for most subgroups except for those who had either negative tumor margins or one to three positive lymph nodes. For these two subgroups, sequence assignment made little difference in local or distant recurrence rates, although the statistical power of these subgroup analyses was low.
- Potential explanations for the increase in distant recurrence noted in the radiation-first group are that chemotherapy was delayed for a median of 17 weeks after surgery, and that this group received lower chemotherapy dosages because of increased myelosuppression.
- Two additional randomized trials, though not specifically designed to address
the timing of radiation therapy and adjuvant chemotherapy, do add useful
information.
- In the NSABP-B-15 trial, patients who had undergone
breast-conserving surgery received either one course of cyclophosphamide, methotrexate, and 5-FU (CMF) (n = 194) followed by
radiation therapy followed by five additional cycles of CMF, or they received four
cycles of doxorubicin and cyclophosphamide (n = 199) followed by radiation therapy.[28][Level of evidence A1]
- No differences in DFS, distant DFS, and OS were observed between these two arms.
- The International Breast Cancer Study Group trials VI and VII also varied the timing of radiation therapy with CMF adjuvant chemotherapy and reported results similar to NSABP-B-15.[29]
- In the NSABP-B-15 trial, patients who had undergone
breast-conserving surgery received either one course of cyclophosphamide, methotrexate, and 5-FU (CMF) (n = 194) followed by
radiation therapy followed by five additional cycles of CMF, or they received four
cycles of doxorubicin and cyclophosphamide (n = 199) followed by radiation therapy.[28][Level of evidence A1]
These studies showed that delaying radiation therapy for 2 to 7 months after surgery had no effect on the rate of local recurrence. These findings have been confirmed in a meta-analysis.[30][Level of evidence A1]
In an unplanned analysis of patients treated on a phase III trial evaluating the benefit of adding trastuzumab in HER2-positive breast cancer patients, there was no associated increase in acute adverse events or frequency of cardiac events in patients who received concurrent adjuvant radiation therapy and trastuzumab.[31] Therefore, delivering radiation therapy concomitantly with trastuzumab appears to be safe and avoids additional delay in radiation therapy treatment initiation.
Acute and Late Toxicities of Radiation Therapy
Acute toxicities of radiation therapy include radiation dermatitis, breast swelling and/or itching, tightness in the axillary area, and fatigue. If regional nodes are being treated, patients can also experience nausea and a sore throat due to radiation esophagitis. Symptoms typically peak 1 to 2 weeks after radiation therapy, then decrease slowly over the next 4 to 6 weeks.[32]
Late toxicities of radiation therapy are uncommon and can be minimized with radiation delivery techniques and with careful delineation of the target volume. Late effects of radiation include:
- Radiation pneumonitis. In a retrospective analysis of 1,624 women treated with conservative surgery and adjuvant breast radiation at a single institution, the overall incidence of symptomatic radiation pneumonitis was 1.0% at a median follow-up of 77 months.[33] The incidence of pneumonitis increased to 3.0% with the use of a supraclavicular radiation field and to 8.8% when concurrent chemotherapy was administered. The incidence was only 1.3% in patients who received sequential chemotherapy.[33][Level of evidence C1]
- Cardiac events. Controversy existed as to whether adjuvant radiation therapy to the left chest wall or breast, with or without inclusion of the regional lymphatics, was associated with increased cardiac mortality. In women treated with radiation therapy before 1980, an increased cardiac death rate was noted after 10 to 15 years, compared with women with nonradiated or right-side-only radiated breast cancer.[24,34-36] This was probably caused by the radiation received by the left myocardium.
Modern radiation therapy techniques introduced in the 1990s minimized deep radiation to the underlying myocardium when left-sided chest wall or left-breast radiation was used. Cardiac mortality decreased accordingly.[37,38]
An analysis of the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER) data from 1973 to 1989 that reviewed deaths caused by ischemic heart disease in women who received breast or chest wall radiation showed that since 1980, no increased death rate resulting from ischemic heart disease in women who received left chest wall or breast radiation was found.[39,40][Level of evidence C1]
A population-based case-control study evaluated major coronary events (i.e., myocardial infarction, coronary revascularization, or death from ischemic heart disease) in 2,168 women who underwent radiation therapy for breast cancer. The study found the overall average mean dose to the whole heart was 4.9 Gy (range, 0.03–27.72). The rates of major coronary events increased linearly with the mean dose to the heart by 7.4% per Gy (95% CI, 2.9%–14.5%; P < .001), with no apparent threshold.[41]
- Arm lymphedema. Lymphedema remains a major quality-of-life concern for breast cancer patients. Single-modality treatment of the axilla (surgery or radiation) is associated with a low incidence of arm edema. In patients who receive axillary dissection, adjuvant radiation therapy increases the risk of arm edema. Edema occurs in 2% to 10% of patients who receive axillary dissection alone compared with 13% to 18% of patients who receive axillary dissection and adjuvant radiation therapy.[42-44] For more information, see Lymphedema.
- Brachial plexopathy. Radiation injury to the brachial plexus after adjuvant nodal radiation therapy is a rare clinical entity for breast cancer patients. In a single-institution study using current radiation techniques, 449 breast cancer patients treated with postoperative radiation therapy to the breast and regional lymphatics were monitored for 5.5 years to assess the rate of brachial plexus injury.[45] The diagnosis of such injury was made clinically with computed tomography to distinguish radiation injury from tumor recurrence. When 54 Gy in 30 fractions was delivered to the regional nodes, the incidence of symptomatic brachial plexus injury was 1.0%, compared with 5.9% when increased fraction sizes (45 Gy in 15 fractions) were used.
- Contralateral breast cancer. One report suggested an increase in contralateral breast cancer for women younger than 45 years who received chest wall radiation therapy after mastectomy.[46] No increased risk of contralateral breast cancer occurred in women aged 45 years and older who received radiation therapy.[47] Techniques to minimize the radiation dose to the contralateral breast are used to keep the absolute risk as low as possible.[48]
- Risk of second malignancy. The rate of second malignancy after adjuvant radiation therapy is very low. Sarcomas in the treated field are rare, with a long-term risk of 0.2% at 10 years.[49] In nonsmokers, the risk of lung cancer as a result of radiation exposure during treatment is minimal when current dosimetry techniques are used. Smokers, however, may have a small increased risk of lung cancer in the ipsilateral lung.[50]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Clarke M, Collins R, Darby S, et al.: Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366 (9503): 2087-106, 2005. [PUBMED Abstract]
- Eifel P, Axelson JA, Costa J, et al.: National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst 93 (13): 979-89, 2001. [PUBMED Abstract]
- Darby S, McGale P, Correa C, et al.: Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378 (9804): 1707-16, 2011. [PUBMED Abstract]
- Romestaing P, Lehingue Y, Carrie C, et al.: Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol 15 (3): 963-8, 1997. [PUBMED Abstract]
- Bartelink H, Horiot JC, Poortmans P, et al.: Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med 345 (19): 1378-87, 2001. [PUBMED Abstract]
- Bartelink H, Maingon P, Poortmans P, et al.: Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 16 (1): 47-56, 2015. [PUBMED Abstract]
- Wazer DE, Kramer B, Schmid C, et al.: Factors determining outcome in patients treated with interstitial implantation as a radiation boost for breast conservation therapy. Int J Radiat Oncol Biol Phys 39 (2): 381-93, 1997. [PUBMED Abstract]
- King MT, Link EK, Whelan TJ, et al.: Quality of life after breast-conserving therapy and adjuvant radiotherapy for non-low-risk ductal carcinoma in situ (BIG 3-07/TROG 07.01): 2-year results of a randomised, controlled, phase 3 trial. Lancet Oncol 21 (5): 685-698, 2020. [PUBMED Abstract]
- Whelan TJ, Pignol JP, Levine MN, et al.: Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 362 (6): 513-20, 2010. [PUBMED Abstract]
- Haviland JS, Owen JR, Dewar JA, et al.: The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 14 (11): 1086-94, 2013. [PUBMED Abstract]
- Hickey BE, James ML, Lehman M, et al.: Fraction size in radiation therapy for breast conservation in early breast cancer. Cochrane Database Syst Rev 7: CD003860, 2016. [PUBMED Abstract]
- Hughes KS, Schnaper LA, Bellon JR, et al.: Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 31 (19): 2382-7, 2013. [PUBMED Abstract]
- Kunkler IH, Williams LJ, Jack WJL, et al.: Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer. N Engl J Med 388 (7): 585-594, 2023. [PUBMED Abstract]
- Shaitelman SF, Anderson BM, Arthur DW, et al.: Partial Breast Irradiation for Patients With Early-Stage Invasive Breast Cancer or Ductal Carcinoma In Situ: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol 14 (2): 112-132, 2024. [PUBMED Abstract]
- Whelan TJ, Julian JA, Berrang TS, et al.: External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet 394 (10215): 2165-2172, 2019. [PUBMED Abstract]
- Vicini FA, Cecchini RS, White JR, et al.: Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet 394 (10215): 2155-2164, 2019. [PUBMED Abstract]
- Meattini I, Marrazzo L, Saieva C, et al.: Accelerated Partial-Breast Irradiation Compared With Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J Clin Oncol 38 (35): 4175-4183, 2020. [PUBMED Abstract]
- Whelan TJ, Olivotto IA, Parulekar WR, et al.: Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 373 (4): 307-16, 2015. [PUBMED Abstract]
- Poortmans PM, Collette S, Kirkove C, et al.: Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 373 (4): 317-27, 2015. [PUBMED Abstract]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG): Radiotherapy to regional nodes in early breast cancer: an individual patient data meta-analysis of 14 324 women in 16 trials. Lancet 402 (10416): 1991-2003, 2023. [PUBMED Abstract]
- Ragaz J, Jackson SM, Le N, et al.: Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 337 (14): 956-62, 1997. [PUBMED Abstract]
- Overgaard M, Hansen PS, Overgaard J, et al.: Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 337 (14): 949-55, 1997. [PUBMED Abstract]
- Fowble B, Gray R, Gilchrist K, et al.: Identification of a subgroup of patients with breast cancer and histologically positive axillary nodes receiving adjuvant chemotherapy who may benefit from postoperative radiotherapy. J Clin Oncol 6 (7): 1107-17, 1988. [PUBMED Abstract]
- Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet 355 (9217): 1757-70, 2000. [PUBMED Abstract]
- McGale P, Taylor C, Correa C, et al.: Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383 (9935): 2127-35, 2014. [PUBMED Abstract]
- Taghian AG, Jeong JH, Mamounas EP, et al.: Low locoregional recurrence rate among node-negative breast cancer patients with tumors 5 cm or larger treated by mastectomy, with or without adjuvant systemic therapy and without radiotherapy: results from five national surgical adjuvant breast and bowel project randomized clinical trials. J Clin Oncol 24 (24): 3927-32, 2006. [PUBMED Abstract]
- Recht A, Come SE, Henderson IC, et al.: The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med 334 (21): 1356-61, 1996. [PUBMED Abstract]
- Fisher B, Brown AM, Dimitrov NV, et al.: Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 8 (9): 1483-96, 1990. [PUBMED Abstract]
- Wallgren A, Bernier J, Gelber RD, et al.: Timing of radiotherapy and chemotherapy following breast-conserving surgery for patients with node-positive breast cancer. International Breast Cancer Study Group. Int J Radiat Oncol Biol Phys 35 (4): 649-59, 1996. [PUBMED Abstract]
- Hickey BE, Francis DP, Lehman M: Sequencing of chemotherapy and radiotherapy for early breast cancer. Cochrane Database Syst Rev 4: CD005212, 2013. [PUBMED Abstract]
- Halyard MY, Pisansky TM, Dueck AC, et al.: Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol 27 (16): 2638-44, 2009. [PUBMED Abstract]
- Lapen K, Sabol C, Tin AL, et al.: Development and Pilot Implementation of a Remote Monitoring System for Acute Toxicity Using Electronic Patient-Reported Outcomes for Patients Undergoing Radiation Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys 111 (4): 979-991, 2021. [PUBMED Abstract]
- Lingos TI, Recht A, Vicini F, et al.: Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 21 (2): 355-60, 1991. [PUBMED Abstract]
- Paszat LF, Mackillop WJ, Groome PA, et al.: Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol 16 (8): 2625-31, 1998. [PUBMED Abstract]
- Rutqvist LE, Johansson H: Mortality by laterality of the primary tumour among 55,000 breast cancer patients from the Swedish Cancer Registry. Br J Cancer 61 (6): 866-8, 1990. [PUBMED Abstract]
- Darby SC, McGale P, Taylor CW, et al.: Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 6 (8): 557-65, 2005. [PUBMED Abstract]
- Højris I, Overgaard M, Christensen JJ, et al.: Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomised trials. Radiotherapy Committee of the Danish Breast Cancer Cooperative Group. Lancet 354 (9188): 1425-30, 1999. [PUBMED Abstract]
- Nixon AJ, Manola J, Gelman R, et al.: No long-term increase in cardiac-related mortality after breast-conserving surgery and radiation therapy using modern techniques. J Clin Oncol 16 (4): 1374-9, 1998. [PUBMED Abstract]
- Giordano SH, Kuo YF, Freeman JL, et al.: Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 97 (6): 419-24, 2005. [PUBMED Abstract]
- Harris EE, Correa C, Hwang WT, et al.: Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 24 (25): 4100-6, 2006. [PUBMED Abstract]
- Darby SC, Ewertz M, McGale P, et al.: Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368 (11): 987-98, 2013. [PUBMED Abstract]
- Meek AG: Breast radiotherapy and lymphedema. Cancer 83 (12 Suppl American): 2788-97, 1998. [PUBMED Abstract]
- Larson D, Weinstein M, Goldberg I, et al.: Edema of the arm as a function of the extent of axillary surgery in patients with stage I-II carcinoma of the breast treated with primary radiotherapy. Int J Radiat Oncol Biol Phys 12 (9): 1575-82, 1986. [PUBMED Abstract]
- Swedborg I, Wallgren A: The effect of pre- and postmastectomy radiotherapy on the degree of edema, shoulder-joint mobility, and gripping force. Cancer 47 (5): 877-81, 1981. [PUBMED Abstract]
- Powell S, Cooke J, Parsons C: Radiation-induced brachial plexus injury: follow-up of two different fractionation schedules. Radiother Oncol 18 (3): 213-20, 1990. [PUBMED Abstract]
- Boice JD, Harvey EB, Blettner M, et al.: Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med 326 (12): 781-5, 1992. [PUBMED Abstract]
- Storm HH, Andersson M, Boice JD, et al.: Adjuvant radiotherapy and risk of contralateral breast cancer. J Natl Cancer Inst 84 (16): 1245-50, 1992. [PUBMED Abstract]
- Fraass BA, Roberson PL, Lichter AS: Dose to the contralateral breast due to primary breast irradiation. Int J Radiat Oncol Biol Phys 11 (3): 485-97, 1985. [PUBMED Abstract]
- Taghian A, de Vathaire F, Terrier P, et al.: Long-term risk of sarcoma following radiation treatment for breast cancer. Int J Radiat Oncol Biol Phys 21 (2): 361-7, 1991. [PUBMED Abstract]
- Inskip PD, Stovall M, Flannery JT: Lung cancer risk and radiation dose among women treated for breast cancer. J Natl Cancer Inst 86 (13): 983-8, 1994. [PUBMED Abstract]
Systemic Therapy for Stages I, II, and III Breast Cancer
The first decision about the use of systemic therapy in patients with stages I, II and III breast cancer is whether it should be given before or after surgery. This section outlines factors to consider when making this decision. Information about the treatment of locally advanced or inflammatory breast cancer is also included in this section.
Preoperative chemotherapy, also known as primary or neoadjuvant chemotherapy, has traditionally been given to patients with locally advanced breast cancer to reduce tumor volume and allow for definitive surgery. Treatment with preoperative chemotherapy can also allow for breast conservation therapy in patients who are not candidates for breast conservation at initial presentation. Preoperative chemotherapy may also reduce the need for an axillary lymph node dissection (ALND) in patients presenting with node-positive disease.
Much of the evidence presented in the following sections on preoperative chemotherapy is discussed in an American Society of Clinical Oncology guideline that describes the selection of options for the management of these patients.[1]
A 2005 meta-analysis of multiple randomized clinical trials demonstrated that preoperative chemotherapy is associated with identical disease-free survival (DFS) and overall survival (OS) as the same therapy in the adjuvant setting.[2][Level of evidence A1]
In 2019, the Early Breast Cancer Trialists’ Collaborative Group performed a meta-analysis using individual patient data from 4,756 women who participated in 10 trials that compared neoadjuvant chemotherapy with the same regimen given in the adjuvant setting.[3] Compared with adjuvant therapy, neoadjuvant therapy was associated with an increased frequency of breast conservation (65% vs. 49%). There were no differences between neoadjuvant chemotherapy and adjuvant therapy in distant recurrence, breast cancer mortality, or death from any cause. However, neoadjuvant therapy was associated with higher 15-year local recurrence rates (21.4% vs. 15.9%; relative risk [RR], 1.37; 95% confidence interval [CI], 1.17−1.61; P = .001).[3][Level of evidence A1]
Pathological complete response (pCR) has been used as a surrogate end point for long-term outcomes, such as DFS, event-free survival (EFS), and OS, in preoperative clinical trials in breast cancer. A pooled analysis (CTNeoBC) of 11 preoperative randomized trials (n = 11,955) determined that pCR, defined as no residual invasive cancer in the breast and axillary nodes with presence or absence of in situ cancer (ypT0/is ypN0 or ypT0 ypN0), was associated with improved outcomes compared with eradication of invasive tumor from the breast alone (ypT0/is).[4] pCR could not be validated in this study as a surrogate end point for improved EFS and OS.[4][Level of evidence C2] Because of a strong association between pCR and substantially improved outcomes in individual patients with more aggressive subtypes of breast cancer, the U.S. Food and Drug Administration (FDA) has supported use of pCR as an end point in preoperative clinical trials for patients with high-risk, early-stage breast cancer.
Unfortunately, categorizing patients as having pCR or residual disease offers no distinction among patients with varied amounts of residual disease. The residual cancer burden (RCB) method was designed to address this and other prognostic deficits. The RCB method provides a standard to evaluate and quantify the extent of residual disease in breast and axillary lymph nodes following neoadjuvant chemotherapy. It is reported as a continuous score, with pCR being scored as RCB-0. There are four RCB classes ranging from RCB-0 to RCB-3. Determining RCB after neoadjuvant treatment has been validated as a prognostic predictor in early breast cancer.
A pooled, multinational, multi-institutional analysis was performed, using participant-level RCB results and clinicopathological data. Data from 5,161 patients were analyzed to assess the association between the continuous RCB score and the primary study outcome, EFS. With a median follow-up of 56 months, the RCB score was prognostic within each breast cancer subtype, with a higher RCB score significantly associated with worse EFS. RCB score was prognostic for EFS in multivariable models adjusted for age, grade, T (tumor) category, and nodal status at baseline. The adjusted hazard ratio (HR) associated with a one-unit increase in RCB ranged from 1.52 in the HER2-negative hormone receptor–positive group to 2.09 in the HER2-positive hormone receptor–negative group (P < .0001 for all subtypes).[5]
Neoadjuvant therapy is particularly favored in patients with triple-negative or HER2-positive disease, when pathological response is used as a guide in choosing the optimal adjuvant therapy after surgery. For more information, see the sections on Stages I, II, and III Triple-Negative Breast Cancer and Stages I, II, and III HER2-Positive Breast Cancer.
Omission of postoperative radiation therapy to the regional nodes in patients who initially present as node positive and become node negative after neoadjuvant therapy is currently being evaluated.
Patient Selection, Staging, Treatment, and Follow-Up
Multidisciplinary management of patients undergoing preoperative therapy by an experienced team is essential to optimize the following:
- Patient selection.
- Choice of systemic therapy.
- Management of the axilla and surgical approach.
- Decision to administer adjuvant radiation therapy.
The tumor histology, grade, and hormone receptor status are carefully evaluated before preoperative therapy is initiated. Patients whose tumors have a pure lobular histology, low grade, or high hormone receptor expression and HER2-negative status are less likely to respond to chemotherapy and should consider primary surgery, especially if the nodes are clinically negative. Even if adjuvant chemotherapy is given after surgery in these cases, a third-generation regimen (anthracycline/taxane based) may be avoided.
Before beginning preoperative therapy, the extent of the disease within the breast and regional lymph nodes should be assessed. Staging of systemic disease may include:[6]
- Computed tomography scan of the chest and abdomen and a bone scan.
- Positron emission tomography.
When breast-conserving therapy is desired, baseline breast imaging is performed to identify the tumor location and exclude multicentric disease. Suspicious abnormalities are usually biopsied before beginning treatment, and a marker is placed at the center of the breast tumor(s). When possible, suspicious axillary nodes should be biopsied before initiation of systemic treatment.
In patients with clinically negative nodes who receive neoadjuvant chemotherapy, a sentinel lymph node (SLN) biopsy is typically performed at the time of surgery. In patients presenting with positive lymph nodes, detected by either clinical examination or imaging, SLN biopsy may be performed in a patient who becomes clinically node negative after preoperative therapy.[7] The use of dual mapping with both radiocolloid and blue dye and retrieval of at least three negative lymph nodes was associated with a lower false-negative rate and ALND may be omitted in these patients.[8][Level of evidence B4]; [9][Level of evidence C2]; [10][Level of evidence C3]
When considering preoperative therapy, treatment options include:
- For HER2-negative breast tumors, an anthracycline/taxane-based chemotherapy regimen.
- For HER2-positive disease, chemotherapy and HER2-targeted therapy.
- Ideally, the entire treatment regimen is administered before surgery.
- For postmenopausal women with hormone receptor–positive breast cancer, chemotherapy is an option. For those who cannot be given chemotherapy, preoperative endocrine therapy may be an option.
- For premenopausal women with hormone-responsive cancer, the use of preoperative endocrine therapy is under investigation.
Regular clinical assessment of response to therapy is necessary after beginning preoperative therapy. Repeat radiographic assessment is also required if breast conservation is the surgical goal. Patients with progressive disease during preoperative therapy may either transition to a non–cross-resistant regimen or proceed to surgery, if feasible.[11,12] Although switching to a non–cross-resistant regimen results in a higher pCR rate than continuing the same therapy, there is no clear evidence that other breast cancer outcomes are improved with this approach.
Stages I, II, and III HER2-Negative Hormone Receptor–Positive Breast Cancer
Most studies that support the use of chemotherapy were conducted after patients had surgery and prior to the widespread use of preoperative therapy. In general, their results are still applicable to preoperative treatment, and these regimens are the ones most commonly used in the neoadjuvant space for this subtype. The following section describes studies that examined the use of chemotherapy in the preoperative setting.
Early trials examined whether anthracycline-based regimens used in the adjuvant setting would prolong DFS and OS when used in the preoperative setting. The evidence supports higher rates of breast-conserving therapy with the use of a preoperative anthracycline chemotherapy regimen than with postoperative use, but no improvement in survival was noted with the preoperative strategy.
Typically, an anthracycline-and-taxane–based regimen is used if chemotherapy is administered in the neoadjuvant setting for patients with HER2-negative breast cancer.
Evidence (anthracycline/taxane–based chemotherapy regimen):
- In an effort to improve on the results observed with doxorubicin and cyclophosphamide (AC) alone, the NSABP B-27 trial (NCT00002707) was conducted. Patients were randomly assigned to receive (1) four cycles of preoperative AC followed by surgery, (2) four cycles of AC followed by four cycles of docetaxel and then surgery, or (3) four cycles of AC followed by surgery and then four cycles of docetaxel.[13][Level of evidence B1]
- The administration of preoperative AC followed by docetaxel was associated with a higher clinical complete response rate compared with the administration of AC alone (63.6% for AC followed by docetaxel and 40.1% for AC alone; P < .001); a higher pCR rate was also observed (26.1% for AC followed by docetaxel and 13.7% for AC alone; P < .001).
- Data from NSABP B-27 and the Aberdeen Breast Group Trial support the use of anthracycline/taxane–based regimens in women with initial response or with relative resistance to anthracyclines.[11]
- Alternative anthracycline/taxane schedules have also been evaluated (concurrent docetaxel, doxorubicin, and cyclophosphamide) and appear similar in efficacy to the sequential approach described above.[14][Level of evidence B3]
- The phase III GeparSepto trial (NCT01583426) investigated an alternative taxane (nab-paclitaxel) in patients with untreated primary breast cancer.[15] Patients (n = 1,229) were randomly assigned to receive 12 weeks of nab-paclitaxel or paclitaxel followed by epirubicin and cyclophosphamide for four cycles.
- The pCR rate was higher in the nab-paclitaxel arm (233 patients, 38%; 95% CI, 35%–42%) when compared with the paclitaxel arm (174 patients, 29%; 95% CI, 25%–33%).[15][Level of evidence B3]
- However, in the ETNA trial (NCT01822314) that compared neoadjuvant nab-paclitaxel with paclitaxel followed by anthracycline-based therapy, no significant difference in pCR was observed, and neutropenia and peripheral neuropathy were more frequent in the nab-paclitaxel arm.[16]
- Differences in taxane dose and schedule may explain the different findings in the GeparSepto and ETNA trials.
- The incorporation of other cytotoxic agents to anthracycline/taxane–based regimens has not offered a significant additional benefit to breast conservation or pCR rate in unselected breast cancer populations.[17][Level of evidence B3]
Preoperative endocrine therapy for HER2-negative hormone receptor–positive breast cancer
Preoperative endocrine therapy may be an option for postmenopausal women with hormone receptor–positive breast cancer when chemotherapy is not a suitable option because of comorbidities or performance status. Although the toxicity profile of preoperative hormonal therapy over the course of 3 to 6 months is favorable, the pCR rates obtained (1%–8%) are far lower than have been reported with chemotherapy in unselected populations.[18][Level of evidence B3]
Longer duration of preoperative therapy may be required in this patient population. Preoperative tamoxifen was associated with an overall response rate of 33%, with maximum response occurring up to 12 months after therapy in some patients.[19] A randomized study of 4, 8, or 12 months of preoperative letrozole in older patients who were not fit for chemotherapy indicated that the longer duration of therapy resulted in the highest pCR rate (17.5% vs. 5% vs. 2.5%, P-value for trend < .04).[20][Level of evidence B3]
Aromatase inhibitors (AIs) have also been compared with tamoxifen in the preoperative setting. Overall objective response and breast-conserving therapy rates with 3 to 4 months of preoperative therapy were either statistically significantly improved in the AI-treated women [18] or comparable to tamoxifen-associated outcomes.[20]
The use of preoperative endocrine therapy in premenopausal women with hormone-responsive breast cancer remains investigational.
Postoperative systemic therapy for HER2-negative hormone receptor–positive breast cancer
Stage and molecular features determine the need for adjuvant systemic therapy and the choice of modalities for patients who have not been treated with preoperative systemic therapy. The selection of therapy is most appropriately based on knowledge of an individual’s risk of tumor recurrence balanced against the short-term and long-term risks of adjuvant treatment. This approach allows clinicians to help individuals determine if the gains anticipated from treatment are reasonable for their situation.
Anthracycline-containing chemotherapy
An Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta-analysis included 11 trials that began from 1976 to 1989 in which women were randomly assigned to receive regimens containing anthracyclines (e.g., doxorubicin or epirubicin) or CMF (cyclophosphamide, methotrexate, and fluorouracil [5-FU]). The result of the overview analysis comparing CMF and anthracycline-containing regimens suggested a slight advantage for the anthracycline regimens in both premenopausal and postmenopausal women. The HER2 status of the women in these trials was unknown.[21]
Several trials have addressed the benefit of adding a taxane (paclitaxel or docetaxel) to an anthracycline-based adjuvant chemotherapy regimen.[22-26]
A literature-based meta-analysis of 13 studies demonstrated that the inclusion of a taxane improved both DFS and OS (DFS: HR, 0.83; 95% CI, 0.79–0.87; P < .001; OS: HR, 0.85; 95% CI, 0.79–0.91; P < .001). Five-year absolute survival differences were 5% for DFS and 3% for OS, in favor of taxane-containing regimens.[22][Level of evidence A1]
A number of studies have addressed the optimal chemotherapy schedule and taxane selection.
An Eastern Cooperative Oncology Group–led intergroup trial (E1199 [NCT00004125]) involving 4,950 patients compared, in a factorial design, two schedules (weekly and every 3 weeks) of the two drugs (docetaxel vs. paclitaxel) after standard-dose AC chemotherapy given every 3 weeks.[27][Level of evidence A1] Study findings include the following:
- There was no difference observed in the overall comparison of docetaxel to paclitaxel with regard to DFS (odds ratio [OR], 1.03; 95% CI, 0.91–1.16; P = .61) or between the 1-week and 3-week schedules (OR, 1.06; 95% CI, 0.94–1.20; P = .33).
- There was a significant association between the drug administered and schedule for both DFS (0.003) and OS (0.01). Thus, compared with paclitaxel given every 3 weeks, paclitaxel given weekly improved both DFS (OR, 1.27; 95% CI, 1.01–1.57; P = .006) and OS (OR, 1.32; 95% CI, 1.02–1.72; P = .01).
- Docetaxel given every 3 weeks was also superior in DFS to paclitaxel given every 3 weeks (OR, 1.23; 95% CI, 1.00–1.52; P = .02), but the difference was not statistically significant for OS (OR, 1.13; 95% CI, 0.88–1.46; P = .25).
- Docetaxel given weekly was not superior to paclitaxel given every 3 weeks. There was no stated a priori basis for expecting that varying the schedule of administration would have opposite effects for the two drugs.
Several studies sought to determine whether decreasing the duration between chemotherapy cycles could improve clinical outcomes. The overall results of these studies support the use of dose-dense chemotherapy for women with HER2-negative breast cancer.
Evidence (administration of dose-dense chemotherapy in women with HER2-negative breast cancer):
- A U.S. intergroup trial (CALGB-9741 [NCT00003088]) of 2,005 node-positive patients compared, in a 2 × 2 factorial design, the use of concurrent AC followed by paclitaxel with sequential doxorubicin, paclitaxel, and cyclophosphamide given every 2 weeks with filgrastim or every 3 weeks.[28][Level of evidence A1]
- At a median follow-up of 68 months, dose-dense treatment improved DFS, the primary end point, in all patient populations (HR, 0.80; P = .018), but not OS (HR, 0.85; P = .12).[29][Level of evidence A1]
- There was no interaction between density and sequence.
- Severe neutropenia was less frequent in patients who received the dose-dense regimens.[30][Level of evidence A1]
- An Italian trial (NCT00433420) compared two versus three weekly doses of epirubicin plus cyclophosphamide (with or without 5-FU) in a factorial design, with a result similar to a U.S. intergroup trial; however, this trial also demonstrated a difference in OS.[31]
- For the dose-density comparison, the 5-year DFS rate was 81% (95% CI, 79%–84%) in patients treated every 2 weeks and 76% (95% CI, 74%–79%) in patients treated every 3 weeks (HR, 0.77; 95% CI, 0.65–0.92; P = .004).
- Five-year OS rates were 94% (95% CI, 93%–96%) and 89% (95% CI, 87%–91%; HR, 0.65; 0.51–0.84; P = .001).[31][Level of evidence A1]
- A meta-analysis of 26 randomized trials that included 37,298 women treated with anthracycline- and taxane-containing chemotherapy compared standard regimens (given every 3–4 weeks) with more dose-intense regimens. Regimens that increased dose intensity by shortening the interval between cycles (i.e., dose-dense therapy or administration of the same dose over a shorter time period) and regimens that increased dose intensity by administering individual drugs in sequence to allow for higher doses (i.e., sequential scheduling).[32]
- Patients who received more dose-intense regimens had superior recurrence-free survival (RFS) (28.0% vs. 31.4%; RR, 0.86; 95% CI, 0.82–0.89; P < .0001) and OS (18.9% vs. 21.3%; RR, 0.87; 95% CI, 0.83–0.92; P < .0001) at 10 years. The difference was present and statistically significant in receptor-positive and receptor-negative subgroups.
Non–anthracycline-containing regimens
Because of potential long-term toxicities from anthracyclines, the efficacy and toxicity of non–anthracycline-containing regimens have been studied. For more information, see the Toxicity of Adjuvant Chemotherapy section.
Data are inconsistent regarding whether an anthracycline-containing regimen is more efficacious than a non–anthracycline-containing regimen. Both types of regimens are acceptable, and the choice must be individualized on the basis of risk and other patient characteristics.
Evidence (non–anthracycline-containing regimens):
- The ABC trials were three open-label, randomized, phase III trials comparing TC (taxane and cyclophosphamide) with regimens containing an anthracycline/cyclophosphamide plus a taxane (TaxAC) for the adjuvant treatment of patients with HER2-negative early-stage breast cancer.[33] The three trials were analyzed together with a primary end point of invasive disease-free survival (IDFS). The primary aim was to determine if TC (the non-anthracycline arm) was noninferior to the TaxAC arms. Inferiority for TC was predefined as an HR exceeding 1.18 for the TC versus TaxAC arms. Participants were randomly assigned to receive TC (n = 2, 125) or TaxAC (n = 2,127).
- In an interim futility analysis, the HR for IDFS was 1.202 (95% CI, 0.97–1.49) for TC versus TaxAC, which exceeded the predetermined limit to define TC as inferior.
- The 4-year IDFS rate was 88.2% for patients who received TC and 90.7% for patients who received TaxAC (P = .04).
- Although the findings favored treatment with regimens containing an anthracycline/cyclophosphamide plus a taxane, absolute differences between TC and TaxAC were small. Exploratory analyses suggested the greatest benefits from the TaxAC regimens were seen in patients with triple-negative disease and hormone receptor–positive disease with involved axillary lymph nodes, supporting a role for non–anthracycline-containing regimens in patients with lower-risk disease.
- The West German Plan B trial (NCT01049425) randomly assigned 2,499 women with node-positive or high-risk node-negative disease to receive either four cycles of epirubicin/cyclophosphamide plus four cycles of docetaxel (EC-T) or six cycles of TC.[34] After an early amendment, women with hormone receptor–positive disease and a recurrence score below 12 were excluded.
- After a median follow-up of 60 months, 5-year outcomes were similar in the EC-T and TC arms for DFS (HR, 1.004; 95% CI, 0.776–1.299) and OS (HR, 0.937; 95% CI, 0.654–1.342).[34][Level of evidence B1]
- The upper 90% confidence limit for DFS did not exceed the noninferiority boundary of 1.467.
- There were five treatment-related deaths among patients who received TC and one death among those who received EC-T, but symptomatic adverse events were more frequent in patients who received EC-T.
Timing of postoperative chemotherapy
The optimal time to initiate adjuvant therapy is uncertain. Studies have reported the following:
- A retrospective, observational, single-institution study of patients with early-stage breast cancer who were diagnosed between 1997 and 2011 revealed that delays in initiation of adjuvant chemotherapy adversely affected survival outcomes.[35][Level of evidence C1]
- Initiation of chemotherapy 61 days or more after surgery was associated with adverse outcomes among patients with stage II breast cancer (distant RFS [DRFS]: HR, 1.20; 95% CI, 1.02–1.43) and stage III breast cancer (OS: HR, 1.76; 95% CI, 1.26–2.46; RFS: HR, 1.34; 95% CI, 1.01–1.76; and DRFS: HR, 1.36; 95% CI, 1.02–1.80).
- Patients with triple-negative breast cancer (TNBC) and those with HER2-positive tumors treated with trastuzumab who started chemotherapy 61 days or more after surgery had worse survival (TNBC: HR, 1.54; 95% CI, 1.09–2.18; HER2-positive: HR, 3.09; 95% CI, 1.49–6.39) than did those who initiated treatment in the first 30 days after surgery.
- Because of the weaknesses and limitations of this study design, the optimal time to initiate adjuvant chemotherapy remains uncertain.
- A population-based study from California included 24,843 patients and found that delays in initiating adjuvant chemotherapy of 90 days or less had no impact on OS, but found a substantial effect for delays over 90 days (HR, 1.27; 95% CI, 1.05–1.53), particularly among patients with TNBC.[36][Level of evidence C1]
- Multiple other studies have examined the effect of treatment delays of 90 days or less and have reported inconsistent results, with the possible exception of patients with TNBC.[36]
Endocrine therapy
Much of the evidence presented in the following sections on therapy for women with hormone receptor–positive disease has been considered in an American Society of Clinical Oncology guideline that describes several options for the management of these patients.[37] Five years of adjuvant endocrine therapy has been shown to substantially reduce the risks of locoregional and distant recurrence, contralateral breast cancer, and death from breast cancer.
The optimal duration of endocrine therapy is unclear, with the preponderance of evidence supporting at least 5 years of endocrine therapy. A meta-analysis of 88 clinical trials involving 62,923 women with hormone receptor–positive breast cancer who were disease free after 5 years of endocrine therapy showed a steady risk of late recurrence 5 to 20 years after diagnosis.[38][Level of evidence C2] The risk of distant recurrence correlated with the original tumor (T) and node (N) status, with risks ranging from 10% to 41%.
Tamoxifen
Tamoxifen has been shown to benefit women with hormone receptor–positive breast cancer.
Evidence (tamoxifen for hormone receptor–positive early breast cancer):
- The EBCTCG performed a meta-analysis of systemic treatment of early breast
cancer by hormone, cytotoxic, or biological therapy methods in randomized trials
involving 144,939 women with stage I or stage II breast cancer. An analysis published in 2005 included information on 80,273 women in 71 trials of adjuvant tamoxifen.[21][Level of evidence A1]
- In this analysis, the benefit of tamoxifen was found to be restricted to women with hormone receptor–positive or hormone receptor–unknown breast tumors. In these women, the 15-year absolute reduction associated with 5 years of use was 12% for recurrence and 9% for mortality.
- Allocation to approximately 5 years of adjuvant tamoxifen reduces the annual breast cancer death rate by 31%, largely irrespective of the use of chemotherapy, age (<50 years, 50–69 years, ≥70 years), progesterone receptor (PR) status, or other tumor characteristics.
- The meta-analysis also confirmed the benefit of adjuvant tamoxifen in hormone receptor–positive premenopausal women. Women younger than 50 years obtained a degree of benefit from 5 years of tamoxifen similar to that obtained by older women. In addition, the proportional reductions in both recurrence and mortality associated with tamoxifen use were similar in women with either node-negative or node-positive breast cancer, but the absolute improvement in survival at 10 years was greater in the node-positive breast cancer group (5.3% vs. 12.5% with 5 years of use).
- Similar results were found in the IBCSG-13-93 trial.[39] Of 1,246 women with stage II disease, only the women with hormone receptor–positive disease benefited from tamoxifen.
The optimal duration of tamoxifen use has been addressed by the EBCTCG meta-analysis and by several large randomized trials.[21,40-43] Ten years of tamoxifen therapy has shown superiority to shorter durations of tamoxifen therapy.
Evidence (duration of tamoxifen therapy):
- The EBCTCG
meta-analysis demonstrated that 5 years of tamoxifen was superior to shorter durations. The following results were reported:[21]
- A highly significant advantage of 5 years versus 1 to 2 years of tamoxifen with respect to the risk of recurrence (proportionate reduction, 15.2%; P < .001) and a less significant advantage with respect to mortality (proportionate reduction, 7.9%; P = .01) was observed.
- Long-term follow-up of the Adjuvant Tamoxifen Longer Against Shorter (ATLAS [NCT00003016]) trial demonstrated that 10 years of tamoxifen therapy was superior to 5 years of tamoxifen therapy. Between 1996 and 2005, 12,894 women with early breast cancer were randomly assigned to receive 10 years or 5 years of tamoxifen therapy. The following results were reported:[43][Level of evidence A1]
- Study results revealed that 10 years of tamoxifen reduced the risk of breast cancer recurrence (617 recurrences for 10 years of tamoxifen vs. 711 recurrences for 5 years of tamoxifen; P = .002), reduced breast cancer mortality (331 deaths for 10 years of tamoxifen vs. 397 deaths for 5 years of tamoxifen; P = .01), and reduced overall mortality (639 deaths for 10 years of tamoxifen vs. 722 deaths for 5 years of tamoxifen; P = .01).
- Of note, from the time of the original breast cancer diagnosis, the benefits of 10 years of therapy were less extreme before than after year 10. At 15 years from the time of diagnosis, breast cancer mortality was 15% at 10 years and 12.2% at 5 years.
- Compared with 5 years, 10 years of tamoxifen therapy increased the risk of the following:
- Pulmonary embolus: RR, 1.87 (95% CI, 1.13–3.07; P = .01).
- Stroke: RR, 1.06 (95% CI, 0.83–1.36).
- Ischemic heart disease: RR, 0.76 (95% CI, 0.6–0.95; P = .02).
- Endometrial cancer: RR, 1.74 (95% CI, 1.30–2.34; P = .0002). Notably, the cumulative risk of endometrial cancer during years 5 to 14 from breast cancer diagnosis was 3.1% for women who received 10 years of tamoxifen versus 1.6% for women who received 5 years of tamoxifen. The mortality for years 5 to 14 was 12.2 versus 15 for an absolute mortality reduction of 2.8%.
The results of the ATLAS trial indicated that for women who remained premenopausal after 5 years of adjuvant tamoxifen, continued tamoxifen for 5 more years was beneficial.[43] Women who have become menopausal after 5 years of tamoxifen may also be treated with AIs. For more information, see the Aromatase inhibitors section.
Tamoxifen and chemotherapy
Because of the results of an EBCTCG analysis, the use of tamoxifen in women who received adjuvant chemotherapy does not attenuate the benefit of chemotherapy.[21] However, concurrent use of tamoxifen with chemotherapy is less effective than sequential administration.[44]
Ovarian ablation, tamoxifen, and chemotherapy
Evidence suggests ovarian ablation alone is not an effective substitute for other systemic therapies.[45-49] Further, the addition of ovarian ablation to chemotherapy and/or tamoxifen has not been found to significantly improve outcomes.[47,49-52]
Evidence (tamoxifen plus ovarian suppression):
- The largest study (SOFT [NCT00066690]) to examine the addition of ovarian ablation to tamoxifen with or without chemotherapy randomly assigned 2,033 premenopausal women (53% of whom had received previous chemotherapy) to receive tamoxifen or tamoxifen plus ovarian suppression with triptorelin or ablation with surgery or radiation therapy.[53][Level of evidence B1]
- Upon initial report, with a median follow-up of 5.6 years, there was no significant difference in the primary outcome of DFS (HR, 0.83; 95% CI, 0.66–1.04; P = .10); the 5-year DFS rate was 86% in the tamoxifen-plus-ovarian-suppression group versus 84.7% in the tamoxifen-alone group. However, updated results with a median follow-up of 8 years, demonstrated improved DFS with tamoxifen plus ovarian suppression compared with tamoxifen alone (HR, 0.76; 95% CI, 0.62–0.93; P = .009); the 8-year DFS rate was 83.2% in the tamoxifen-plus-ovarian-suppression group versus 78.9% in the tamoxifen-alone group.
- In addition, OS at 8 years was improved with tamoxifen plus ovarian suppression compared with tamoxifen alone (HR, 0.67; 95% CI, 0.48–0.92; P = .01); the 8-year OS rate was 93.3% in the tamoxifen-plus-ovarian-suppression group versus 91.5% in the tamoxifen-alone group.
Despite overall negative initial results, subgroup analysis suggested a benefit with ovarian suppression in women who underwent chemotherapy and remained premenopausal afterwards. Follow-up results at 8 years, however, did not demonstrate heterogeneity of treatment effect according to whether chemotherapy was administered, although recurrences were more frequent among patients who received chemotherapy.[54]
- A Korean Breast Cancer Study Group trial (NCT00912548) included 1,293 premenopausal women younger than 45 years, all of whom had received adjuvant chemotherapy and either retained ovarian function or regained it after 2 years of tamoxifen. Patients were randomly assigned to receive either ovarian function suppression with goserelin plus tamoxifen or tamoxifen alone.[55]
- In the intent-to-treat analysis of 1,282 patients, the 5-year DFS rate was 89.8% for patients in the goserelin-plus-tamoxifen group and 87.3% for patients in the tamoxifen-alone group (HR, 0.69; 95% CI, 0.49–0.98; P = .036).
- OS was a secondary end point and was also improved for patients in the goserelin-plus-tamoxifen group (HR, 0.31; 95% CI, 0.10–0.95; P = .039).[55][Level of evidence A1]
Aromatase inhibitors
Premenopausal women
AIs have been compared with tamoxifen in premenopausal women in whom ovarian function was suppressed or ablated. The results of these studies have been conflicting.
Evidence (comparison of an AI with tamoxifen in premenopausal women):
- In one study (NCT00295646), 1,803 women who received goserelin were randomly assigned to a 2 × 2 factorial design trial that compared anastrozole and tamoxifen, with or without zoledronic acid.[56]
- At a median follow-up of 62 months, there was no difference in DFS (HR, 1.08; 95% CI, 0.81–1.44; P = .59).
- OS was superior with tamoxifen (HR, 1.75; 95% CI, 1.08–2.83; P = .02).
- In two unblinded studies that were analyzed together (TEXT [NCT00066703] and SOFT [NCT00066690]), exemestane was also compared with tamoxifen in 4,690 premenopausal women who underwent ovarian ablation.[57]
- The use of exemestane resulted in a significant difference in DFS. The 8-year DFS rate was 86.8% in the exemestane-ovarian suppression group vs. 82.8% in the tamoxifen-ovarian suppression group (HR, 0.77; 95% CI, 0.67–0.90; P < .001).[57][Level of evidence B1]
- The 8-year rate of freedom from distant recurrence was also higher in the exemestane-ovarian suppression group (91.8% vs. 89.7%; HR, 0.80; 95% CI, 0.66–0.96; P = .02).
- Despite improvements in DFS and freedom from distant recurrence, no difference in OS was observed in the exemestane-ovarian suppression group compared with the tamoxifen-ovarian suppression group (93.4% vs. 93.3%; HR, 0.98; 95% CI, 0.79–1.22; P = .84).[57][Level of evidence A1] However, after a median follow-up of 12 years in the SOFT trial, the 12-year OS rate was 89.4% in the exemestane-ovarian suppression group and 86.8% in a tamoxifen-alone group from that study (HR, 0.80; 95% CI, 0.62–1.04).[58]
- A follow-up report on the differences in quality of life (QOL) for the exemestane-ovarian suppression group versus the tamoxifen-ovarian suppression group observed the following results (the differences cited below were all significant at P < .001 and occurred in patients who did and did not receive chemotherapy):[59]
- Patients who received tamoxifen plus ovarian function suppression were more affected by hot flashes and sweats over 5 years than were those who received exemestane plus ovarian function suppression, although these symptoms improved.
- Patients who received exemestane plus ovarian function suppression reported more vaginal dryness, greater loss of sexual interest, and more difficulties becoming aroused than did patients who received tamoxifen plus ovarian function suppression. These differences persisted over time.
- An increase in bone or joint pain was more pronounced, particularly in the short term, in patients who received exemestane plus ovarian function suppression than in patients who received tamoxifen plus ovarian function suppression.
- Changes in global QOL indicators from baseline were small and similar between treatments over the 5 years.[59][Level of evidence A3]
Postmenopausal women
In postmenopausal women, the use of AIs in sequence with or as a substitute for tamoxifen has been the subject of multiple studies, the results of which have been summarized in an individual patient-level meta-analysis.[60]
Initial therapy
Evidence (AI vs. tamoxifen as initial therapy in postmenopausal women):
- A large randomized trial of 9,366 patients compared the use of the AI anastrozole and the combination of anastrozole and tamoxifen with tamoxifen alone as adjuvant therapy for postmenopausal patients with lymph node–negative or lymph node–positive disease. Most (84%) of the patients in the study were hormone receptor–positive. Slightly more than 20% had received chemotherapy.[61]; [62][Level of evidence B1]
- With a median follow-up of 33.3 months, no benefit in DFS was observed for the combination arm relative to tamoxifen alone.[61]
- Patients on anastrozole, however, had a significantly longer DFS (HR, 0.83) than those on tamoxifen. In an analysis conducted after a median follow-up of 100 months among hormone receptor–positive patients, DFS was significantly (P = .003) longer in patients on anastrozole (HR, 0.85; 95% CI, 0.76–0.94), but OS was not improved (HR, 0.97; 95% CI, 0.86–1.11; P = .7).[62]
- Patients on tamoxifen more frequently developed endometrial cancer and cerebrovascular accidents, whereas patients on anastrozole had more fracture episodes. The frequency of myocardial infarction was similar in both groups. Except for a continued increased frequency of endometrial cancer in the tamoxifen group, these differences did not persist in the posttreatment period.[62]
- A large, double-blind, randomized trial of 8,010 postmenopausal women with hormone receptor–positive breast cancer compared the use of letrozole with tamoxifen given continuously for 5 years or with crossover to the alternate drug at 2 years.[63] An updated analysis from the International Breast Cancer Study Group (IBCSG-1-98 [NCT00004205]) reported results on the 4,922 women who received tamoxifen or letrozole for 5 years at a median follow-up of 51 months.[64][Level of evidence B1]
- DFS was significantly superior in patients treated with letrozole (HR, 0.82; 95% CI, 0.71–0.95; P = .007; 5-year DFS, 84.0% vs. 81.1%).
- OS was not significantly different in patients treated with letrozole (HR, 0.91; 95% CI, 0.75–1.11; P = .35).
- In a meta-analysis, which included 9,885 women from multiple trials, the 10-year recurrence risk was 19.1% in the AI group versus 22.7% in the tamoxifen group (RR, 0.80; 95% CI, 0.73–0.88; P < .001). The overall 10-year mortality rate was also reduced from 24.0% to 21.3%. (RR, 0.89; 95% CI, 0.8–0.97; P = .01).[60][Level of evidence A2]
Sequential tamoxifen and AI versus 5 years of tamoxifen
Several trials and meta-analyses have examined the effect of switching to anastrozole or exemestane to complete a total of 5 years of therapy after 2 to 3 years of tamoxifen.[65-67] The evidence, as described below, indicates that sequential tamoxifen and AI is superior to remaining on tamoxifen for 5 years.
Evidence (sequential tamoxifen and AI vs. 5 years of tamoxifen):
- Two trials carried out in sequence by the same group enrolled a total of 828 patients and were reported together; one trial used aminoglutethimide as the AI, and the other trial used anastrozole.[67]
- After a median follow-up of 78 months, an improvement in all-cause mortality (HR, 0.61; 95% CI, 0.42–0.88; P = .007) was observed in the AI groups.[67][Level of evidence A1]
- Two other trials were reported together.[66] A total of 3,224 patients were randomly assigned after 2 years of tamoxifen to continue tamoxifen for a total of 5 years or to take anastrozole for 3 years.[67]
- There was a significant difference in EFS (HR, 0.80; 95% CI; P = .0009), but not in OS (5-year OS, 97% CI for the switched arm vs. 96% CI for the tamoxifen-alone arm; P = .16).[67][Level of evidence B1]
- A large, double-blind, randomized trial (EORTC-10967 [ICCG-96OEXE031-C1396-BIG9702]) (NCT00003418) of 4,742 patients compared continuing tamoxifen with switching to exemestane for a total of 5 years of therapy in women who had received 2 to 3 years of tamoxifen.[68][Level of evidence B1]
- After the second planned interim analysis, when median follow-up for patients on the study was 30.6 months, the results were released because of a highly significant (P < .005) difference in DFS (HR, 0.68) favoring the exemestane arm.[68]
- After a median follow-up of 55.7 months, the HR for DFS was 0.76 (95% CI, 0.66–0.88; P = .001) in favor of exemestane.[69][Level of evidence A1]
- At 2.5 years after random assignment, 3.3% fewer patients on exemestane had developed a DFS event (95% CI, 1.6–4.9). The HR for OS was 0.85 (95% CI, 0.7–1.02; P = .08).[69]
- In a meta-analysis, which included 11,798 patients from six trials, the 10-year recurrence rate was reduced from 19% to 17% in the AI-containing groups (RR, 0.82; 95% CI, 0.75–0.91; P = .0001). The 10-year overall mortality rate was 17.5% in the tamoxifen group and 14.6% in the AI-containing group (RR, 0.82; 95% CI, 0.73–0.91; P = .0002).[60][Level of evidence A2]
Sequential tamoxifen and AI for 5 years versus an AI for 5 years
The evidence indicates that there is no benefit to the sequential use of tamoxifen and an AI for 5 years over 5 years of an AI.
Evidence (sequential tamoxifen and AI vs. an AI for 5 years):
- A large, randomized trial of 9,779 patients compared DFS of postmenopausal women with hormone receptor–positive breast cancer between initial treatment with sequential tamoxifen for 2.5 to 3 years followed by exemestane for a total of 5 years versus exemestane alone for 5 years. The primary end points were DFS at 2.75 years and 5.0 years.[70][Level of evidence B1]
- The 5-year DFS rate was 85% in the sequential group and 86% in the exemestane-alone group (HR, 0.97; 95% CI, 0.88–1.08; P = .60).
- Similarly in the IBCSG 1-98 trial (NCT00004205), two sequential arms were compared with 5 years of letrozole.[71][Level of evidence B1]
- There was no difference in DFS when the two sequential arms were compared with 5 years of letrozole (letrozole to tamoxifen HR, 1.06; 95% CI, 0.91–1.23; P = .45 and tamoxifen to letrozole HR, 1.07; 95% CI, 0.92–1.25; P = .36).
- The FATA-GIM3 trial (NCT00541086), which was not included in the meta-analysis, compared 2 years of tamoxifen followed by 3 years of one of the three AIs with 5 years of an AI.[72]
- No significant difference in the 5-year DFS rate was found between the two approaches (88.5% for switching; 89.8% for upfront AI; HR, 0.89; 95% CI, 0.73–1.08; P = .23).
- In a meta-analysis, which included 12,779 patients from the trials, the 7-year recurrence rate was slightly reduced from 14.5% to 13.8% in the groups that received 5 years of an AI (RR, 0.90; 95% CI, 0.81–0.99; P = .045). The overall mortality rate at 7 years was 9.3% in the tamoxifen-followed-by-AI groups and 8.2% in the AI-alone groups (RR, 0.89; 95% CI, 0.78–1.03; P = .11).[60][Level of evidence A2]
One AI versus another for 5 years
- The mild androgen activity of exemestane prompted a randomized trial that evaluated whether exemestane might be preferable to anastrozole, in terms of its efficacy (i.e., EFS) and toxicity, as upfront therapy for postmenopausal women diagnosed with hormone receptor–positive breast cancer.[73][Level of evidence A1] The MA27 trial (NCT00066573) randomly assigned 7,576 postmenopausal women to receive 5 years of anastrozole or exemestane.
- At a median follow-up of 4.1 years, no difference in efficacy was seen (HR, 1.02; 95% CI, 0.87–1.18; P = .86).[73][Level of evidence B1]
- The two therapies also were not significantly different in terms of impact on bone mineral density or fracture rates.[74][Level of evidence B1]
- In the Femara Versus Anastrozole Clinical Evaluation (FACE) study (NCT00248170), 4,136 patients with hormone receptor–positive disease were randomly assigned to receive either letrozole or anastrozole.[75]
- There was no significant difference in DFS (HR, 0.93; 95% CI, 0.80–1.07; P = .3150) at the time of a final analysis that was conducted when there were 709 of the planned 959 events.
- There were no substantial differences in adverse events between the arms.
- In the FATA-GIM3 trial, 3,697 patients with hormone receptor–positive disease were randomly assigned among the three AIs either for 5 years or after 2 years of tamoxifen.[72]
- No significant difference in 5-year DFS (90.0% for anastrozole, 88.0% for exemestane, and 89.4% for letrozole; P = .24) was noted among the three AIs.
Switching to an AI after 5 years of tamoxifen
The evidence, as described below, indicates that switching to an AI after 5 years of tamoxifen is superior to stopping tamoxifen at that time.
- A large, double-blinded, randomized trial (CAN-NCIC-MA17 [NCT00003140]) of 5,187 patients compared the use of letrozole versus placebo in receptor-positive postmenopausal women who received tamoxifen for approximately 5 years (range, 4.5–6.0) years.[76][Level of evidence B1]
- After the first planned interim analysis, when median follow-up for patients in the study was 2.4 years, the results were unblinded because of a highly significant (P < .008) difference in DFS (HR, 0.57), favoring the letrozole arm.[76]
- After 3 years of follow-up, 4.8% of the women on the letrozole arm had developed recurrent disease or new primaries versus 9.8% on the placebo arm (95% CI for the difference, 2.7%–7.3%). Because of the early unblinding of the study, longer-term comparative data on the risks and benefits of letrozole in this setting will not be available.[77,78]
- An updated analysis including all events before unblinding confirmed the results of the interim analysis.[79] In addition, a statistically significant improvement in distant DFS was found for patients who received letrozole (HR, 0.60; 95% CI, 0.43–0.84; P = .002). Although no statistically significant difference was found in the total study population, the lymph node-positive patients who received letrozole also experienced a statistically significant improvement in OS (HR, 0.61; 95% CI, 0.38–0.98; P = .04), although the P value was not corrected for multiple comparisons.
- The NSABP B-33 trial (NCT00016432) that was designed to compare 5 years of exemestane with placebo after 5 years of tamoxifen was stopped prematurely when the results of CAN-NCIC-MA17 became available. At the time of analysis, 560 of the 783 patients who were randomly assigned to receive exemestane remained on that drug and 344 of the 779 patients who were randomly assigned to receive placebo had crossed over to exemestane.[80][Level of evidence B1]
- An intent-to-treat analysis of the primary study end point, DFS, demonstrated a nonsignificant benefit of exemestane (HR, 0.68; P = .07).
Duration of AI therapy
The optimal duration of AI therapy is uncertain, and multiple trials have evaluated courses longer than 5 years.
Evidence regarding extension of endocrine therapy beyond 5 years of initial AI-based adjuvant therapy:
- A double-blind, randomized, phase III trial assessed the effect of an additional 5 years of letrozole versus placebo in 1,918 women who had received 5 years of an AI.[81] Patients who received previous tamoxifen therapy were included. Most women on the study (70.6%) had received 4.5 to 6 years of adjuvant tamoxifen, but a significant proportion of them (20.7%) had been treated initially with an AI. The primary study end point was DFS.
- At a median follow-up of 6.3 years, DFS was significantly improved in patients randomly assigned to receive letrozole (HR, 0.66; 95% CI, 0.48–0.91; P = .01). The 5-year DFS rate was improved from 91% to 95%.[81][Level of evidence B1]
- OS rates showed no difference (HR, 0.97; 95% CI, 0.73–1.28; P = .83). More patients who received letrozole had fractures (14%) than did patients who received placebo (9%) (P = .001).
- QOL was assessed with the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) and Menopause-Specific QOL (MENQOL) instruments. More than 85% of participants completed yearly assessments over a 5-year period.
- No between-group differences were found on the four MENQOL subscales or on the SF-36 summary score.
- SF-36 role-emotional and bodily pain scores were statistically significantly worse (P = .03) among patients receiving letrozole, but the differences observed were fewer than the minimum clinically important differences for the SF-36 instrument.
- A randomized phase III study assessed the effect of an additional 2.5 years of letrozole versus 5 years of letrozole in 1,824 women who received 5 years of an AI.[Level of evidence B1] [82]
- DFS events were similar in both groups (HR, 0.92; 95% CI, 0.74–1.16). The distant metastasis-free interval was also similar (HR, 1.06; 95% CI, 0.78–1.45).
- A subgroup analysis did not identify patients who benefited from 5-year extended therapy.
- This study did not show that 10 years of AI therapy was superior to 7.5 years of AI therapy.
- A phase III trial (NSABP-B42 [NCT00382070]) randomly assigned, in a double-blind fashion, 3,966 women who received 5 years of initial adjuvant therapy with an AI or received tamoxifen for 2 to 3 years followed by an AI to receive 5 mg of letrozole or placebo for 5 additional years.[83][Level of evidence B1] The planned analysis of DFS was carried out after a median follow-up of 6.9 years.
- The 7-year DFS rate was 81.3% in the placebo group and 84.7% in the letrozole group (HR, 0.85; 95% CI, 0.73–0.999; P = .048). The observed difference was not statistically significant when interim analyses were accounted for.
- There were no statistically significant differences in adverse events between the arms.
- A phase III trial conducted by the IBCSG (SOLE [NCT00553410]) included 4,851 receptor-positive postmenopausal women who had completed 5 years of adjuvant therapy with an AI, a selective estrogen receptor (ER) modulator, or both. Patients were randomly assigned to receive 2.5 mg of letrozole daily for 5 years or to an intermittent schedule in which there was a 3-month break at the end of each of the first 4 years, but not in the final year.[84]
- There was no observed advantage to the intermittent schedule with respect to DFS (HR, 1.08; 95% CI, 0.93–1.26; P = .31) or in the frequency of adverse events.[84][Level of evidence B1]
- A phase III trial conducted by the Dutch Breast Cancer Study Group (DATA [NCT00301457]) randomly assigned 1,860 eligible receptor-positive postmenopausal women who had received 2 to 3 years of tamoxifen to receive either 3 or 6 years of anastrozole (1 mg daily).[85]
- At 3 years, 1,660 of these women were free of disease: among them, DFS was observed to be improved, but not statistically significantly so, on the extended-therapy arm (HR, 0.79; 95% CI, 0.62–1.02).[85][Level of evidence B1] Myalgia and osteoporosis/osteopenia were more frequent on the extended-therapy arm.
- A phase III, open-label, Italian trial (NCT01064635) included 2,056 hormone receptor–positive postmenopausal women who had received 2 to 3 years of tamoxifen treatment. Patients were randomly assigned to receive letrozole for either 2 to 3 years (control) or 5 years (extended therapy). The primary end point was IDFS.[86]
- After 11.7 years of follow-up, the 12-year DFS rate was 62% in the control group and 67% in the extended-therapy group (HR, 0.78; 95% CI, 0.65–0.93; P = .0064). These results were confirmed in a landmark analysis that excluded patients who experienced a DFS event or who were lost to follow-up before treatment divergence (2–3 years after randomization).
- The 12-year OS rate was 84% in the control group and 88% in the extended-therapy group (HR, 0.77; 95% CI, 0.60–0.98; P = .036).[86][Level of evidence A1]
- Grade 3 or greater arthralgia and myalgia were slightly more frequent in the extended-therapy group than in the control group (3.0% vs. 2.2% and 0.9% vs. 0.7%, respectively).
- The phase III ABCSG-16 study enrolled 3,484 postmenopausal women with hormone receptor–positive breast cancer who had completed 5 years of endocrine therapy with tamoxifen and/or an AI to either 2 or 5 years of extended therapy with anastrozole. The primary end point was DFS in the 3,208 patients who remained in the study after 2 years. Secondary end points included OS, time to contralateral breast cancer, time to second primary cancer, fractures, and toxicity.[87]
- After 10 years of follow-up, there was no difference in DFS between the two arms (73.6% for the 2-year course vs. 73.9% for the 5-year course [HR, 0.99; 95% CI, 0.85–1.15; P = .90]). In addition, there was no difference in OS, time to second primary cancer, and time to contralateral breast cancer between the arms.[87][Level of evidence B1]
- There was a trend toward more fractures in the 5-year arm (4.7% vs. 6.3%; HR, 1.35; 95% CI, 1.00–1.84).
- An open-label trial in Japan included 1,697 patients who had received either (1) 5 years of anastrozole or (2) 2 to 3 years of tamoxifen, followed by 2 to 3 years of anastrozole. Patients were randomly assigned to either discontinue anastrozole or continue it for 5 years. The primary end point was DFS.[88]
- In an analysis done after all patients had completed protocol therapy, DFS was improved in the continued-therapy group (5-year DFS rate, 91% vs. 86%; HR, 0.62; 95% CI, 0.46–0.83; P < .0010).[88][Level of evidence B1]
- There was no difference in distant DFS or OS.
Endocrine therapy and cyclin-dependent kinase (CDK) inhibitor therapy
CDK4 and CDK6 have been implicated in the continued proliferation of hormone receptor–positive breast cancer that is resistant to endocrine therapy. CDK inhibitors, in combination with endocrine therapy, have been approved by the FDA in both first-line and later-line treatment of patients with advanced, HER2-negative, hormone receptor–positive breast cancer and are now being studied in the adjuvant setting. Abemaciclib is currently the only FDA-approved CDK inhibitor in the adjuvant setting.
Evidence (CDK inhibitors in the adjuvant setting):
- The monarchE trial (NCT03155997) examined the effect of adding abemaciclib to standard endocrine therapy in women with HER2-negative hormone receptor–positive breast cancer who were at high risk of recurrence.[89] The trial enrolled 5,637 women who met one of the following criteria: four or more positive nodes; or one to three positive nodes and either tumor size 5 cm or larger, histological grade 3, or central Ki67 20% or greater. The women were randomly assigned in a 1:1 ratio to standard-of-care adjuvant endocrine therapy with or without open-label abemaciclib (150 mg twice daily for 2 years). The primary end point was IDFS and secondary end points included DRFS, OS, and safety.
- At a median follow-up of 54 months, an analysis showed that adding abemaciclib resulted in a substantial improvement in IDFS (5-year IDFS: 76% vs. 83.6%; HR, 0.680; 95% CI, 0.599–0.772; P < .001) and DRFS (5-year DRFS: 79.2% vs. 86%; HR, 0.675; 95% CI, 0.588–0.774; P < .001).
- There were 208 deaths in the abemaciclib arm and 234 deaths in the endocrine therapy-alone arm. This difference was not statistically significant.[90][Level of evidence B1]
- Because of adverse events, abemaciclib dose adjustments occurred in 1,901 patients (68.1%); 56.9% of these patients had dose omissions and 41.2% had dose reductions. In the abemaciclib arm, 463 patients (16.6%) discontinued abemaciclib because of adverse events, 306 of whom remained on endocrine therapy.[89]
- Similar results were found in a prespecified subgroup analysis of patients who had received neoadjuvant chemotherapy and had residual disease after surgery.[91]
- The NATALEE trial (NCT03701334) evaluated adding ribociclib to standard adjuvant therapy in women and men with stage II to stage III HER2-negative hormone receptor–positive breast cancer.[92] A total of 5,101 patients were randomly assigned without blinding to receive either a nonsteroidal AI alone or in combination with ribociclib within 12 months of starting a nonsteroidal AI. The primary end point was IDFS.
- At the time of the second planned interim analysis, when the median duration of follow-up was 28 months, there was a significant difference in IDFS favoring the ribociclib arm (3-year IDFS, 90.4% for ribociclib plus nonsteroidal AI vs 87.1% for nonsteroidal AI alone) (HR, 0.75; 95% CI, 0.62–0.91; P = .003). This P-value exceeded the prespecified cutoff of .0256.[92][Level of evidence B1]
- The exploratory end points of distant DFS, RFS, DRFS, and OS favored the ribociclib arm, but the differences were not formally tested for significance in this analysis.
- Neutropenia (62.15% vs. 4.5%) and liver-related events (25.4% vs. 10.6%) were more common in the ribociclib-plus-nonsteroidal AI group than the nonsteroidal AI-alone group. A total of 477 patients discontinued ribociclib, and 554 patients had dose reductions. There were no treatment-related deaths.
- The PALLAS trial (NCT02513394) studied the effect of adding palbociclib to standard adjuvant therapy in women with stage II to stage III HER2-negative hormone receptor–positive breast cancer.[93] In the trial, 5,760 women who were within 6 months of initiating adjuvant endocrine therapy were randomly assigned in a 1:1 ratio without blinding to receive palbociclib plus endocrine therapy or to continue endocrine therapy alone. The primary end point was IDFS.
- At the time of the second interim analysis, no significant difference between the treatment arms was found (HR, 0.93; 95% CI, 0.76–1.15; 3-year IDFS rates, 88.2% vs. 88.5%; P = .51).[93][Level of evidence B1] The planned final analysis yielded similar results.[94]
- As the test statistic for futility crossed the prespecified boundary, the data safety monitoring committee recommended that patients discontinue palbociclib therapy.
- Neutropenia and leukopenia were much more common in patients on the palbociclib arm, and fatigue was slightly more common. There were no treatment-related deaths.
Bone-Modifying Therapy for Stages I, II, and III Breast Cancer
Both bisphosphonates and denosumab have been evaluated as adjuvant therapies for early-stage breast cancer; however, their role is unclear. Compared with denosumab, the amount of evidence supporting bisphosphonates is greater, and there is evidence supporting a reduction in breast cancer mortality—an end point that is more clinically relevant. The optimal duration of bisphosphonate therapy is uncertain.
Evidence (bisphosphonates in the treatment of early breast cancer):
- A meta-analysis included data from 18,766 patients from 26 adjuvant trials of bisphosphonates of any type.[95] Overall, reductions associated with bisphosphonate use in recurrence (RR, 0.94; 95% CI, 0.87–1.01; 2-sided P = .08), distant recurrence (RR, 0.92; 95% CI, 0.85–0.99; 2-sided P = .03), and breast cancer mortality (RR, 0.91; 95% CI, 0.83–0.99; 2-sided P = .04) were of only borderline significance, but the reduction in bone recurrence was more definite (RR, 0.83; 95% CI, 0.73–0.94; 2-sided P = .004).
- In a prespecified subgroup analysis among premenopausal women, treatment had no apparent effect on any outcome, but among 11,767 postmenopausal women, it produced highly significant reductions in recurrence (RR, 0.86; 95% CI, 0.78–0.94; 2-sided P = .002), distant recurrence (RR, 0.82; 95% CI, 0.74–0.92; 2-sided P = .0003), bone recurrence (RR, 0.72; 95% CI, 0.60–0.86; 2-sided P = .0002), and breast cancer mortality (RR, 0.82; 95% CI, 0.73–0.93; 2-sided P = .002).[95]
- The ABCSG-18 trial (NCT00556374) included 3,435 postmenopausal women with receptor-positive breast cancer who were receiving an AI. Patients were randomly assigned to receive denosumab or a placebo every 6 months during AI therapy.[96] The patients were unblinded when results related to bone events were reported, and patients on placebo were allowed to cross over to the active drug.
- In an intent-to-treat analysis according to the original assignment, DFS, a secondary end point, was improved in patients who received denosumab (5-year DFS rate, 89.2% vs. 87.3%; HR, 0.82; 95% CI, 0.69–0.98; P = .0260).[96][Level of evidence B1]
- The frequency of adverse events was similar in the two groups.
- The D-CARE trial (NCT01077154) randomly assigned 4,509 women with stage II or stage III breast cancer to receive denosumab or placebo.[97]
- The primary end point of bone metastasis–free survival was not significantly different between the groups (median, not reached in either group; HR, 0.97; 95% CI, 0.82−1.14; P = .70).[97][Level of evidence B1]
- The SUCCESS trial (NCT02181101) included 3,421 patients with node-positive or high-risk (≥pT2, grade 3, hormone receptor–negative, or aged 35 years or younger) node-negative breast cancer who completed adjuvant chemotherapy. Patients were randomly assigned to receive zoledronate 4 mg intravenously (IV) for either 2 years (every 3 months) or 5 years (every 3 months for 2 years and then every 6 months for 3 years). Only those patients who completed 2 years of zoledronate treatment (1,447 on the 2-year arm; 1,729 on the 5-year arm) were included in the final analysis. The main study end points were DFS and OS.[98]
- Outcomes were similar between the two arms for DFS (HR, 0.97; 95% CI, 0.76–1.25; P = .83) and OS (HR, 0.93; 95% CI, 0.65–1.34; P = .71).[98][Level of evidence A1]
- An accompanying editorial explained why the results of this study do not definitively establish how long bisphosphonates should be administered.[99]
Adjuvant PARP Inhibitors for Patients with Germline BRCA1 and BRCA2 Variants
The role of adjuvant poly (ADP-ribose) polymerase (PARP) inhibition has been evaluated in patients with early-onset breast cancer and germline BRCA1 or BRCA2 pathogenic variants. BRCA1 and BRCA2 are tumor suppressor genes that encode proteins involved in DNA repair (via the homologous recombination repair pathway). PARP plays a critical role in DNA repair.
Evidence (olaparib):
- The OlympiA trial (NCT02032823) randomly assigned 1,836 patients with HER2-negative breast cancer and germline BRCA1 or BRCA2 pathogenic variants to receive either 1 year of adjuvant olaparib (300 mg twice daily) or placebo. All women completed surgery and adjuvant or neoadjuvant chemotherapy or radiation therapy. Patients were considered at higher risk of recurrence on the basis of tumor size, node involvement, or the presence of residual cancer after neoadjuvant therapy.[100]
Eligibility criteria for patients who underwent initial surgery and received adjuvant chemotherapy
- Patients with triple-negative breast cancer (TNBC) had axillary node-positive (≥pN1, any tumor size) disease OR axillary node-negative disease with an invasive primary tumor larger than 2 cm (pN0, ≥pT2).
- At least four pathologically confirmed positive lymph nodes were required for ER- and/or PR-positive/HER2-negative patients.
Eligibility criteria for patients who underwent neoadjuvant chemotherapy followed by surgery
- Patients with TNBC had residual invasive cancer in the breast and/or resected lymph nodes (i.e., no pCR).
- ER- and/or PR-positive/HER2-negative patients had residual invasive cancer in the breast and/or resected lymph nodes (i.e., no pCR) AND a CPS+EG (Clinical stage/Pathological Stage + ER status/nuclear Grade) score of 3 or higher.[101]
The primary study end point was invasive disease-free survival (IDFS).[100][Level of evidence B1]
- At the time of the first (and only) planned interim analysis, when 284 events (i.e., invasive disease or death) had occurred, the HR for IDFS strongly favored the olaparib arm, and the prespecified stopping boundary for significance was exceeded (3-year IDFS rate, 85.9% vs 77.1%; HR, 0.58; 99.5% CI, 0.41–0.82; P < .001).[100][Level of evidence B1]
- Distant DFS was also statistically significantly improved for patients who received olaparib (HR, 0.57; 99.5% CI, 0.39–0.83; P < .001).
- A difference in OS was also observed (HR, 0.68; 99% CI, 0.44–1.05; P = .02), but it did not meet the prespecified level of significance when corrected for multiple testing (P < .01).
- Grade 3 or higher adverse events that occurred in more than 1% of patients on the olaparib arm included anemia (8.7%), decreased neutrophil count (4.8%), decreased white cell count (3.0%), fatigue (1.8%), and lymphopenia (1.2%). No adverse events of grade 3 or higher occurred in more than 1% of the patients on the placebo arm.
- Patient-reported outcomes were assessed among the 1,538 patients who completed both a baseline questionnaire and at least one subsequent questionnaire.[102]
- Patients who received olaparib experienced statistically significant greater fatigue than patients who received placebo, but the differences did not meet the criteria for clinical significance. The fatigue resolved over time after cessation of therapy.
- Patients who received olaparib had small but statistically and clinically significant increases in nausea, vomiting, and appetite loss scores during treatment.[102][Level of evidence A3]
Stages I, II, and III Triple-Negative Breast Cancer (TNBC)
TNBC is defined as the absence of staining for ER, PR, and HER2. TNBC is insensitive to some of the most effective therapies for patients with breast cancer, including HER2-directed therapy such as trastuzumab and endocrine therapies such as tamoxifen or AIs.
Preoperative therapy for TNBC
Patients with TNBC are frequently treated with preoperative systemic therapy.
Chemotherapy
Promising results have been observed with the addition of carboplatin to anthracycline/taxane combination chemotherapy regimens in patients with TNBC.
Evidence (adding carboplatin to an anthracycline/taxane–based chemotherapy regimen in patients with TNBC):
- In the GeparSixto trial (NCT01426880), carboplatin was added to an anthracycline/taxane–based backbone.[103][Level of evidence B3]
- Higher pCR rates were observed with the addition of carboplatin to an anthracycline/taxane–based backbone compared with anthracycline/taxane alone (36.9% vs. 53.2%; P = .005) in patients with TNBC.
- Patients with BRCA1 or BRCA2 variants had a higher rate of pCR, which was not increased by the addition of carboplatin (66.7% in the nonplatinum arm vs. 65.7% in the platinum-containing arm).
- The 3-year DFS rate was higher for patients with TNBC randomly assigned to the carboplatin arm (86.1% vs. 75.8%; HR, 0.56; 95% CI, 0.34−0.93), but OS did not differ.[104]
- The more intensive regimen was also associated with increased toxicity and treatment discontinuations (39% vs. 48%).
- The CALGB 40603 trial (NCT00861705) compared an anthracycline/taxane backbone alone with an anthracycline/taxane backbone plus carboplatin in patients with stage II and stage III TNBC.[105][Level of evidence B3]
- The pCR rate for the breast and axilla was 54% for the anthracycline/taxane backbone-plus-carboplatin group versus 41% for the anthracycline/taxane backbone-alone group (P = .0029).
Immunotherapy
Evidence (adding pembrolizumab to a chemotherapy regimen in patients with stage II or stage III TNBC):
- The randomized, double blind, phase III KEYNOTE-522 trial (NCT03036488) evaluated the addition of immunotherapy to neoadjuvant chemotherapy for patients with stage II and stage III TNBC.[106][Level of evidence B1] Participants were randomly assigned in a 2:1 ratio to receive neoadjuvant chemotherapy (paclitaxel plus carboplatin, followed by doxorubicin plus cyclophosphamide) with either neoadjuvant and adjuvant pembrolizumab or neoadjuvant and adjuvant placebo. Co-primary end points were pCR rate and EFS. The pCR rate, as reported at the time of the first interim analysis for the first 602 participants (pembrolizumab arm, n = 401; placebo arm, n = 201), favored the pembrolizumab arm.
- A pCR was observed in 64.8% of patients in the pembrolizumab arm and 51.2% of patients in the placebo arm (estimated treatment difference, 13.6%; 95% CI, 5.4%−21.8%; P < .001). Approximately 80% of tumors were positive for programmed death-ligand 1 (PD-L1), but the benefits of pembrolizumab regarding pCR were observed regardless of PD-L1 status.
- At the time of the fourth interim analysis, when the median follow-up was 39 months, an improved EFS was observed in patients who received pembrolizumab. The 36-month EFS rate was 84.5% for patients who received pembrolizumab and 76.8% for patients who received placebo. (HR, 0.63; 95% CI, 0.48–0.82; P < .001).[107][Level of evidence B1]
- EFS data are immature.
- Grade 3 or higher adverse events occurred in 76.8% of participants in the pembrolizumab arm and 72.2% of participants in the placebo arm. Serious treatment-related adverse events occurred in 32.5% of participants in the pembrolizumab arm and 19.5% of participants in the placebo arm. Grade 3 or higher skin rashes, infusion reactions, and adrenal insufficiency were more frequent in the pembrolizumab arm.
Postoperative therapy for TNBC
For patients who undergo surgery first, combination chemotherapy is typically given in the adjuvant setting. While there is no established standard therapy in this setting, the following trial provides evidence that a non–anthracycline-based regimen may be suitable:
Evidence (adjuvant non–anthracycline-containing regimens):
- The PATTERN trial (NCT01216111) compared an anthracycline-based regimen (cyclophosphamide, 5-FU, epirubicin, and docetaxel [CEF-T]) with paclitaxel and carboplatin (PCb) in 647 Chinese women with TNBC who had completed definitive surgery. The primary end point was DFS.[108]
- At a median follow-up of 62 months, the 5-year DFS rate was 86.5% for patients who received PCb and 80.3% for patients who received CEF-T (HR, 0.65; 95% CI, 0.44–0.96; P = .03).[108][Level of evidence B1]
- There was no statistically significant difference in OS between the groups (HR, 0.71; 95% CI, 0.42–1.22; P = .22).
Capecitabine therapy
Capecitabine therapy increased DFS when given after conventional adjuvant therapy.
Evidence (capecitabine therapy for patients who have not been treated with preoperative systemic therapy):
- The SYSUCC-001 trial (NCT01112826) included 443 women (434 analyzed) with TNBC from 13 Chinese institutions. The women had received adjuvant chemotherapy and were randomly assigned to receive either no further therapy or capecitabine at a dose of 650 mg/m2 twice daily for 1 year. The primary study end point was DFS.[109]
- After a median follow-up of 61 months, the 5-year DFS rate was 82.5% for patients who received capecitabine maintenance therapy compared with 73.0% for patients who received no further therapy (HR, 0.64; 95% CI, 0.42–0.95; P = .03).[109][Level of evidence B1]
- Forty-five percent of patients who received capecitabine developed hand-foot syndrome, which was grade 3 in 7.7% of patients.
- The rate of patients who completed 1 year of therapy was 82.8%.
Evidence (capecitabine therapy for patients who have been treated with preoperative systemic therapy):
- In a study conducted in Japan and Korea, 910 women with HER2-negative breast cancers were randomly assigned in a nonblinded fashion to receive six to eight 3-week cycles of capecitabine or no further chemotherapy. Patients had residual disease after preoperative chemotherapy with anthracyclines, taxanes, or both, and 30% of the patients also had hormone receptor–negative disease. The primary end point was DFS.[110] The study was terminated because of the results of a planned interim analysis, and a final analysis was done.
- In the final analysis, which included 887 eligible patients the 5-year DFS rate was 74.1% in the capecitabine group and 67.6% in the no-further-chemotherapy group (HR, 0.70; 95% CI, 0.53–0.92; P = .01).[Level of evidence B1]
- OS was a secondary end point. The 5-year OS rate was 89.2% in the capecitabine group and 83.6% in the no-further-chemotherapy group (HR, 0.59; 95% CI, 0.39–0.90; P = .01).
- In a subset analysis, OS was significantly prolonged only in the patients with hormone receptor–negative disease. Among those patients, the 5-year OS rate was 78.8% in the capecitabine group and 70.3% in the no-further-chemotherapy group (HR, 0.52; 95% CI, 0.30–0.90).
- In the capecitabine group, 73.4% of the patients experienced hand-foot syndrome of varying degrees of severity.
These approaches should be considered for patients with residual disease after preoperative therapy. Patients may also consider participation in clinical trials of novel therapies. Clinical trials for this patient population have included EA1131 (NCT02445391), a phase III clinical trial that randomly assigned patients with residual basal-like TNBC after preoperative therapy to receive either platinum-based chemotherapy or capecitabine, and S1418/BR006 (NCT02954874), a phase III trial that evaluated the efficacy of pembrolizumab as adjuvant therapy for patients with residual TNBC (≥1 cm invasive cancer or residual nodes) after preoperative therapy. Information about ongoing clinical trials is available from the NCI website.
Immunotherapy
One completed trial showed no benefit to adding atezolizumab to postoperative chemotherapy.
Evidence (postoperative atezolizumab):
- In the ALEXANDRA/IMpassion030 trial (NCT03498716), 2,199 women who had not received preoperative systemic therapy were randomly assigned postoperatively to receive chemotherapy with or without atezolizumab. The chemotherapy regimen included paclitaxel and cyclophosphamide with doxorubicin or epirubicin. The primary end point was IDFS. Trial accrual was prematurely terminated by the data safety monitoring committee for futility.[111]
- The final analysis demonstrated no benefit in IDFS with the addition of atezolizumab (HR, 1.11; 95% CI, 0.87–1.42; P = .38).[111][Level of evidence B1]
Stages I, II, and III HER2-Positive Breast Cancer
Patients with HER2-positive breast cancer who are also hormone receptor–positive receive hormone therapy as described in the Stages I, II, and III HER2-Negative Hormone Receptor–Positive Breast Cancer section.
Preoperative therapy for HER2-positive breast cancer
After the success in the adjuvant setting, initial reports from phase II studies indicated improved pCR rates when trastuzumab, a monoclonal antibody that binds the extracellular domain of HER2, was added to preoperative anthracycline/taxane–based regimens.[112][Level of evidence B3] This has been confirmed in phase III studies.[113,114]
Trastuzumab
Evidence (trastuzumab):
- The phase III NeOAdjuvant Herceptin (NOAH) study randomly assigned patients with HER2-positive locally advanced or inflammatory breast cancers to undergo preoperative chemotherapy with or without 1 year of trastuzumab therapy.[114][Level of evidence A1]
- Study results confirmed that the addition of trastuzumab to preoperative chemotherapy resulted not only in improved clinical responses (87% vs. 74%) and pathological responses (breast and axilla, 38% vs. 19%) but also in EFS, the primary outcome.[114][Level of evidence A1]
- After a median follow-up of 5.4 years, the EFS benefit was 58% with the addition of trastuzumab to chemotherapy (95% CI, 48%–66%) and 43% (95% CI, 34%–52%) in patients in the chemotherapy group. The unadjusted HR for EFS between the two randomized HER2-positive treatment groups was 0.64 (95% CI, 0.44–0.93; two-sided log-rank P = .016). EFS was strongly associated with pCR in patients who received trastuzumab.[115]
- Symptomatic cardiac failure occurred in two patients who received concurrent doxorubicin and trastuzumab for two cycles. Close cardiac monitoring of left ventricular ejection fraction (LVEF) and the total dose of doxorubicin not exceeding 180 mg/m2 accounted for the relatively low number of declines in LVEF and only two cardiac events. For more information, see the Cardiac Toxic Effects With Adjuvant Trastuzumab section.[114][Level of evidence B1]
- Due to concern about coadministration of trastuzumab and anthracyclines, a phase III trial (Z1041 [NCT00513292]) randomly assigned patients with operable HER2-positive breast cancer to receive trastuzumab sequential to or concurrent with the anthracycline component (5-FU, epirubicin, and cyclophosphamide [FEC]) of the preoperative chemotherapy regimen.[116][Level of evidence B3]
- The primary outcome was pCR. There was no significant difference in pCR rate in the breast between the arms (56.5% sequential, 54.2% concurrent; difference, 2.3%; 95% CI, -9.3 to 13.9).
- Asymptomatic declines in LVEF during preoperative chemotherapy were identified in similar proportions of patients in each arm.
- DFS and OS were secondary outcomes. After median follow-up of 5.1 years, there was no difference in DFS (HR, 1.02; 95% CI, 0.56−1.83; P = .96) or OS (HR, 1.17; 95% CI, 0.48−2.88; P = .73) between the sequential and concurrent arms.[117]
- Based on these findings, concurrent administration of trastuzumab with anthracyclines is not warranted.
A subcutaneous (SQ) formulation of trastuzumab has also been approved.
The SafeHer trial (NCT01566721) evaluated the safety and tolerability of self-administered versus clinician-administered SQ trastuzumab in patients with stage I to stage III HER2-positive breast cancer.[118] Chemotherapy was administered concurrently or sequentially.
A phase III (HannaH [NCT00950300]) trial also demonstrated that the pharmacokinetics and efficacy of preoperative SQ trastuzumab is noninferior to the IV formulation. This international open-label trial (n = 596) randomly assigned women with operable, locally advanced, or inflammatory HER2-positive breast cancer to undergo preoperative chemotherapy (anthracycline/taxane–based), with either SQ-administered or IV-administered trastuzumab every 3 weeks before surgery. Patients received adjuvant trastuzumab to complete 1 year of therapy.[119][Level of evidence B1] The pCR rates between the arms differed by 4.7% (95% CI, 4.0%–13.4%); 40.7% in the IV-administered group versus 45.4% in the SQ-administered group, demonstrating noninferiority for the SQ formulation. EFS and OS were secondary end points. The 6-year EFS rate was 65% in both arms (HR, 0.98; 95% CI, 0.74−1.29). The 6-year OS rate was 84% in both arms (HR, 0.94; 95% CI, 0.61−1.45).[120]
Newer HER2-targeted therapies (lapatinib, pertuzumab) have also been investigated. It appears that dual targeting of the HER2 receptor results in an increase in pCR rate. However, no survival advantage has been demonstrated to date with this approach.[121,122]
Pertuzumab
Pertuzumab is a humanized monoclonal antibody that binds to a distinct epitope on the extracellular domain of the HER2 receptor and inhibits dimerization. Pertuzumab, in combination with trastuzumab with or without chemotherapy, has been evaluated in two preoperative clinical trials to improve on the pCR rates observed with trastuzumab and chemotherapy.
Evidence (pertuzumab):
- In the open-label, randomized, phase II NeoSPHERE trial (NCT00545688),[121] 417 women with tumors that were larger than 2 cm or node-positive, and who had HER2-positive breast cancer, were randomly assigned to one of four preoperative regimens:[121][Level of evidence B3]
- Docetaxel plus trastuzumab.
- Docetaxel plus trastuzumab and pertuzumab.
- Pertuzumab plus trastuzumab.
- Docetaxel plus pertuzumab.
The following results were observed:
- The pCR rates were 29% for docetaxel plus trastuzumab, 46% for docetaxel plus trastuzumab and pertuzumab, 17% for pertuzumab plus trastuzumab, and 24% for docetaxel plus pertuzumab. Therefore, the highest pCR rate was seen in the preoperative treatment arm with dual HER2 blockade plus chemotherapy.
- The addition of pertuzumab to the docetaxel-plus-trastuzumab combination did not appear to increase toxic effects, including the risk of cardiac adverse events.
- Despite the high pCR rate observed with dual HER2 blockade plus chemotherapy, PFS and DFS rates were not improved, although the NeoSPHERE trial was not powered to detect differences in long-term efficacy outcomes.[123]
- The open-label, randomized, phase II TRYPHAENA trial (NCT00976989) sought to evaluate the tolerability and activity associated with trastuzumab and pertuzumab.[124][Level of evidence B3] All 225 women with tumors that were larger than 2 cm or node positive, and who had operable, locally advanced, or inflammatory HER2-positive breast cancer, were randomly assigned to one of three preoperative regimens:
- Concurrent FEC plus trastuzumab plus pertuzumab (×3) followed by concurrent docetaxel plus trastuzumab plus pertuzumab.
- FEC alone (×3) followed by concurrent docetaxel plus trastuzumab plus pertuzumab (×3).
- Concurrent docetaxel and carboplatin plus trastuzumab plus pertuzumab (×6).
The following results were observed:
- The pCR rate was equivalent across all three treatment arms: (62% for concurrent FEC plus trastuzumab plus pertuzumab followed by concurrent docetaxel plus trastuzumab plus pertuzumab; 57% for FEC alone followed by concurrent docetaxel plus trastuzumab plus pertuzumab; and 66% for concurrent docetaxel and carboplatin plus trastuzumab plus pertuzumab).
- All three arms were associated with a low incidence of cardiac adverse events of 5% or less.
Because of these studies, the FDA granted accelerated approval of pertuzumab as part of a preoperative treatment for women with early-stage, HER2-positive breast cancer whose tumors are larger than 2 cm or node-positive.
The FDA approval of pertuzumab was subsequently converted to regular approval following the results of the confirmatory APHINITY trial (NCT01358877), a randomized, phase III, adjuvant study for women with HER2-positive breast cancer. Study results demonstrated improved IDFS with the combination of chemotherapy and dual HER2-targeted therapy with pertuzumab plus trastuzumab compared with chemotherapy and trastuzumab alone.[125] Pertuzumab is now approved both in combination with trastuzumab and chemotherapy for the neoadjuvant therapy of locally advanced, inflammatory, or early-stage HER2-positive breast cancer, which is larger than 2 cm or node-positive, as part of a complete treatment regimen and in combination with chemotherapy and trastuzumab as adjuvant treatment for HER2-positive early breast cancer at a high risk of recurrence.
The randomized, open-label, multicenter TRAIN-2 trial (NCT01996267) evaluated the optimal chemotherapy backbone to use with neoadjuvant pertuzumab and trastuzumab in patients with stage II to stage III HER2-positive breast cancer (i.e., an anthracycline-containing or non–anthracycline-containing regimen).[126,127][Level of evidence B3] A total of 438 patients were randomly assigned to receive one of the following regimens:
- FEC every 3 weeks for three cycles followed by paclitaxel and carboplatin every 3 weeks for six cycles. Paclitaxel was administered on days 1 and 8 and carboplatin was administered either on day 1 alone or on days 1 and 8. Trastuzumab and pertuzumab were given every 3 weeks throughout chemotherapy. Primary prophylaxis with filgrastim was not administered during the FEC portion of therapy.
- Paclitaxel and carboplatin according to the same schedule for nine cycles. Trastuzumab and pertuzumab were given every 3 weeks throughout chemotherapy.
The primary end point was pCR (ypT0/is, ypN0). Secondary end point data on EFS, OS, toxicity, and breast conservation are available. The following results were observed:
- There was no statistically significant difference in the proportion of patients with pCR between the anthracycline (67%) and non-anthracycline (68%) arm.
- There was no difference in the proportion of patients in each arm who underwent breast-conserving surgery.
- Irrespective of hormone receptor and nodal status, 3-year EFS estimates were 92.7% in the anthracycline group and 93.6% in the non-anthracycline group. The 3-year OS estimates were 97.7% in the anthracycline group and 98.2% in the non-anthracycline group.
- A decline in LVEF of 10% or more from baseline to less than 50% was more common in patients who received anthracyclines than those who did not (7.7% vs. 3.2%; P = .04).
- Two patients treated with anthracyclines developed acute leukemia.
- There was no difference in the proportion of patients in each arm with at least grade 2 peripheral neuropathy: 66 patients (30%) in the anthracycline arm versus 68 patients (31%) in the non-anthracycline arm.
- Grade 4 neutropenia and febrile neutropenia were more common in the anthracycline arm (23 patients [10%]) than in the non-anthracycline arm (3 patients [1%], P < .0001).
Postoperative therapy for HER2-positive breast cancer
Patients who have not been treated with preoperative systemic therapy.
Standard treatment for HER2-positive early breast cancer is 1 year of adjuvant HER2-targeted therapy.
Trastuzumab
Several phase III clinical trials have addressed the role of the anti-HER2 antibody, trastuzumab, as adjuvant therapy for patients with HER2-overexpressing cancers. Study results confirm the benefit of 1 year of adjuvant trastuzumab therapy.
Evidence (including duration of trastuzumab therapy for patients who have not been treated with preoperative systemic therapy):
- The Herceptin Adjuvant (HERA) (BIG-01-01 [NCT00045032]) trial examined the efficacy of trastuzumab as adjuvant treatment for HER2-positive breast cancer if used after completion of the primary treatment. For most patients, primary treatment consisted of an anthracycline-containing chemotherapy regimen given preoperatively or postoperatively, with or without locoregional radiation therapy. Trastuzumab was given every 3 weeks starting within 7 weeks of the completion of primary treatment.[128][Level of evidence A1] Patients were randomly assigned to one of three study arms:
- Observation (n = 1,693).
- 1 year of trastuzumab (n = 1,694).
- 2 years of trastuzumab (n = 1,694).
Of the patients in the group comparing 1 year of trastuzumab versus observation, the median age was 49 years, about 33% had node-negative disease, and nearly 50% had hormone receptor–negative disease.[129]
The following results were observed for patients assigned to 1 year of trastuzumab versus patients assigned to observation:
- After a median follow-up of 11 years,[129] 1 year of trastuzumab improved DFS (10-year DFS rate, 72% vs. 66%; HR, 0.76; 95% CI, 0.68–0.86; P < .0001), despite a crossover of 52% of the patients on observation.
- One year of trastuzumab also improved OS (12-year OS rate, 79% vs. 73%; HR, 0.74; 95% CI, 0.64–0.86; P < .0001).[129][Level of evidence A1]
The following results were observed for patients assigned to 1 year of trastuzumab versus patients assigned to 2 years of trastuzumab:
- After a median follow-up of 11 years, there was no DFS benefit to an additional year of trastuzumab (HR, 1.02; 95% CI, 0.89–1.17).
Symptomatic cardiac events occurred in 1% of the patients who received trastuzumab and in 0.1% of the observation group.
- In the combined analysis of the NSABP-B-31 (NCT00004067) and intergroup NCCTG-N9831 (NCT00005970) trials, trastuzumab was given weekly, concurrently, or immediately after the paclitaxel component of the AC with paclitaxel regimen.[130,131][Level of evidence A1]
- The HERA results were confirmed in a joint analysis of the two studies, with a combined enrollment of 3,676 patients. A highly statistically significant improvement in DFS (3-year DFS rate, 87% vs. 75%; HR, 0.48; P < .001) was observed, as was a significant improvement in OS (3-year OS rate, 94.3% in the trastuzumab group vs. 91.7% in the control group; 4-year OS rate, 91.4% in the trastuzumab group vs. 86.6% in the control group; HR, 0.67; P = .015).[130]
- Patients treated with trastuzumab experienced a longer DFS, with a 52% lower risk of a DFS event (HR, 0.48; 95% CI, 0.39–0.59; P < .001), corresponding to an absolute difference in DFS of 11.8% at 3 years and 18% at 4 years. The risk of distant recurrence in patients treated with trastuzumab was 53% lower (HR, 0.47; 95% CI, 0.37–0.61; P < .001), and the risk of death was 33% lower (HR, 0.67; 95% CI, 0.48–0.93; P = .015).[130]
- In an updated analysis with a median follow-up of 8.4 years, the addition of trastuzumab to chemotherapy led to a 37% relative improvement in OS (HR, 0.63; 95% CI, 0.54–0.73; P < .001) and an increase in the 10-year OS rate from 75.2% to 84%.[132]
- In the BCIRG-006 trial (NCT00021255), 3,222 women with early-stage HER2-overexpressing breast cancer were randomly assigned to receive AC followed by docetaxel (AC-T), AC followed by docetaxel plus trastuzumab (AC-T plus trastuzumab), or docetaxel, carboplatin, plus trastuzumab (TCH, a non–anthracycline-containing regimen).[133][Level of evidence A1]
- A significant DFS and OS benefit was seen in both groups treated with trastuzumab compared with the control group that did not receive trastuzumab.
- For patients receiving AC-T plus trastuzumab, the 5-year DFS rate was 84% (HR for the comparison with AC-T, 0.64; P < .001), and the OS rate was 92% (HR, 0.63; P < .001). For patients receiving TCH, the 5-year DFS rate was 81% (HR, 0.75; P = .04), and the OS rate was 91% (HR, 0.77; P = .04). The control group had a 5-year DFS rate of 75% and an OS rate of 87%.
- The authors stated that there was no significant difference in DFS or OS between the two trastuzumab-containing regimens. However, the study was not powered to detect equivalence between the two trastuzumab-containing regimens.
- The rates of congestive heart failure and cardiac dysfunction were significantly higher in the group receiving AC-T plus trastuzumab than in the TCH group (P < .001).
- These trial findings raise the question of whether anthracyclines are needed for the adjuvant treatment of HER2-overexpressing breast cancer. The group receiving AC-trastuzumab showed a small but not statistically significant benefit over TCH.
- This trial supports the use of TCH as an alternative adjuvant regimen for women with early-stage HER2-overexpressing breast cancer, particularly in those with concerns about cardiac toxic effects.
- The Finland Herceptin (FINHER) study assessed the impact of a much shorter course of trastuzumab. In this trial, 232 women younger than 67 years with node-positive or high-risk (>2 cm tumor size) node-negative HER2-overexpressing breast cancer were given nine weekly infusions of trastuzumab concurrently with docetaxel or vinorelbine followed by FEC.[134][Level of evidence A1]
- At a 3-year median follow-up, the risk of recurrence and/or death was significantly reduced in patients receiving trastuzumab (3-year DFS rate, 89% vs. 78%; HR, 0.41; P = .01; 95% CI, 0.21–0.83).
- The difference in OS (HR, 0.41) was not statistically significant (P = .07; 95% CI, 0.16–1.08).
- Several studies have compared 6 months of trastuzumab administration to 12 months.[135-137]
- In an interim analysis of the PHARE trial (NCT00381901), the 2-year DFS rate was 93.8% (95% CI, 92.6%–94.9%) in the 12-month group and 91.1% (89.7%–92.4%) in the 6-month group (HR, 1.28; 95% CI, 1.05–1.56; noninferiority, P = .29).[135][Level of evidence A1]
- In the final analysis, after 704 events were observed, the adjusted HR was 1.08 (95% CI, 0.93–1.25), and the prespecified noninferiority HR of 1.15 was not excluded.
- Similar results were noted in a much smaller study of 481 patients led by the Hellenic Oncology Research Group.[136][Level of evidence A1]
- In contrast, the PERSEPHONE trial (NCT00712140), which enrolled 4,088 patients who experienced 512 DFS events at the time of analysis, excluded its prespecified noninferiority margin (HR, 1.07; 90% CI, 0.93−1.24; noninferiority, P = .011).[137][Level of evidence A1]
- In an interim analysis of the PHARE trial (NCT00381901), the 2-year DFS rate was 93.8% (95% CI, 92.6%–94.9%) in the 12-month group and 91.1% (89.7%–92.4%) in the 6-month group (HR, 1.28; 95% CI, 1.05–1.56; noninferiority, P = .29).[135][Level of evidence A1]
- The SOLD trial (NCT00593697) compared 9 weeks of trastuzumab with 1 year of trastuzumab in 2,174 women with HER2-positive breast cancer.[138]
- Noninferiority of the 9-week treatment could not be demonstrated for DFS (HR, 1.39; 2-sided 90% CI, 1.12−1.72).[138][Level of evidence B1]
- A meta-analysis that included these trials concluded that, with respect to OS, 1 year of trastuzumab was superior to a shorter duration of therapy; however, there was no significant benefit of 1 year of therapy in patients with low-risk disease.[139][Level of evidence A1]
Several studies have evaluated the use of SQ trastuzumab in the neoadjuvant and adjuvant settings.
Pertuzumab
Pertuzumab is a humanized monoclonal antibody that binds to a distinct epitope on the extracellular domain of the HER2 receptor and inhibits dimerization. Its use, in combination with trastuzumab, has been evaluated in a randomized trial in the postoperative setting.
Evidence (pertuzumab):
- The Breast Intergroup (BIG) trial enrolled 4,805 women with HER2-positive cancer in a blinded comparison study. Patients received 12 months of trastuzumab plus placebo versus 12 months of trastuzumab plus pertuzumab, which were given in conjunction with standard chemotherapy and hormone therapy. The primary end point was IDFS.[125]
- At the time of the final analysis, the 3-year IDFS rate was 94.1% in the trastuzumab-pertuzumab group and 93.2% in the trastuzumab-placebo group (HR, 0.81; 95% CI, 0.66–1.00; P = .045).
- There was no statistically significant difference in OS (a secondary end point) at the first interim analysis. The same observation was made at the third interim analysis.[140]
- Patients who received pertuzumab had more grade 3 diarrhea (9.8% vs. 3.7%) and were more likely to develop heart failure (0.6% vs. 0.2%) than those who received placebo.
Trastuzumab emtansine (T-DM1)
Evidence (T-DM1 for patients who have received preoperative HER2-targeted therapy.):
- In a phase III trial (KATHERINE [NCT01772472]), 1,486 women with HER2-positive disease who received a preoperative taxane-containing chemotherapy (with or without an anthracycline) along with trastuzumab with or without a second HER2 targeted agent, but who had residual disease after surgery, were randomly assigned to receive 14 cycles of adjuvant trastuzumab or T-DM1. The primary end point was IDFS.[141][Level of evidence B1]
- At the time of a planned interim analysis, IDFS was significantly higher in the T-DM1 group than in the trastuzumab group (HRinvasive disease or death, 0.50; 95% CI, 0.39–0.64; P < .001; IDFS at 3 years, 88.3% vs. 77%).
- Data on OS are immature and not significant (HR, 0.70; 95% CI, 0.47–1.05).
- Patients receiving T-DM1 were more likely to discontinue treatment because of an adverse event (18% vs. 2.1%) and had a higher frequency of sensory neuropathy (18.6% vs. 6.9%), most cases of which had resolved at the time of the analysis.
- On subgroup analysis, the benefit of T-DM1 was observed in all subgroups, including participants who received dual HER2-targeted therapy in the preoperative setting.
Neratinib
Neratinib is an irreversible tyrosine kinase inhibitor of HER1, HER2, and HER4, which has been approved by the FDA for the extended adjuvant treatment of patients with early-stage HER2-positive breast cancer, to follow adjuvant trastuzumab-based therapy.
Evidence (neratinib):
- In the ExteNET trial (NCT00878709), the safety and efficacy of 12 months of adjuvant neratinib was investigated in patients with early-stage HER2-positive breast cancer (n = 2,840) who had completed neoadjuvant trastuzumab up to 2 years before randomization. Patients received 240 mg of oral neratinib daily for 1 year or a placebo. The primary end point was IDFS.[142][Level of evidence A1]
- After a median follow-up of 5.2 years (interquartile range, 2.1–5.3), patients in the neratinib group had significantly fewer IDFS events than those in the placebo group (neratinib group, 116 events vs. placebo group, 163 events; stratified HR, 0.73; 95% CI, 0.57–0.92; P = .0083). The 5-year IDFS rate was 90.2% (95% CI, 88.3%–91.8%) in the neratinib group and 87.7% (85.7%–89.4%) in the placebo group.[143]
- OS data are not mature.
- The most common grade 1 to 2 adverse events included diarrhea (neratinib, 55% vs. placebo, 34%), nausea (41% vs. 21%), fatigue (25% vs. 20%), vomiting (23% vs. 8%), and abdominal pain (22% vs. 10%). The FDA label recommends prophylactic loperamide during the first 56 days of therapy, and as needed thereafter to help manage diarrhea.
- The most common grade 3 to 4 adverse event was diarrhea (neratinib, 40% vs. placebo, 2%). All other grade 3 to 4 adverse events occurred in 2% or less of patients.
Node-negative, small, HER2-positive breast cancer
There are no studies comparing different regimens in patients with node-negative, small, HER2-positive breast tumors. The following two large single-arm studies demonstrated outcomes that appear to be superior to previous results in similar patients who did not receive adjuvant therapy.
Evidence (combination regimens for node-negative, small, HER2-positive tumors):
- The single-arm Adjuvant Paclitaxel and Trastuzumab
(APT) trial (NCT00542451) evaluated a non–anthracycline-containing regimen, paclitaxel and trastuzumab. The trial enrolled 410 women with node-negative, small (≤3 cm), HER2-positive tumors.[144]
- After 6.5 years of follow-up, the DFS rate was 93% (95% CI, 90.4%–96.2%).[144][Level of evidence C2]
- The 10-year DFS rate was 91.3% (95% CI, 88.3%–94.4%).[145]
- The ATEMPT trial (NCT01853748) compared paclitaxel and trastuzumab with T-DM1 in patients with node-negative HER2-positive tumors measuring 2 cm or smaller (patients with a single micrometastatic node were eligible). The goals of the trial were to determine if T-DM1 produced fewer clinically relevant toxicities and was associated with an acceptable 3-year IDFS rate. The trial randomly assigned 383 patients to the T-DM1 arm and 114 patients to the paclitaxel and trastuzumab arm.[146]
- Although the types of toxicities differed in the two treatment arms, the total number of clinically relevant toxicities was nearly identical (46% in the T-DM1 arm vs. 47% in the paclitaxel and trastuzumab arm; P = .83). However, patients who received T-DM1 reported more favorable outcomes during treatment.
- The 3-year IDFS rate for patients who received T-DM1 was 97.8% (95% CI, 96.3%–99.3%), which exceeded the protocol-specified rate of 95% to reject the null hypothesis (P = .0001).[146][Level of evidence C1] Results were similar at 5 years.[147]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Korde LA, Somerfield MR, Carey LA, et al.: Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol 39 (13): 1485-1505, 2021. [PUBMED Abstract]
- Mauri D, Pavlidis N, Ioannidis JP: Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97 (3): 188-94, 2005. [PUBMED Abstract]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG): Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 19 (1): 27-39, 2018. [PUBMED Abstract]
- Cortazar P, Zhang L, Untch M, et al.: Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384 (9938): 164-72, 2014. [PUBMED Abstract]
- Yau C, Osdoit M, van der Noordaa M, et al.: Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol 23 (1): 149-160, 2022. [PUBMED Abstract]
- Carlson RW, Allred DC, Anderson BO, et al.: Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 7 (2): 122-92, 2009. [PUBMED Abstract]
- Lyman GH, Temin S, Edge SB, et al.: Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 32 (13): 1365-83, 2014. [PUBMED Abstract]
- Boughey JC, Suman VJ, Mittendorf EA, et al.: Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310 (14): 1455-61, 2013. [PUBMED Abstract]
- Kuehn T, Bauerfeind I, Fehm T, et al.: Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14 (7): 609-18, 2013. [PUBMED Abstract]
- Alvarado R, Yi M, Le-Petross H, et al.: The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol 19 (10): 3177-84, 2012. [PUBMED Abstract]
- Smith IC, Heys SD, Hutcheon AW, et al.: Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20 (6): 1456-66, 2002. [PUBMED Abstract]
- von Minckwitz G, Kümmel S, Vogel P, et al.: Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst 100 (8): 552-62, 2008. [PUBMED Abstract]
- Bear HD, Anderson S, Brown A, et al.: The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 21 (22): 4165-74, 2003. [PUBMED Abstract]
- Vriens BE, Aarts MJ, de Vries B, et al.: Doxorubicin/cyclophosphamide with concurrent versus sequential docetaxel as neoadjuvant treatment in patients with breast cancer. Eur J Cancer 49 (15): 3102-10, 2013. [PUBMED Abstract]
- Untch M, Jackisch C, Schneeweiss A, et al.: Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol 17 (3): 345-56, 2016. [PUBMED Abstract]
- Gianni L, Mansutti M, Anton A, et al.: Comparing Neoadjuvant Nab-paclitaxel vs Paclitaxel Both Followed by Anthracycline Regimens in Women With ERBB2/HER2-Negative Breast Cancer-The Evaluating Treatment With Neoadjuvant Abraxane (ETNA) Trial: A Randomized Phase 3 Clinical Trial. JAMA Oncol 4 (3): 302-308, 2018. [PUBMED Abstract]
- von Minckwitz G, Rezai M, Loibl S, et al.: Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. J Clin Oncol 28 (12): 2015-23, 2010. [PUBMED Abstract]
- Eiermann W, Paepke S, Appfelstaedt J, et al.: Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 12 (11): 1527-32, 2001. [PUBMED Abstract]
- Preece PE, Wood RA, Mackie CR, et al.: Tamoxifen as initial sole treatment of localised breast cancer in elderly women: a pilot study. Br Med J (Clin Res Ed) 284 (6319): 869-70, 1982. [PUBMED Abstract]
- Smith IE, Dowsett M, Ebbs SR, et al.: Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 23 (22): 5108-16, 2005. [PUBMED Abstract]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365 (9472): 1687-717, 2005. [PUBMED Abstract]
- De Laurentiis M, Cancello G, D'Agostino D, et al.: Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 26 (1): 44-53, 2008. [PUBMED Abstract]
- Henderson IC, Berry DA, Demetri GD, et al.: Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21 (6): 976-83, 2003. [PUBMED Abstract]
- Mamounas EP, Bryant J, Lembersky B, et al.: Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23 (16): 3686-96, 2005. [PUBMED Abstract]
- Martin M, Pienkowski T, Mackey J, et al.: Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352 (22): 2302-13, 2005. [PUBMED Abstract]
- Perez EA: TAC--a new standard in adjuvant therapy for breast cancer? N Engl J Med 352 (22): 2346-8, 2005. [PUBMED Abstract]
- Sparano JA, Wang M, Martino S, et al.: Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358 (16): 1663-71, 2008. [PUBMED Abstract]
- Citron ML, Berry DA, Cirrincione C, et al.: Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21 (8): 1431-9, 2003. [PUBMED Abstract]
- Hudis C, Citron M, Berry D, et al.: Five year follow-up of INT C9741: dose-dense (DD) chemotherapy (CRx) is safe and effective. [Abstract] Breast Cancer Research and Treatment 94 (Suppl 1): A-41, 2005.
- Citron ML, Berry DA, Cirrincione C, et al.: Dose-dense (DD) AC followed by paclitaxel is associated with moderate, frequent anemia compared to sequential (S) and/or less DD treatment: update by CALGB on Breast Cancer Intergroup Trial C9741 with ECOG, SWOG, & NCCTG. [Abstract] J Clin Oncol 23 (Suppl 16): A-620, 33s, 2005.
- Del Mastro L, De Placido S, Bruzzi P, et al.: Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised phase 3 trial. Lancet 385 (9980): 1863-72, 2015. [PUBMED Abstract]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG): Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 393 (10179): 1440-1452, 2019. [PUBMED Abstract]
- Blum JL, Flynn PJ, Yothers G, et al.: Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 35 (23): 2647-2655, 2017. [PUBMED Abstract]
- Nitz U, Gluz O, Clemens M, et al.: West German Study PlanB Trial: Adjuvant Four Cycles of Epirubicin and Cyclophosphamide Plus Docetaxel Versus Six Cycles of Docetaxel and Cyclophosphamide in HER2-Negative Early Breast Cancer. J Clin Oncol 37 (10): 799-808, 2019. [PUBMED Abstract]
- Gagliato Dde M, Gonzalez-Angulo AM, Lei X, et al.: Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32 (8): 735-44, 2014. [PUBMED Abstract]
- Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, et al.: Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA Oncol 2 (3): 322-9, 2016. [PUBMED Abstract]
- Burstein HJ, Temin S, Anderson H, et al.: Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol 32 (21): 2255-69, 2014. [PUBMED Abstract]
- Pan H, Gray R, Braybrooke J, et al.: 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 377 (19): 1836-1846, 2017. [PUBMED Abstract]
- Colleoni M, Gelber S, Goldhirsch A, et al.: Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13-93. J Clin Oncol 24 (9): 1332-41, 2006. [PUBMED Abstract]
- Fisher B, Dignam J, Bryant J, et al.: Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 93 (9): 684-90, 2001. [PUBMED Abstract]
- Stewart HJ, Prescott RJ, Forrest AP: Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst 93 (6): 456-62, 2001. [PUBMED Abstract]
- Tormey DC, Gray R, Falkson HC: Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. Eastern Cooperative Oncology Group. J Natl Cancer Inst 88 (24): 1828-33, 1996. [PUBMED Abstract]
- Davies C, Pan H, Godwin J, et al.: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381 (9869): 805-16, 2013. [PUBMED Abstract]
- Albain KS, Barlow WE, Ravdin PM, et al.: Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet 374 (9707): 2055-63, 2009. [PUBMED Abstract]
- Eisen A, Messersmith J, Franek M, et al.: Adjuvant ovarian ablation in the treatment of premenopausal women with early stage invasive breast cancer. Ontario, Canada: Cancer Care, 2010. Evidence-based Series # 1-9: Section 1. Available online. Last accessed April 24, 2025.
- Adjuvant ovarian ablation versus CMF chemotherapy in premenopausal women with pathological stage II breast carcinoma: the Scottish trial. Scottish Cancer Trials Breast Group and ICRF Breast Unit, Guy's Hospital, London. Lancet 341 (8856): 1293-8, 1993. [PUBMED Abstract]
- Schmid P, Untch M, Kossé V, et al.: Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: the TABLE study. J Clin Oncol 25 (18): 2509-15, 2007. [PUBMED Abstract]
- Ejlertsen B, Mouridsen HT, Jensen MB, et al.: Similar efficacy for ovarian ablation compared with cyclophosphamide, methotrexate, and fluorouracil: from a randomized comparison of premenopausal patients with node-positive, hormone receptor-positive breast cancer. J Clin Oncol 24 (31): 4956-62, 2006. [PUBMED Abstract]
- Wolff AC, Davidson NE: Still waiting after 110 years: the optimal use of ovarian ablation as adjuvant therapy for breast cancer. J Clin Oncol 24 (31): 4949-51, 2006. [PUBMED Abstract]
- Boccardo F, Rubagotti A, Amoroso D, et al.: Cyclophosphamide, methotrexate, and fluorouracil versus tamoxifen plus ovarian suppression as adjuvant treatment of estrogen receptor-positive pre-/perimenopausal breast cancer patients: results of the Italian Breast Cancer Adjuvant Study Group 02 randomized trial. boccardo@hp380.ist.unige.it. J Clin Oncol 18 (14): 2718-27, 2000. [PUBMED Abstract]
- Winer EP, Hudis C, Burstein HJ, et al.: American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol 20 (15): 3317-27, 2002. [PUBMED Abstract]
- Tevaarwerk AJ, Wang M, Zhao F, et al.: Phase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in premenopausal women with node-negative, hormone receptor-positive breast cancer (E-3193, INT-0142): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 32 (35): 3948-58, 2014. [PUBMED Abstract]
- Francis PA, Regan MM, Fleming GF, et al.: Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 372 (5): 436-46, 2015. [PUBMED Abstract]
- Francis PA, Pagani O, Fleming GF, et al.: Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N Engl J Med 379 (2): 122-137, 2018. [PUBMED Abstract]
- Kim HA, Lee JW, Nam SJ, et al.: Adding Ovarian Suppression to Tamoxifen for Premenopausal Breast Cancer: A Randomized Phase III Trial. J Clin Oncol 38 (5): 434-443, 2020. [PUBMED Abstract]
- Gnant M, Mlineritsch B, Stoeger H, et al.: Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 12 (7): 631-41, 2011. [PUBMED Abstract]
- Pagani O, Regan MM, Walley BA, et al.: Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371 (2): 107-18, 2014. [PUBMED Abstract]
- Francis PA, Fleming GF, Láng I, et al.: Adjuvant Endocrine Therapy in Premenopausal Breast Cancer: 12-Year Results From SOFT. J Clin Oncol 41 (7): 1370-1375, 2023. [PUBMED Abstract]
- Bernhard J, Luo W, Ribi K, et al.: Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncol 16 (7): 848-58, 2015. [PUBMED Abstract]
- Dowsett M, Forbes JF, Bradley R, et al.: Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386 (10001): 1341-52, 2015. [PUBMED Abstract]
- The ATAC Trialists' Group. Arimidex, tamoxifen alone or in combination: Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359 (9324): 2131-9, 2002. [PUBMED Abstract]
- Howell A, Cuzick J, Baum M, et al.: Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365 (9453): 60-2, 2005. [PUBMED Abstract]
- Thürlimann B, Keshaviah A, Coates AS, et al.: A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353 (26): 2747-57, 2005. [PUBMED Abstract]
- Coates AS, Keshaviah A, Thürlimann B, et al.: Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol 25 (5): 486-92, 2007. [PUBMED Abstract]
- Boccardo F, Rubagotti A, Guglielmini P, et al.: Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol 17 (Suppl 7): vii10-4, 2006. [PUBMED Abstract]
- Jakesz R, Jonat W, Gnant M, et al.: Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366 (9484): 455-62, 2005 Aug 6-12. [PUBMED Abstract]
- Boccardo F, Rubagotti A, Aldrighetti D, et al.: Switching to an aromatase inhibitor provides mortality benefit in early breast carcinoma: pooled analysis of 2 consecutive trials. Cancer 109 (6): 1060-7, 2007. [PUBMED Abstract]
- Coombes RC, Hall E, Gibson LJ, et al.: A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350 (11): 1081-92, 2004. [PUBMED Abstract]
- Coombes RC, Kilburn LS, Snowdon CF, et al.: Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369 (9561): 559-70, 2007. [PUBMED Abstract]
- van de Velde CJ, Rea D, Seynaeve C, et al.: Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 377 (9762): 321-31, 2011. [PUBMED Abstract]
- Regan MM, Neven P, Giobbie-Hurder A, et al.: Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol 12 (12): 1101-8, 2011. [PUBMED Abstract]
- De Placido S, Gallo C, De Laurentiis M, et al.: Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): a randomised, phase 3 trial. Lancet Oncol 19 (4): 474-485, 2018. [PUBMED Abstract]
- Goss PE, Ingle JN, Pritchard KI, et al.: Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol 31 (11): 1398-404, 2013. [PUBMED Abstract]
- Goss PE, Hershman DL, Cheung AM, et al.: Effects of adjuvant exemestane versus anastrozole on bone mineral density for women with early breast cancer (MA.27B): a companion analysis of a randomised controlled trial. Lancet Oncol 15 (4): 474-82, 2014. [PUBMED Abstract]
- Smith I, Yardley D, Burris H, et al.: Comparative Efficacy and Safety of Adjuvant Letrozole Versus Anastrozole in Postmenopausal Patients With Hormone Receptor-Positive, Node-Positive Early Breast Cancer: Final Results of the Randomized Phase III Femara Versus Anastrozole Clinical Evaluation (FACE) Trial. J Clin Oncol 35 (10): 1041-1048, 2017. [PUBMED Abstract]
- Goss PE, Ingle JN, Martino S, et al.: A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349 (19): 1793-802, 2003. [PUBMED Abstract]
- Bryant J, Wolmark N: Letrozole after tamoxifen for breast cancer--what is the price of success? N Engl J Med 349 (19): 1855-7, 2003. [PUBMED Abstract]
- Burstein HJ: Beyond tamoxifen--extending endocrine treatment for early-stage breast cancer. N Engl J Med 349 (19): 1857-9, 2003. [PUBMED Abstract]
- Goss PE, Ingle JN, Martino S, et al.: Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97 (17): 1262-71, 2005. [PUBMED Abstract]
- Mamounas EP, Jeong JH, Wickerham DL, et al.: Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol 26 (12): 1965-71, 2008. [PUBMED Abstract]
- Goss PE, Ingle JN, Pritchard KI, et al.: Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med 375 (3): 209-19, 2016. [PUBMED Abstract]
- Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al.: Optimal Duration of Extended Adjuvant Endocrine Therapy for Early Breast Cancer; Results of the IDEAL Trial (BOOG 2006-05). J Natl Cancer Inst 110 (1): , 2018. [PUBMED Abstract]
- Mamounas EP, Bandos H, Lembersky BC, et al.: Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20 (1): 88-99, 2019. [PUBMED Abstract]
- Colleoni M, Luo W, Karlsson P, et al.: Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19 (1): 127-138, 2018. [PUBMED Abstract]
- Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, et al.: Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol 18 (11): 1502-1511, 2017. [PUBMED Abstract]
- Del Mastro L, Mansutti M, Bisagni G, et al.: Extended therapy with letrozole as adjuvant treatment of postmenopausal patients with early-stage breast cancer: a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 22 (10): 1458-1467, 2021. [PUBMED Abstract]
- Gnant M, Fitzal F, Rinnerthaler G, et al.: Duration of Adjuvant Aromatase-Inhibitor Therapy in Postmenopausal Breast Cancer. N Engl J Med 385 (5): 395-405, 2021. [PUBMED Abstract]
- Iwase T, Saji S, Iijima K, et al.: Postoperative Adjuvant Anastrozole for 10 or 5 Years in Patients With Hormone Receptor-Positive Breast Cancer: AERAS, a Randomized Multicenter Open-Label Phase III Trial. J Clin Oncol 41 (18): 3329-3338, 2023. [PUBMED Abstract]
- Johnston SRD, Harbeck N, Hegg R, et al.: Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol 38 (34): 3987-3998, 2020. [PUBMED Abstract]
- Rastogi P, O'Shaughnessy J, Martin M, et al.: Adjuvant Abemaciclib Plus Endocrine Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative, High-Risk Early Breast Cancer: Results From a Preplanned monarchE Overall Survival Interim Analysis, Including 5-Year Efficacy Outcomes. J Clin Oncol 42 (9): 987-993, 2024. [PUBMED Abstract]
- Martin M, Hegg R, Kim SB, et al.: Treatment With Adjuvant Abemaciclib Plus Endocrine Therapy in Patients With High-risk Early Breast Cancer Who Received Neoadjuvant Chemotherapy: A Prespecified Analysis of the monarchE Randomized Clinical Trial. JAMA Oncol 8 (8): 1190-1194, 2022. [PUBMED Abstract]
- Slamon D, Lipatov O, Nowecki Z, et al.: Ribociclib plus Endocrine Therapy in Early Breast Cancer. N Engl J Med 390 (12): 1080-1091, 2024. [PUBMED Abstract]
- Mayer EL, Dueck AC, Martin M, et al.: Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 22 (2): 212-222, 2021. [PUBMED Abstract]
- Gnant M, Dueck AC, Frantal S, et al.: Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). J Clin Oncol 40 (3): 282-293, 2022. [PUBMED Abstract]
- Coleman R, Powles T, Paterson A, et al.: Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386 (10001): 1353-61, 2015. [PUBMED Abstract]
- Gnant M, Pfeiler G, Steger GG, et al.: Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20 (3): 339-351, 2019. [PUBMED Abstract]
- Coleman R, Finkelstein DM, Barrios C, et al.: Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 21 (1): 60-72, 2020. [PUBMED Abstract]
- Friedl TWP, Fehm T, Müller V, et al.: Prognosis of Patients With Early Breast Cancer Receiving 5 Years vs 2 Years of Adjuvant Bisphosphonate Treatment: A Phase 3 Randomized Clinical Trial. JAMA Oncol 7 (8): 1149-1157, 2021. [PUBMED Abstract]
- Desnoyers A, Amir E, Tannock IF: Adjuvant Zoledronate Therapy for Women With Breast Cancer-Effective Treatment or Fool's Gold? JAMA Oncol 7 (8): 1121-1123, 2021. [PUBMED Abstract]
- Tutt ANJ, Garber JE, Kaufman B, et al.: Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 384 (25): 2394-2405, 2021. [PUBMED Abstract]
- Mittendorf EA, Jeruss JS, Tucker SL, et al.: Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol 29 (15): 1956-62, 2011. [PUBMED Abstract]
- Ganz PA, Bandos H, Španić T, et al.: Patient-Reported Outcomes in OlympiA: A Phase III, Randomized, Placebo-Controlled Trial of Adjuvant Olaparib in gBRCA1/2 Mutations and High-Risk Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer. J Clin Oncol 42 (11): 1288-1300, 2024. [PUBMED Abstract]
- von Minckwitz G, Schneeweiss A, Loibl S, et al.: Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 15 (7): 747-56, 2014. [PUBMED Abstract]
- Loibl S, Weber KE, Timms KM, et al.: Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol 29 (12): 2341-2347, 2018. [PUBMED Abstract]
- Sikov WM, Berry DA, Perou CM, et al.: Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33 (1): 13-21, 2015. [PUBMED Abstract]
- Schmid P, Cortes J, Pusztai L, et al.: Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 382 (9): 810-821, 2020. [PUBMED Abstract]
- Schmid P, Cortes J, Dent R, et al.: Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med 386 (6): 556-567, 2022. [PUBMED Abstract]
- Yu KD, Ye FG, He M, et al.: Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women With Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 6 (9): 1390-1396, 2020. [PUBMED Abstract]
- Wang X, Wang SS, Huang H, et al.: Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial. JAMA 325 (1): 50-58, 2021. [PUBMED Abstract]
- Masuda N, Lee SJ, Ohtani S, et al.: Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 376 (22): 2147-2159, 2017. [PUBMED Abstract]
- Ignatiadis M, Bailey A, McArthur H, et al.: Adjuvant Atezolizumab for Early Triple-Negative Breast Cancer: The ALEXANDRA/IMpassion030 Randomized Clinical Trial. JAMA 333 (13): 1150-60, 2025. [PUBMED Abstract]
- Buzdar AU, Ibrahim NK, Francis D, et al.: Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23 (16): 3676-85, 2005. [PUBMED Abstract]
- Untch M, Rezai M, Loibl S, et al.: Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol 28 (12): 2024-31, 2010. [PUBMED Abstract]
- Gianni L, Eiermann W, Semiglazov V, et al.: Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375 (9712): 377-84, 2010. [PUBMED Abstract]
- Gianni L, Eiermann W, Semiglazov V, et al.: Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 15 (6): 640-7, 2014. [PUBMED Abstract]
- Buzdar AU, Suman VJ, Meric-Bernstam F, et al.: Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol 14 (13): 1317-25, 2013. [PUBMED Abstract]
- Buzdar AU, Suman VJ, Meric-Bernstam F, et al.: Disease-Free and Overall Survival Among Patients With Operable HER2-Positive Breast Cancer Treated With Sequential vs Concurrent Chemotherapy: The ACOSOG Z1041 (Alliance) Randomized Clinical Trial. JAMA Oncol 5 (1): 45-50, 2019. [PUBMED Abstract]
- Gligorov J, Ataseven B, Verrill M, et al.: Safety and tolerability of subcutaneous trastuzumab for the adjuvant treatment of human epidermal growth factor receptor 2-positive early breast cancer: SafeHer phase III study's primary analysis of 2573 patients. Eur J Cancer 82: 237-246, 2017. [PUBMED Abstract]
- Ismael G, Hegg R, Muehlbauer S, et al.: Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol 13 (9): 869-78, 2012. [PUBMED Abstract]
- Jackisch C, Stroyakovskiy D, Pivot X, et al.: Subcutaneous vs Intravenous Trastuzumab for Patients With ERBB2-Positive Early Breast Cancer: Final Analysis of the HannaH Phase 3 Randomized Clinical Trial. JAMA Oncol 5 (5): e190339, 2019. [PUBMED Abstract]
- Gianni L, Pienkowski T, Im YH, et al.: Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13 (1): 25-32, 2012. [PUBMED Abstract]
- Baselga J, Bradbury I, Eidtmann H, et al.: Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379 (9816): 633-40, 2012. [PUBMED Abstract]
- Gianni L, Pienkowski T, Im YH, et al.: 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 17 (6): 791-800, 2016. [PUBMED Abstract]
- Schneeweiss A, Chia S, Hickish T, et al.: Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24 (9): 2278-84, 2013. [PUBMED Abstract]
- von Minckwitz G, Procter M, de Azambuja E, et al.: Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 377 (2): 122-131, 2017. [PUBMED Abstract]
- van Ramshorst MS, van der Voort A, van Werkhoven ED, et al.: Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19 (12): 1630-1640, 2018. [PUBMED Abstract]
- van der Voort A, van Ramshorst MS, van Werkhoven ED, et al.: Three-Year Follow-up of Neoadjuvant Chemotherapy With or Without Anthracyclines in the Presence of Dual ERBB2 Blockade in Patients With ERBB2-Positive Breast Cancer: A Secondary Analysis of the TRAIN-2 Randomized, Phase 3 Trial. JAMA Oncol 7 (7): 978-984, 2021. [PUBMED Abstract]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al.: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353 (16): 1659-72, 2005. [PUBMED Abstract]
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al.: 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389 (10075): 1195-1205, 2017. [PUBMED Abstract]
- Romond EH, Perez EA, Bryant J, et al.: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353 (16): 1673-84, 2005. [PUBMED Abstract]
- Perez E, Romond E, Suman V, et al.: Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patiens with HER2-positive breast cancer. [Abstract] J Clin Oncol 25 (Suppl 18): 512, 6s, 2007.
- Perez EA, Romond EH, Suman VJ, et al.: Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32 (33): 3744-52, 2014. [PUBMED Abstract]
- Slamon D, Eiermann W, Robert N, et al.: Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365 (14): 1273-83, 2011. [PUBMED Abstract]
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al.: Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354 (8): 809-20, 2006. [PUBMED Abstract]
- Pivot X, Romieu G, Debled M, et al.: 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 393 (10191): 2591-2598, 2019. [PUBMED Abstract]
- Mavroudis D, Saloustros E, Malamos N, et al.: Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 26 (7): 1333-40, 2015. [PUBMED Abstract]
- Earl HM, Hiller L, Vallier AL, et al.: 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 393 (10191): 2599-2612, 2019. [PUBMED Abstract]
- Joensuu H, Fraser J, Wildiers H, et al.: Effect of Adjuvant Trastuzumab for a Duration of 9 Weeks vs 1 Year With Concomitant Chemotherapy for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: The SOLD Randomized Clinical Trial. JAMA Oncol 4 (9): 1199-1206, 2018. [PUBMED Abstract]
- Inno A, Barni S, Ghidini A, et al.: One year versus a shorter duration of adjuvant trastuzumab for HER2-positive early breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 173 (2): 247-254, 2019. [PUBMED Abstract]
- Loibl S, Jassem J, Sonnenblick A, et al.: Adjuvant Pertuzumab and Trastuzumab in Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer in the APHINITY Trial: Third Interim Overall Survival Analysis With Efficacy Update. J Clin Oncol 42 (31): 3643-3651, 2024. [PUBMED Abstract]
- von Minckwitz G, Huang CS, Mano MS, et al.: Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 380 (7): 617-628, 2019. [PUBMED Abstract]
- Chan A, Delaloge S, Holmes FA, et al.: Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17 (3): 367-77, 2016. [PUBMED Abstract]
- Martin M, Holmes FA, Ejlertsen B, et al.: Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18 (12): 1688-1700, 2017. [PUBMED Abstract]
- Tolaney SM, Guo H, Pernas S, et al.: Seven-Year Follow-Up Analysis of Adjuvant Paclitaxel and Trastuzumab Trial for Node-Negative, Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. J Clin Oncol 37 (22): 1868-1875, 2019. [PUBMED Abstract]
- Tolaney SM, Tarantino P, Graham N, et al.: Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol 24 (3): 273-285, 2023. [PUBMED Abstract]
- Tolaney SM, Tayob N, Dang C, et al.: Adjuvant Trastuzumab Emtansine Versus Paclitaxel in Combination With Trastuzumab for Stage I HER2-Positive Breast Cancer (ATEMPT): A Randomized Clinical Trial. J Clin Oncol 39 (21): 2375-2385, 2021. [PUBMED Abstract]
- Tarantino P, Tayob N, Villacampa G, et al.: Adjuvant Trastuzumab Emtansine Versus Paclitaxel Plus Trastuzumab for Stage I Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: 5-Year Results and Correlative Analyses From ATEMPT. J Clin Oncol 42 (31): 3652-3665, 2024. [PUBMED Abstract]
Toxicity of Systemic Therapy
Capecitabine and Fluorouracil Dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[1,2] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[1-3] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient's DPYD genotype and number of functioning DPYD alleles.[4-6] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[7] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[8]
Toxicity of Adjuvant Chemotherapy
The acute toxicities of the drugs used for adjuvant chemotherapy are the same as those observed when these drugs are used in other treatment settings. However, because many patients with early breast cancer have prolonged survival, long-term adverse effects are particularly important in this setting. The following two toxicities are of special concern:
- Cardiotoxicity from anthracyclines.
- Marrow neoplasia. A study of 20,063 patients treated in National Comprehensive Cancer Network centers found an incidence of marrow neoplasia of 0.46 per 1,000 person-years in women treated with anthracycline- and/or cyclophosphamide-containing chemotherapy. This rate was significantly higher than the rate observed in women who were treated with surgery alone (hazard ratio, 6.8; 95% confidence interval [CI], 1.3–36.1).[9]
Cardiac Toxic Effects With Adjuvant Trastuzumab
Cardiac events associated with adjuvant trastuzumab have been reported in multiple studies. Key study results include the following:
- In the HERA (BIG-01-01) trial, severe congestive heart failure (CHF) (New York Heart Association class III–IV) occurred in 0.6% of patients treated with trastuzumab.[10] Symptomatic CHF occurred in 1.7% of patients in the trastuzumab arm and 0.06% of patients in the observation arm.
- In the NSABP B-31 trial (NCT00004067), 31 of 850 patients in the trastuzumab arm had confirmed symptomatic cardiac events, compared with 5 of 814 patients in the control arm.[11] The 3-year cumulative incidence of cardiac events for trastuzumab-treated patients was 4.1%, compared with 0.8% of patients in the control arm (95% CI, 1.7%–4.9%).
- In the NCCTG-N9831 trial, 39 cardiac events were reported in the three arms over a 3-year period. The 3-year cumulative incidence of cardiac events was 0.35% in arm A (no trastuzumab), 3.5% in arm B (trastuzumab after paclitaxel), and 2.5% in arm C, (trastuzumab concomitant with paclitaxel).
- In the AVENTIS-TAX-GMA-302 (BCIRG 006) trial (NCT00021255), clinically symptomatic cardiac events were detected in 0.38% of patients in the doxorubicin and cyclophosphamide (AC)/docetaxel (AC-D) arm, 1.87% of patients in the AC/docetaxel/trastuzumab (AC-DH) arm, and 0.37% of patients in the docetaxel/carboplatin/trastuzumab (DCbH) arm.[12] There was also a statistically significant higher incidence of asymptomatic and persistent decrease in left ventricular ejection fraction (LVEF) in the AC-DH arm than with either the AC-D or DCbH arms.
- In the FINHER trial, none of the patients who received trastuzumab experienced clinically significant cardiac events. LVEF was preserved in all of the women receiving trastuzumab, but the number of patients receiving adjuvant trastuzumab was very low.[13]
Cardiac Toxic Effects With Pertuzumab and Lapatinib
Evidence (cardiac toxic effects with pertuzumab and lapatinib):
- A pooled analysis of cardiac safety in 598 cancer patients treated with pertuzumab was performed using data supplied by Roche and Genentech.[14][Level of evidence C2]
- Asymptomatic left ventricular systolic dysfunction was observed in 6.9% of patients receiving pertuzumab alone (n = 331; 95% CI, 4.5%–10.2%), 3.4% of patients receiving pertuzumab in combination with a non–anthracycline-containing chemotherapy (n = 175; 95% CI, 1.3%–7.3%), and 6.5% of patients receiving pertuzumab in combination with trastuzumab (n = 93; 95% CI, 2.4%–13.5%).
- Symptomatic heart failure was observed in one patient (0.3%) who received pertuzumab alone, two patients (1.1%) who received pertuzumab in combination with a non–anthracycline-containing chemotherapy, and one patient (1.1%) who received pertuzumab in combination with trastuzumab.
- A meta-analysis of randomized trials (n = 6) that evaluated the administration of anti-HER2 monotherapy (trastuzumab or lapatinib or pertuzumab) versus dual anti-HER2 therapy (trastuzumab plus lapatinib or trastuzumab plus pertuzumab) was performed.[15][Level of evidence C2]
- LVEF decline was observed in 3.1% of the patients who received monotherapy (95% CI, 2.2%–4.4%) and 2.9% of the patients who received dual therapy (95% CI, 2.1%–4.1%).
- Symptomatic heart failure was observed in 0.88% of the patients who received monotherapy (95% CI, 0.47%–1.64%) and 1.49% of the patients who received dual therapy (95% CI, 0.98%–2.23%).
Toxicity of Endocrine Therapy
Tamoxifen
- Long-term follow-up of the ATLAS trial (NCT00003016) included toxicity data. Compared with 5 years, 10 years of tamoxifen therapy increased the risk of the following:[16]
- Pulmonary embolus: relative risk (RR), 1.87 (95% CI, 1.13–3.07; P = .01).
- Stroke: RR, 1.06 (95% CI, 0.83–1.36).
- Ischemic heart disease: RR, 0.76 (95% CI, 0.6–0.95; P = .02).
- Endometrial cancer: RR, 1.74 (95% CI, 1.30–2.34; P = .0002). Notably, the cumulative risk of endometrial cancer during years 5 to 14 from breast cancer diagnosis was 3.1% for women who received 10 years of tamoxifen versus 1.6% for women who received 5 years of tamoxifen. The mortality rate for years 5 to 14 was 12.2% for women who received 10 years of tamoxifen versus 15% for women who received 5 years of tamoxifen, for an absolute mortality reduction of 2.8%.
Aromatase Inhibitors
Patients on tamoxifen more frequently developed endometrial cancer and cerebrovascular accidents, whereas patients on anastrozole had more fracture episodes. The frequency of myocardial infarction was similar in both groups. Except for a continued increased frequency of endometrial cancer in the tamoxifen group, these differences did not persist in the posttreatment period.[17]
References
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PUBMED Abstract]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PUBMED Abstract]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PUBMED Abstract]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PUBMED Abstract]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PUBMED Abstract]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PUBMED Abstract]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PUBMED Abstract]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PUBMED Abstract]
- Wolff AC, Blackford AL, Visvanathan K, et al.: Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol 33 (4): 340-8, 2015. [PUBMED Abstract]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al.: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353 (16): 1659-72, 2005. [PUBMED Abstract]
- Tan-Chiu E, Yothers G, Romond E, et al.: Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 23 (31): 7811-9, 2005. [PUBMED Abstract]
- Slamon D, Eiermann W, Robert N, et al.: BCIRG 006: 2nd interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC->T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC->TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients. [Abstract] 29th Annual San Antonio Breast Cancer Symposium, December 14-17, 2006, San Antonio, Texas. A-52, 2006.
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al.: Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354 (8): 809-20, 2006. [PUBMED Abstract]
- Lenihan D, Suter T, Brammer M, et al.: Pooled analysis of cardiac safety in patients with cancer treated with pertuzumab. Ann Oncol 23 (3): 791-800, 2012. [PUBMED Abstract]
- Valachis A, Nearchou A, Polyzos NP, et al.: Cardiac toxicity in breast cancer patients treated with dual HER2 blockade. Int J Cancer 133 (9): 2245-52, 2013. [PUBMED Abstract]
- Davies C, Pan H, Godwin J, et al.: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381 (9869): 805-16, 2013. [PUBMED Abstract]
- Howell A, Cuzick J, Baum M, et al.: Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365 (9453): 60-2, 2005. [PUBMED Abstract]
Posttherapy Surveillance of Stages I, II, and III Breast Cancer
The frequency of follow-up and the appropriateness of screening tests after the completion of primary treatment for stage I, stage II, or stage III breast cancer remain controversial.
Evidence from randomized trials indicates that periodic follow-up with bone scans, liver sonography, chest x-rays, and blood tests of liver function does not improve survival or quality of life when compared with routine physical examinations.[1-3] Even when these tests permit earlier detection of recurrent disease, patient survival is unaffected.[2] On the basis of these data, acceptable follow-up can be limited to the following for asymptomatic patients who complete treatment for stages I to III breast cancer:
- Physical examination.
- Annual mammography.
References
- Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. The GIVIO Investigators. JAMA 271 (20): 1587-92, 1994. [PUBMED Abstract]
- Rosselli Del Turco M, Palli D, Cariddi A, et al.: Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA 271 (20): 1593-7, 1994. [PUBMED Abstract]
- Khatcheressian JL, Wolff AC, Smith TJ, et al.: American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol 24 (31): 5091-7, 2006. [PUBMED Abstract]
Treatment of Locoregional Recurrent Breast Cancer
Recurrent breast cancer often responds to therapy, although treatment is rarely curative at this stage of disease. Patients with locoregional breast cancer recurrence may become long-term survivors with appropriate therapy.
The rates of locoregional recurrence have declined over time, and a meta-analysis suggests a recurrence rate of less than 3% in patients treated with breast-conserving surgery and radiation therapy.[1] The rates are somewhat higher (up to 10%) for those treated with mastectomy.[2] Nine percent to 25% of patients with locoregional recurrence will have distant metastases or locally extensive disease at the time of recurrence.[3-5]
Before treatment for recurrent breast cancer, restaging to evaluate the extent of disease is indicated. Cytological or histological documentation of recurrent disease is obtained whenever possible. When therapy is selected, the estrogen-receptor (ER) status, progesterone-receptor (PR) status, and human epidermal growth factor receptor 2 (HER2) status at the time of recurrence and previous treatment are considered, if known.
ER status may change at the time of recurrence. In a single small study by the Cancer and Leukemia Group B (MDA-MBDT-8081), 36% of hormone receptor–positive tumors were found to be receptor negative in biopsy specimens isolated at the time of recurrence.[6] Patients in this study had no interval treatment. If ER and PR statuses are unknown, then the site(s) of recurrence, disease-free interval, response to previous treatment, and menopausal status are useful in the selection of chemotherapy or hormone therapy.[7]
Patients with locoregional recurrence should be considered for further local treatment (e.g., mastectomy). In one series, the 5-year actuarial rate of relapse for patients treated for invasive recurrence after initial breast conservation and radiation therapy was 52%.[4]
Treatment options also depend on the site of recurrence, as follows:
- Chest wall: Local chest wall recurrence after mastectomy is often a sign of widespread disease, but, in a subset of patients, it may be the only site of recurrence. For patients in this subset, surgery and/or radiation therapy may be curative.[8,9] Patients with chest wall recurrences of less than 3 cm, axillary and internal mammary node recurrence (not supraclavicular, which has a poorer survival), and a greater-than-2-year disease-free interval before recurrence have the best chance for prolonged survival.[9] The 5-year disease-free survival (DFS) rate in one series of such patients was 25%, with a 10-year rate of 15%.[10] The locoregional control rate was 57% at 10 years. Systemic therapy should be considered in patients with locoregional recurrence.
- Breast: In the Chemotherapy as Adjuvant for Locally Recurrent Breast Cancer (CALOR [NCT00074152]) trial, patients with a history of breast-conserving surgery or mastectomy with clear margins and complete excision of an isolated local recurrence of their breast cancer were randomly assigned to receive either chemotherapy of the physician's choice or no chemotherapy. The study was closed early because of poor accrual. The original sample size for a hazard ratio (HR) of 0.74 was 977 patients (347 DFS events) and was revised subsequently to 265 patients (HR, 0.6; 124 DFS events), with only 162 enrolled at the time of study closure.[11][Level of evidence B1]
- In ER-negative patients, the HR for DFS for chemotherapy versus no chemotherapy was 0.29 (95% confidence interval [CI], 0.13–0.67; 10-year DFS, 70% vs. 34%), whereas in ER-positive patients, the HR was 1.07 (95% CI, 0.57–2.00; 10-year DFS, 50% vs. 59%). The interaction between chemotherapy and ER status with respect to DFS was significant (P = .013).[12]
- This trial supports consideration of adjuvant chemotherapy after complete resection of isolated locoregional recurrence of breast cancer in patients with ER-negative tumors.
All patients with recurrent breast cancer are considered candidates for ongoing clinical trials. For more information, see the Treatment of Metastatic Breast Cancer section.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Darby S, McGale P, Correa C, et al.: Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378 (9804): 1707-16, 2011. [PUBMED Abstract]
- Buchanan CL, Dorn PL, Fey J, et al.: Locoregional recurrence after mastectomy: incidence and outcomes. J Am Coll Surg 203 (4): 469-74, 2006. [PUBMED Abstract]
- Aberizk WJ, Silver B, Henderson IC, et al.: The use of radiotherapy for treatment of isolated locoregional recurrence of breast carcinoma after mastectomy. Cancer 58 (6): 1214-8, 1986. [PUBMED Abstract]
- Abner AL, Recht A, Eberlein T, et al.: Prognosis following salvage mastectomy for recurrence in the breast after conservative surgery and radiation therapy for early-stage breast cancer. J Clin Oncol 11 (1): 44-8, 1993. [PUBMED Abstract]
- Haffty BG, Fischer D, Beinfield M, et al.: Prognosis following local recurrence in the conservatively treated breast cancer patient. Int J Radiat Oncol Biol Phys 21 (2): 293-8, 1991. [PUBMED Abstract]
- Kuukasjärvi T, Kononen J, Helin H, et al.: Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol 14 (9): 2584-9, 1996. [PUBMED Abstract]
- Perry MC, Kardinal CG, Korzun AH, et al.: Chemohormonal therapy in advanced carcinoma of the breast: Cancer and Leukemia Group B protocol 8081. J Clin Oncol 5 (10): 1534-45, 1987. [PUBMED Abstract]
- Schwaibold F, Fowble BL, Solin LJ, et al.: The results of radiation therapy for isolated local regional recurrence after mastectomy. Int J Radiat Oncol Biol Phys 21 (2): 299-310, 1991. [PUBMED Abstract]
- Halverson KJ, Perez CA, Kuske RR, et al.: Survival following locoregional recurrence of breast cancer: univariate and multivariate analysis. Int J Radiat Oncol Biol Phys 23 (2): 285-91, 1992. [PUBMED Abstract]
- Halverson KJ, Perez CA, Kuske RR, et al.: Isolated local-regional recurrence of breast cancer following mastectomy: radiotherapeutic management. Int J Radiat Oncol Biol Phys 19 (4): 851-8, 1990. [PUBMED Abstract]
- Aebi S, Gelber S, Anderson SJ, et al.: Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol 15 (2): 156-63, 2014. [PUBMED Abstract]
- Wapnir IL, Price KN, Anderson SJ, et al.: Efficacy of Chemotherapy for ER-Negative and ER-Positive Isolated Locoregional Recurrence of Breast Cancer: Final Analysis of the CALOR Trial. J Clin Oncol 36 (11): 1073-1079, 2018. [PUBMED Abstract]
Treatment of Metastatic Breast Cancer
Treatment of metastatic disease is palliative in intent. Goals of treatment include prolonging life and improving quality of life. The 5-year relative survival rate for women with metastatic breast cancer is 31.9%.[1] The longest median survival outcomes have been observed in patients with human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor–positive metastatic breast cancer, and less favorable outcomes have been observed in patients with metastatic triple-negative breast cancer (TNBC).[2]
Treatment Option Overview for Metastatic Breast Cancer
Treatment options for metastatic breast cancer include:
- Hormone therapy (tamoxifen, aromatase inhibitors, selective estrogen receptor [ER] degraders).
- HER2-targeted therapy.
- CDK4/6 inhibitors.
- mTOR inhibitors.
- PIK3CA inhibitors.
- Chemotherapy.
- Immunotherapy.
- Surgery for patients with limited symptomatic metastases.
- Radiation therapy for patients with limited symptomatic metastases.
- Bone-modifying therapy for patients with bone metastases.
In many cases, these therapies are given in sequence and used in various combinations.
Cytological or histological documentation of metastatic disease, with testing of ER, progesterone receptor, and HER2 statuses, should be obtained at the time of metastatic presentation, if possible. If not possible, it is appropriate to consider liquid biopsy (via circulating tumor cell and/or circulating tumor DNA testing).
All patients with metastatic breast cancer are considered candidates for ongoing clinical trials.
Metastatic HER2-Negative Hormone Receptor–Positive Breast Cancer
Endocrine therapy and cyclin-dependent kinase (CDK) inhibitor therapy
CDK4 and CDK6 have been implicated in the continued proliferation of hormone receptor–positive breast cancer resistant to endocrine therapy. CDK inhibitors have been approved by the U.S. Food and Drug Administration (FDA) in combination with endocrine therapy in both first-line and later-line treatment of advanced, HER2-negative hormone receptor–positive breast cancer. Three oral CDK4/6 inhibitors are currently available: palbociclib, ribociclib, and abemaciclib.
Overall, the addition of CDK4/6 inhibitors to endocrine therapy is associated with improved breast cancer outcomes and, in general, either maintained or improved quality of life.[3-8] This benefit was observed across multiple clinicopathological subgroups of breast cancer.[9]
First-line palbociclib and endocrine therapy
Evidence (first-line palbociclib and endocrine therapy):
- PALOMA-2 (NCT01740427) confirmed the results of the phase II PALOMA-1 trial.[10] This phase III, double-blind trial compared placebo plus letrozole with palbociclib plus letrozole as initial therapy for ER-positive postmenopausal patients with advanced disease (n = 666). The primary end point was investigator-assessed progression-free survival (PFS).[11]
- The median PFS was 24.8 months in the palbociclib-plus-letrozole group compared with 14.5 months in the placebo-plus-letrozole group (hazard ratio [HR], 0.58; 95% confidence interval [CI], 0.46–0.72; P < .001).[11][Level of evidence B1]
- Overall survival (OS) data are not yet mature.
- Patients who received palbociclib experienced more frequent cytopenias (66.4% grade 3 to 4 in palbociclib-treated patients vs. 1.4% in placebo-treated patients). Other common adverse events included nausea, arthralgia, fatigue, and alopecia. The most common grade 3 to 4 adverse events other than neutropenia included leukopenia (24.8% vs. 0%), anemia (5.4% vs. 1.8%), and fatigue (1.8% vs. 0.5%).
First-line ribociclib and endocrine therapy
Ribociclib, another CDK4/6 inhibitor, has also been tested in the first-line setting for postmenopausal patients and premenopausal patients with HER2-negative hormone receptor–positive, recurrent or metastatic breast cancer.
Evidence (first-line ribociclib and endocrine therapy):
- The phase III placebo-controlled MONALEESA-2 trial (NCT01958021) randomly assigned 668 patients to receive either first-line ribociclib plus letrozole or placebo plus letrozole.[12,13]
- The primary end point (investigator-assessed PFS) was met. A preplanned interim analysis was performed after 243 patients had disease progression or died, and median duration of follow-up was 15.3 months. After 18 months, the PFS rate was 63.0% (95% CI, 54.6%–70.3%) in the ribociclib group and 42.2% (95% CI, 34.8%–49.5%) in the placebo group.[12]
- OS was a secondary end point. A protocol-specified final analysis of OS was published after 400 deaths and a median follow-up of 6.6 years. Patients who received ribociclib plus letrozole had a significant OS benefit compared with patients who received placebo plus letrozole. Median OS was 63.9 months in the ribociclib group and 51.4 months in the placebo group (HRdeath, 0.76; 95% CI, 0.63–0.93; two-sided P = .008).[14][Level of evidence A1]
- Grade 3 to 4 neutropenia occurred in 63.8% of patients in the ribociclib group and 1.2% of patients in the placebo group. Grade 1 to 2 nausea, infection, fatigue, and diarrhea were also noted. Grade 3 to 4 hepatobiliary toxic effects occurred in 14.4% of patients who received ribociclib and 4.8% of patients who received placebo. Prolonged QTcF interval occurred in 4.5% of patients in the ribociclib group and 2.1% of patients in the placebo group.
- Ribociclib has also been tested in combination with fulvestrant in postmenopausal patients with HER2-negative hormone receptor–positive, recurrent or metastatic breast cancer. The MONALEESA-3 trial (NCT02422615) included patients receiving first-line or second-line therapy. This phase III, placebo-controlled trial randomly assigned 726 patients in a 2:1 ratio to receive ribociclib plus fulvestrant or placebo plus fulvestrant.[15]
- The primary end point (investigator-assessed PFS) was met. At the time of final analysis for PFS, the median PFS for the ribociclib group was 20.5 months versus 12.8 months in the placebo group (HR, 0.593; 95% CI, 0.480–0.732; P < .001).[15][Level of evidence B1]
- OS was superior in the ribociclib group (HR, 0.724; 95% CI, 0.568–0.924; P = .004). The result crossed the prespecified stopping boundary (P = .011) for superior efficacy. Results were similar in all subgroups.[16][Level of evidence A1]
- Adverse events were similar to those in other studies of CDK4/6 inhibitors.
- Grade 3 to 4 neutropenia occurred in 53.4% of patients in the ribociclib group and 0.0% of patients in the placebo group.
- The rate of febrile neutropenia was 1.0% in the ribociclib group and 0% in the placebo group.
- An increase in QTcF interval of more than 60 milliseconds from baseline was observed in 6.5% of patients in the ribociclib arm and 0.4% in the placebo arm.
- Ribociclib was also assessed in the first-line setting in a study conducted solely in premenopausal or perimenopausal women receiving either tamoxifen or a nonsteroidal aromatase inhibitor (AI) plus goserelin.[17] In the MONALEESA-7 trial (NCT02278120), 672 premenopausal patients with HER2-negative hormone receptor–positive, recurrent or metastatic breast cancer, who had not received endocrine therapy for advanced disease, were randomly assigned in a 1:1 ratio to ribociclib or placebo.
- The primary end point (investigator-assessed PFS) was met. At the time of final analysis for PFS, the median PFS for the ribociclib group was 23.8 months versus 13.0 months in the placebo group (HR, 0.55; 95% CI, 0.44–0.69; P < .0001).[17][Level of evidence A3]
- OS was a secondary end point. The combination of ribociclib plus endocrine therapy was associated with longer OS than was endocrine therapy alone (42-month OS, 70.2% vs. 46%; HRdeath, 0.71; 95% CI, 0.54−0.95; P = .01).[18][Level of evidence A1] The survival benefit was observed both in patients who received an AI plus goserelin and in those who received tamoxifen, but it was not statistically significant in the much-smaller tamoxifen group.
- Adverse events were similar to those in other studies of CDK4/6 inhibitors.
- Grade 3 to 4 neutropenia occurred in 61% of patients in the ribociclib group and 4% of patients in the placebo group.
- The rate of febrile neutropenia was 2.0% in the ribociclib group and 1.0% in the placebo group.
- An increase in QTcF interval of more than 60 milliseconds from baseline was observed in 10.0 % of patients in the ribociclib arm and 2.0% in the placebo arm. Sixty-millisecond increases were more common in patients receiving tamoxifen (16% on ribociclib and 7% on placebo).
First-line abemaciclib and endocrine therapy
Abemaciclib, another CDK4/6 inhibitor, has also been tested in the first-line setting for postmenopausal patients with HER2-negative hormone receptor–positive, recurrent or metastatic breast cancer.
Evidence (first-line abemaciclib and endocrine therapy):
- MONARCH 3 (NCT02246621) was a randomized, double-blind, phase III trial that evaluated first-line abemaciclib or placebo plus a nonsteroidal AI in 493 postmenopausal women with HER2-negative hormone receptor–positive, advanced breast cancer.[19]
- The primary end point, investigator-assessed PFS, was met. After a median follow-up of 17.8 months, the PFS was not reached in the abemaciclib arm and was reached at 14.7 months in the placebo arm (HR, 0.54; 95% CI, 0.41–0.72; P = .000021).
- In the final analysis of OS, there was a nonsignificant difference in median survival (median OS, 66.8 months [abemaciclib arm] vs. 53.7 months [placebo arm]; HR, 0.804; 95% CI, 0.637–1.015; P = .066).[20][Level of evidence B1]
- The side effect profile of abemaciclib differs from the other CDK4/6 inhibitors. Diarrhea was the most frequent adverse event in the abemaciclib arm, although most of the diarrhea cases were grade 1.
- Neutropenia was more common in the abemaciclib arm; however, only 21.1% of participants experienced grade 3 to 4 neutropenia.
Second-line palbociclib and endocrine therapy
Evidence (second-line palbociclib and endocrine therapy):
- PALOMA-3 (NCT01942135) was a double-blind, phase III trial of 521 patients with HER2-negative hormone receptor–positive, advanced breast cancer who had relapsed after or progressed on previous endocrine therapy and were randomly assigned to receive either fulvestrant plus placebo or fulvestrant plus palbociclib. Premenopausal and postmenopausal patients were eligible. Premenopausal patients received goserelin.[5][Level of evidence A3]
- The final PFS analysis showed a median PFS of 9.5 months on the palbociclib-fulvestrant arm versus 4.6 months on the placebo-fulvestrant arm (HR, 0.46; 95% CI, 0.36–0.59; P < .0001).[21][Level of evidence A3]
- Cytopenias, particularly neutropenia, were much more frequent on the palbociclib-containing arm, but febrile neutropenia was very uncommon (1%) in both groups. Patients receiving palbociclib had more-frequent fatigue, nausea, and headache.
- A prespecified analysis of OS was made after 310 patients had died. A 6.9 month difference in median OS favoring the palbociclib-fulvestrant arm (34.9 months vs. 28.0 months) was found, which did not reach statistical significance (HR, 0.81; 95% CI, 0.64–1.03; P = .09).[22]
- In a preplanned subgroup analysis, improved OS was observed in patients who had demonstrated sensitivity to hormone therapy (HR, 0.72; 95% CI, 0.55−0.94), whereas in patients without sensitivity, OS was not improved in the palbociclib group (HR, 1.14; 95% CI, 0.71−1.84; P = .12 for interaction).
Second-line ribociclib and endocrine therapy
The MONALEESA-3 trial included patients receiving second-line therapy. For more information, see the evidence on first-line ribociclib and endocrine therapy above.
Second-line abemaciclib and endocrine therapy
Evidence:
- The MONARCH 2 study (NCT02107703) tested abemaciclib (CDK4/6 inhibitor) in a phase III, placebo-controlled trial that randomly assigned 669 patients with HER2-negative, hormone receptor–positive, advanced breast cancer (with previous progression on endocrine therapy) to receive abemaciclib plus fulvestrant or placebo plus fulvestrant.[23]
- The primary end point (investigator-assessed PFS) was met, with median duration of follow-up of 19.5 months. The median PFS was 16.4 months for the abemaciclib-fulvestrant arm versus 9.3 months for the placebo-fulvestrant arm (HR, 0.55; 95% CI, 0.45–0.68; P < .001).[23][Level of evidence B1]
- OS data are mature and demonstrate an improvement in OS for patients receiving abemaciclib, and showed a median OS of 46.7 months for abemaciclib plus fulvestrant versus 37.3 months for placebo (HR, 0.757; 95% CI, 0.606–0.945; P = .01).[24][Level of evidence A1]
- Adverse events included diarrhea in the abemaciclib group (86.4%) and in the placebo group (24.7%), neutropenia (46% and 4%), nausea (45.1% and 22.9%), fatigue (39.9% and 26.9%), and abdominal pain (35.4% and 15.7%).
- These events were mostly grade 1 to 2. Grade 1 to 2 diarrhea occurred in 73% of the patients in the abemaciclib group and in 24.2% of the placebo group. Anti-diarrheal medicine effectively managed this symptom in most cases, according to the study report.
- Grade 3 diarrhea occurred in 13.4% of patients in the abemaciclib group and 0.4% of patients in the placebo group. No grade 4 diarrhea was reported.
- Grade 3 to 4 neutropenia occurred in 25.5% of patients in the abemaciclib group and 1.7% of patients in the placebo group. Febrile neutropenia was reported in six patients in the abemaciclib arm.
CDK inhibitor therapy after disease progression on a prior CDK inhibitor
Evidence (CDK inhibitor therapy after disease progression on a prior CDK inhibitor):
- The global, double-blind, placebo-controlled postMONARCH trial (NCT05169567), reported in abstract form, included 368 patients with HER2-negative ER-positive advanced breast cancer. Patients were randomly assigned in a 1:1 ratio to receive either abemaciclib plus fulvestrant or fulvestrant alone. Eligible patients had (1) disease progression during initial therapy for metastatic breast cancer, which included a CDK4/6 inhibitor and aromatase inhibitor (99% of patients), or (2) disease relapse during or after adjuvant therapy for early breast cancer with a CDK4/6 inhibitor plus endocrine therapy. The CDK inhibitor was palbociclib in 59% of patients, ribociclib in 33% of patients, and abemaciclib in 8% of patients. No other prior treatment was permitted for metastatic disease. The primary end point was investigator-assessed PFS.[25]
- The 6-month PFS rate was 50% in the abemaciclib-plus-fulvestrant group and 37% in the fulvestrant-alone group (HR, 0.73; 95% CI, 0.57–0.95).[25][Level of evidence B1]
- Overall response rate, a secondary end point, was 17% in the abemaciclib-plus-fulvestrant group and 7% in the fulvestrant-alone group.
Single-agent CDK inhibitor therapy
Evidence (single-agent CDK inhibitor therapy):
- Single-agent abemaciclib was approved by the FDA for use in HER2-negative hormone receptor–positive breast cancer with disease progression on or after endocrine therapy and chemotherapy on the basis of results of the MONARCH 1 trial (NCT02102490).[26] Abemaciclib is the only CDK4/6 inhibitor approved as a single agent. MONARCH 1 was a single-arm phase II study of single-agent abemaciclib in 132 women with HER2-negative hormone receptor–positive, advanced breast cancer that had progressed on at least one line of previous endocrine therapy and at least two lines of previous chemotherapy. The study population was heavily pretreated, and most participants had visceral disease. Patients who had previous CDK inhibitors were excluded. The primary end point was investigator-assessed objective response rate.
- The objective response rate was 19.7% at 12 months (95% CI, 13.3%–27.5%).
- The clinical benefit rate was 42.4%.
- Median PFS was 6.0 months (95% CI, 4.2–7.5).
- The most common adverse event was diarrhea, which occurred in 90.2% of the participants. However, the majority was grade 1 to 2, and only 19.7% of participants experienced grade 3 diarrhea. There was no grade 4 diarrhea.
- Neutropenia occurred in 97.7% of participants; however, the majority was grade 1 to 2, and only 26.9% of participants experienced grade 3 to 4 neutropenia.
Mammalian target of rapamycin (mTOR) inhibitor therapy plus endocrine therapy
Preclinical models and clinical studies suggest that mTOR inhibitors might overcome endocrine resistance.
Evidence (mTOR inhibitor therapy):
- The Breast Cancer Trial of Oral Everolimus (BOLERO-2 [NCT00863655]) was a randomized, phase III, placebo-controlled trial in which patients with hormone receptor–positive metastatic breast cancer that is resistant to nonsteroidal aromatase inhibition were randomly assigned to receive either the mTOR inhibitor everolimus plus exemestane, or placebo plus exemestane.[27][Level of evidence B1]
- At the interim analysis, median PFS was 6.9 months for everolimus plus exemestane and 2.8 months for placebo plus exemestane (HR, 0.43; 95% CI, 0.35–0.54; P < .001).
- The addition of everolimus to exemestane was more toxic than was placebo plus exemestane, with the most-common grade 3 or 4 adverse events being stomatitis (8% vs. 1%), anemia (6% vs. <1%), dyspnea (4% vs. 1%), hyperglycemia (4% vs. <1%), fatigue (4% vs. 1%), and pneumonitis (3% vs. 0%).
- OS differences were not significant after further follow-up.[28]
- TAMRAD (NCT01298713) was an open-label, randomized, phase II trial comparing tamoxifen with tamoxifen plus everolimus in postmenopausal women whose disease had progressed after receiving an AI in the adjuvant or metastatic setting. The trial randomly assigned 57 women to receive tamoxifen and 54 women to receive the combination therapy.[29]
- Median time to progression was 8.6 months in the combination group and 4.5 months in the tamoxifen group (HR, 0.54; 95% CI, 0.56−0.81; P = .002).
- Toxicities were greater on the everolimus arm and similar to those in the BOLERO2 trial.
- In an exploratory analysis, OS was 32.9 months in the tamoxifen group and not reached in the combination group (HR, 0.45; 95% CI, 0.24−0.81; P = .007).[29][Level of evidence A1]
- PrE0102 (NCT01797120) was a double-blind, randomized, phase II trial comparing fulvestrant with fulvestrant plus everolimus in postmenopausal women whose disease had progressed after receiving an AI in the adjuvant or metastatic setting. Sixty-six women were randomly assigned to the combination arm and 65 to fulvestrant alone.[30]
- Median PFS was 10.3 months on the combination arm and 5.1 months on the fulvestrant-alone arm (HR, 0.61; 95% CI, 0.40−0.92; P = .02).[30][Level of evidence B1]
- Toxicities were similar to those in previous studies.
- The was no observed difference in OS between the arms.
- The single-arm SWISH trial (NCT02069093) assessed the efficacy of a dexamethasone oral solution (0.5 mg per 5 mL) in the prevention of stomatitis in women receiving exemestane plus everolimus.[31] The incidence of grade 2 or worse stomatitis was 2% in the 85 evaluable patients in this study compared with 33% in the BOLERO-2 trial.
AKT inhibitor therapy
Activating AKT1 variants are found in 5% to 10% of advanced breast cancers. AKT is downstream from both PIK3CA and PTEN in the PIK3CA/AKT/PTEN pathway.
Capivasertib
Overactivation of the PIK3CA/AKT/PTEN signaling pathway occurs in approximately one-half of HER2-negative hormone receptor–positive breast cancers. Activating PIK3CA and AKT1 variants and inactivating alterations in PTEN can cause this overactivation. Capivasertib is an oral small-molecule inhibitor of all three AKT isoforms (AKT1, AKT2, and AKT3).
Evidence (capivasertib):
- The phase III, randomized, double-blind CAPITELLO-291 trial (NCT04305496) enrolled women and men with HER2-negative ER-positive advanced breast cancer. Patients had disease relapse or progression during or after treatment with an aromatase inhibitor, with or without previous CDK4/6 inhibitor therapy. The trial included 708 patients: 289 patients (40.8%) had PIK3CA/AKT/PTEN pathway alterations and 489 (69.1%) received a prior CDK4/6 inhibitor for advanced breast cancer. Patients were randomly assigned in a 1:1 ratio to receive either capivasertib plus fulvestrant or placebo plus fulvestrant. The dual primary end point was investigator-assessed PFS which was measured both in the overall population and in patients with PIK3CA/AKT/PTEN pathway–altered tumors.[32]
- In the overall population, the median PFS was 7.2 months in the capivasertib-fulvestrant group versus 3.6 months in the placebo-fulvestrant group (HR, 0.60; 95% CI, 0.51–0.71; P < .001).[32][Level of evidence B1]
- In the PIK3CA/AKT/PTEN pathway-altered population, the median PFS was 7.3 months in the capivasertib-fulvestrant group and 3.1 months in the placebo-fulvestrant group (HR, 0.50; 95% CI, 0.38–0.65; P < .001).
- In patients who received capivasertib and fulvestrant, the most frequent grade 3 or higher adverse events were rash (12.1%) and diarrhea (9.3%). Grade 3 or higher rash and diarrhea occurred in 0.3% of patients who received placebo and fulvestrant. Adverse events sometimes led to treatment discontinuation. This occurred in 13.0% of the capivasertib-fulvestrant group and 2.3% of the placebo-fulvestrant group.
Based on this trial, the FDA approved capivasertib in 2023.
Alpelisib plus endocrine therapy
Activating PIK3CA variants are identified in approximately 40% of HER2-negative hormone receptor–positive breast cancers. Alpelisib is an alpha-specific PIK3CA inhibitor.
Evidence (alpelisib plus endocrine therapy):
- SOLAR-1 (NCT02437318) was a randomized phase III trial comparing alpelisib plus fulvestrant with placebo plus fulvestrant. The trial included 572 postmenopausal women with HER2-negative hormone receptor–positive advanced breast cancer who had received previous endocrine therapy.[33][Level of evidence B1]
PIK3CA variants were confirmed in 341 participants. The primary end point was PFS in the cohort of patients with PIK3CA variants.
- In this cohort, median PFS was 11 months in the alpelisib-plus-fulvestrant arm compared with 5.7 months in the placebo-plus-fulvestrant arm (HRprogression or HRdeath, 0.65; 95% CI, 0.50−0.85; P < .001).
- PFS did not differ between arms in the cohort of participants without PIK3CA variants (median PFS, 7.4 months in the alpelisib-plus-fulvestrant arm vs. 5.6 months in the placebo-plus-fulvestrant arm).
- OS in the cohort with PIK3CA variants was a secondary end point. OS data are not yet mature.
- Very few study participants had received previous CDK4/6 inhibitor therapy.
- Common toxicities associated with alpelisib included hyperglycemia, diarrhea, nausea, anorexia, and rash. Careful monitoring and management of hyperglycemia are required during alpelisib use.
The FDA approved alpelisib for use in combination with fulvestrant in advanced PIK3CA-mutated, HER2-negative hormone receptor–positive breast cancer after previous endocrine therapy.
Elacestrant
Elacestrant is an oral selective ER degrader (SERD). It degrades ER alpha in a dose-dependent manner and inhibits estradiol-dependent ER-directed gene transcription and tumor growth, including in cells with ESR1 variants. ESR1 variants result in estrogen-independent ER activation and, consequently, resistance to AIs, but not necessarily to SERDs and selective ER modulators.
Evidence (elacestrant):
- A randomized, open-label, phase III trial (EMERALD [NCT03778931]) enrolled patients with ER-positive HER2-negative metastatic breast cancer. Eligible patients had previously received one or two lines of endocrine therapy, a CDK4/6 inhibitor, and no more than one line of chemotherapy. A total of 477 patients were randomly assigned in a 1:1 ratio to receive either elacestrant 400 mg orally once daily or standard-of-care endocrine monotherapy. Primary end points were PFS by blinded independent central review in all patients and in patients with detectable ESR1 variants. ESR1 variants were found in 47.8% of patients, and 43.4% of patients had received two prior endocrine therapies. Twenty-nine percent of patients in the elacestrant arm and 31% of patients in the standard-of-care arm had received prior fulvestrant therapy. Less than 5% of patients in either arm had received prior mTOR inhibitor therapy.[34]
- PFS was prolonged in the elacestrant arm in all patients (HR, 0.70; 95% CI, 0.55–0.88; P = .002) and in elacestrant-treated patients with ESR1 variants (HR, 0.55; 95% CI, 0.39–0.77; P = .0005). Among all patients, 6-month PFS rates were 34.3% for patients in the elacestrant arm and 20.4% for patients in the standard-of-care arm. In patients with ESR1 variants, 6-month PFS rates were 40.8% for patients in the elacestrant arm and 19.1% for patients in the standard-of-care arm. Similarly, for all patients, 12-month PFS rates were 22.3% for patients in the elacestrant arm and 9.4% for patients in the standard-of-care arm. For patients with ESR1 variants, 12-month PFS rates were 26.8% for patients in the elacestrant arm and 8.2% for patients in the standard-of-care arm.[34][Level of evidence B1]
- The most common adverse events observed with elacestrant versus standard-of-care therapy included nausea (35.0% vs. 18.8%), fatigue (19.0% vs. 18.8%), vomiting (19.0% vs. 8.3%), decreased appetite (14.8% vs. 9.2%), and arthralgia (14.3% vs. 16.2%). Grade 3 or 4 adverse events occurred in 64 patients (27.0%) who received elacestrant and 47 patients (20.5%) who received standard-of-care therapy. The most common grade 3 or 4 adverse events in the elacestrant arm were nausea (six patients, 2.5%), back pain (six patients, 2.5%), and increased alanine aminotransferase (five patients, 2.1%). The most common grade 3 or 4 adverse events in the standard-of-care arm were nausea, fatigue, diarrhea, and increased aspartate aminotransferase (each occurring in two patients, 0.9%). Adverse events led to treatment discontinuation in 15 patients (6.3%) in the elacestrant arm and 10 patients (4.4%) in the standard-of-care arm.
HDAC inhibitor therapy
Epigenetic modification alters gene expression. This can lead to endocrine therapy resistance and may be reversed by epigenetic modifiers such as histone deacetylase (HDAC) inhibitors. Entinostat, an oral HDAC inhibitor, induces downregulation of estrogen-independent growth factor signaling pathways and normalization of estrogen receptor levels. Entinostat was evaluated in a phase III trial and showed no benefit.[35,36]
Endocrine therapy alone
With the PFS and OS advantages associated with combination therapy with targeted agents and endocrine therapy as discussed above, single-agent endocrine therapy is less frequently used, especially in the first-line setting. However, its use remains appropriate in select cases as first-line therapy and in later-line therapy after progression on targeted therapies and before the use of chemotherapy in cases in which endocrine-sensitive disease is still thought to be present.
Commonly used single-agent endocrine therapies include tamoxifen, nonsteroidal AI (letrozole, anastrozole), the steroidal AI exemestane, and fulvestrant. In general, premenopausal women with metastatic breast cancer undergo ovarian suppression or ablation and are treated in the same manner as postmenopausal women.
Tamoxifen and AI therapy
While tamoxifen has been used for many years in treating postmenopausal women with newly metastatic disease that is ER positive, PR positive, or ER/PR unknown, several randomized trials suggest equivalent or superior response rates and PFS for AIs compared with tamoxifen.[37-39][Level of evidence B1]
Evidence (tamoxifen and AI therapy):
- A meta-analysis evaluated patients with metastatic disease who were randomly assigned to receive either an AI as their first or second hormone therapy, or standard therapy (tamoxifen or a progestational agent).[40][Level of evidence A1]
- Patients who received an AI as either their first or second hormone therapy for metastatic disease and were randomly assigned to receive a third-generation drug (anastrozole, letrozole, exemestane, or vorozole) lived longer (HRdeath, 0.87; 95% CI, 0.82–0.93) than those who received standard therapy (tamoxifen or a progestational agent).
Fulvestrant
Fulvestrant is a selective estrogen receptor degrader that has been studied in the first-line and second-line setting in women with advanced or metastatic breast cancer.
First-line fulvestrant
Evidence (first-line fulvestrant):
- FALCON (NCT01602380) was a phase III, double-blind, randomized trial that compared fulvestrant (500 mg) with anastrozole (1 mg) in patients with advanced or metastatic receptor-positive breast cancer who had not received previous endocrine therapy.[41] The trial randomly assigned 230 patients to receive fulvestrant and 232 patients to receive anastrozole.
- Median PFS was 16.6 months in the fulvestrant group and 13.8 months in the anastrozole group (HR, 0.797; 95% CI, 0.637−0.999; P = .049).[41][Level of evidence B1]
- The frequency of adverse events was similar in the two groups, and there was no difference in quality of life.
- OS results were not reported.
Second-line fulvestrant
Evidence (second-line fulvestrant):
- Two randomized trials that enrolled 400 and 451 patients whose disease had progressed after they received tamoxifen demonstrated that fulvestrant yielded results similar to those of anastrozole in terms of its impact on PFS.[42,43] The proper sequence of these therapies is not known.[44,45]
- EFECT (NCT00065325) was a phase III, double-blind, randomized trial that compared fulvestrant given in a loading-dose regimen (500 mg day 0, 250 mg days 14 and 28, and 250 mg every 28 days thereafter) with exemestane (25 mg) in women who had developed progressive disease after previous nonsteroidal AI (anastrozole or letrozole) therapy.[46] The trial randomly assigned 351 women to receive fulvestrant and 342 women to receive exemestane.
- Median time to progression was 3.7 months in both groups (HR, 0.93; 95% CI, 0.819−1.133; P = .65).[46][Level of evidence B1]
- The frequency of adverse events was similar in both groups, and there was no difference in quality of life.
- OS results were not reported.
- CONFIRM (NCT00099437) was a double-blind phase III trial that compared two doses of fulvestrant (500 mg vs. 250 mg, each given in a loading-dose schedule) in 736 women whose disease had progressed on previous endocrine therapy.[47]
- PFS was significantly better on the higher-dose arm (HR, 0.80; 95% CI, 0.68–0.94; P = .006).[47][Level of evidence B1]
- Adverse events and quality of life were similar on the two arms.
Combination endocrine therapy with an AI and fulvestrant
Conflicting results were found in two trials that compared the combination of the antiestrogen fulvestrant and anastrozole with anastrozole alone in the first-line treatment of hormone receptor–positive postmenopausal patients with recurrent or metastatic disease. For more information, see the Fulvestrant section.[48,49] In both studies, fulvestrant was given as a 500-mg loading dose on day 1; 250 mg was given on days 15 and 29, and monthly thereafter; plus, 1 mg of anastrozole was given daily. The Southwest Oncology Group (SWOG) trial included more patients who presented with metastatic disease; the Fulvestrant and Anastrozole Combination Therapy (FACT [NCT00256698]) study enrolled more patients who had previously received tamoxifen.[48,49]
Evidence (combination endocrine therapy with an AI and fulvestrant):
- The SWOG-0226 trial (NCT00075764), which enrolled 707 patients, demonstrated a statistically significant difference in PFS (HR, 0.80; 95% CI, 0.68–0.94; P = .007) and OS (HR, 0.81; 95% CI, 0.65–1.00; P = .05).[48][Level of evidence A1]
- In an analysis done after 5 more years of follow-up, the observed benefits of combined therapy were still present, and the level of significance with respect to OS was greater (HR, 0.82; 95% CI, 0.69–0.98; P = .03).[50][Level of evidence A1]
- In contrast, the FACT trial, which enrolled 514 patients, found no difference in either disease-free survival (DFS) (HR, 0.99; 95% CI, 0.81–1.20; P = .91) or OS (HR, 1.0; 95% CI, 0.76–1.32; P = 1.00).[49][Level of evidence A1]
Sequencing therapy for hormone receptor–positive metastatic breast cancer
The optimal sequence of therapies for hormone receptor–positive metastatic breast cancer is not known. In general, in the absence of a visceral crisis, most patients receive sequential endocrine-based regimens before transitioning to chemotherapy. On the basis of the PFS and OS improvements mentioned above, a combination of a CDK4/6 inhibitor therapy and endocrine therapy in the first line is an appropriate choice.
Poly (ADP-ribose) polymerase (PARP) inhibitor therapy
Patients with hormone receptor-positive metastatic breast cancer and a germline BRCA variant are eligible for PARP inhibitor therapy. For more information, see the Germline BRCA-Mutated Metastatic Breast Cancer section.
Sacituzumab govitecan
Sacituzumab govitecan is an antibody-drug conjugate that combines an anti–trophoblast cell-surface antigen 2 (TROP2) antibody with an active metabolite of irinotecan (SN-38). TROP2 is a transmembrane calcium signal transducer highly expressed in HER2-negative ER-positive breast cancer. Internalization of TROP2–bound sacituzumab govitecan delivers SN-38 into the tumor cell through hydrolysis of the linker.[51]
Evidence (sacituzumab govitecan):
- The global phase III TROPiCS-02 trial (NCT03901339) randomly assigned 543 patients to receive either sacituzumab govitecan or physician’s choice chemotherapy (eribulin, vinorelbine, capecitabine, or gemcitabine). Patients had locally recurrent inoperable or metastatic breast cancer that was endocrine-resistant, chemotherapy-treated, hormone receptor–positive, and HER2-negative. Patients had received three median lines of chemotherapy for advanced disease, and 99% of patients had previously received a CDK4/6 inhibitor. The primary end point was PFS by blinded independent central review.[52][Level of evidence B1]
- An analysis noted a 34% reduction in the risk of progression or death (HR, 0.66; 95% CI, 0.53–0.83; P = .0003). The median PFS was 5.5 months in the sacituzumab govitecan group and 4.0 months in the chemotherapy group. The PFS at 6 and 12 months was 46% and 21% for patients who received sacituzumab govitecan and 30% and 7% for patients who received chemotherapy.
- Median OS data are not mature.
- Key grade 3 or higher treatment-related adverse events were neutropenia (occurring in 51% of patients who received sacituzumab govitecan and 38% of patients who received chemotherapy) and diarrhea (occurring in 9% of patients who received sacituzumab govitecan and 1% of patients who received chemotherapy). One patient in the sacituzumab govitecan group died of a treatment-related adverse event (septic shock related to neutropenic colitis).
- The objective response rate by blinded independent central review was 21% in the sacituzumab govitecan group and 14% in the chemotherapy group. The clinical benefit rate was higher with sacituzumab govitecan than with chemotherapy (34% vs. 22%).
The FDA has approved sacituzumab govitecan for patients with metastatic unresectable breast cancer, including patients with TNBC who have received at least two systemic therapies, including at least one for metastatic cancer. It is also approved in patients with HER2-negative hormone receptor–positive breast cancer who have received hormone therapy and at least two systemic therapies for metastatic cancer.
Chemotherapy
Chemotherapy may be appropriate for patients with HER2-negative hormone receptor–positive breast cancer. For more information, see the Chemotherapy for Metastatic Breast Cancer section.
Metastatic HER2-Low Breast Cancer
Sixty percent of HER2-negative metastatic breast cancers express low levels of HER2, defined as a score of 1+ on immunohistochemical (IHC) analysis or as an IHC score of 2+ and negative results on in situ hybridization. These are referred to as HER2-low tumors. Historically, HER2-directed therapies have not improved outcomes in these patients. Early-phase trials of trastuzumab deruxtecan showed potential efficacy in this subgroup.[53] A subsequent randomized phase III trial showed OS benefit in this subgroup.[54] Trastuzumab deruxtecan is an antibody-drug conjugate consisting of a humanized anti-HER2 monoclonal antibody linked to a topoisomerase-I inhibitor payload through a cleavable linker. This agent offers bystander effect to surrounding HER2-low cells via uptake into HER2-amplified cells, intracellular cleavage of payload, and release of payload into the surrounding tumor.
Evidence (trastuzumab deruxtecan for HER2-low hormone receptor–positive and hormone receptor–negative tumors):
- The DESTINY-Breast04 trial (NCT03734029) randomly assigned 557 women in a 2:1 ratio to receive either trastuzumab deruxtecan or the physician’s choice of chemotherapy (capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel). Patients had received one or two prior lines of chemotherapy. The primary end point was PFS in the hormone receptor–positive group (88.7% of patients). The secondary end points were PFS in the total population, OS in the total population, and OS in the hormone receptor–positive group.[54][Level of evidence A1]
- In the hormone receptor–positive cohort, the median PFS was 10.1 months for patients who received trastuzumab deruxtecan and 5.4 months for patients who received the physician’s choice of chemotherapy (HRdisease progression or death, 0.51; P < .001). The OS was 23.9 months for patients who received trastuzumab deruxtecan and 17.5 months for patients who received the physician's choice of chemotherapy (HRdeath, 0.64; P = .003).
- Among all patients, the median PFS was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice group (HRdisease progression or death, 0.50; P < .001). The OS was 23.4 months in the trastuzumab deruxtecan group and 16.8 months in the physician's choice group (HRdeath, 0.64; P = .001).
- Drug-related interstitial lung disease or pneumonitis occurred in 12.1% of the patients who received trastuzumab deruxtecan; 0.8% had grade 5 events.
- The phase III, randomized, open-label DESTINY-Breast06 trial (NCT04494425) included patients with ER-positive metastatic breast cancer with low HER2 expression (with a score of 1+ or 2+ on IHC analysis and negative results on in situ hybridization) or ultralow HER2 expression (with a score of 0 on IHC analysis with membrane staining). These patients previously received one or more lines of endocrine-based therapy and no previous chemotherapy for metastatic breast cancer. The trial randomly assigned 866 patients in a 1:1 ratio to receive either trastuzumab deruxtecan or the physician’s choice of chemotherapy. A total of 713 patients had HER2-low disease and 153 patients had HER2-ultralow disease. The primary end point was PFS.[55]
- In patients with HER2-low disease, the median PFS was 13.2 months (95% CI, 11.4–15.2) in the trastuzumab deruxtecan group and 8.1 months (95% CI, 7.0–9.0) in the chemotherapy group (HR, 0.62; 95% CI, 0.51–0.74; P < .001).[55][Level of evidence B1]
- Grade 3 or higher adverse events occurred in 52.8% of patients in the trastuzumab deruxtecan group and 44.4% of patients in the chemotherapy group. Interstitial lung disease occurred in 49 patients (11.3%; three events had grade 5 severity) and pneumonitis occurred in one patient (0.2%; grade 2).
Selection of this agent requires careful consideration and discussion between the clinician and patient about (1) the biology of the patient's disease, (2) the toxicity of the agent and risks to the patient compared with other standardly available agents, and (3) the cost of the agent and insurance coverage.
Metastatic Triple-Negative Breast Cancer
Chemotherapy plus immunotherapy
The standard-of-care treatment for first-line metastatic TNBC with a programmed death-ligand 1 (PD-L1) combined positive score (CPS) of 10 or more is chemotherapy plus pembrolizumab. This was evaluated in the KEYNOTE-355 trial.[56,57]
Pembrolizumab
The phase III, randomized, placebo-controlled, double-blind, multinational KEYNOTE-355 trial (NCT02819518) evaluated the addition of pembrolizumab to first-line chemotherapy in patients with metastatic TNBC. This combination was approved for use in this patient population as a result of the trial.[56,57]
Evidence (pembrolizumab):
- Patients were randomly assigned in a 2:1 ratio to receive either pembrolizumab (200 mg every 3 weeks) and chemotherapy (nab-paclitaxel, paclitaxel, or gemcitabine plus carboplatin) or placebo and chemotherapy. Randomization was stratified by the type of on-study chemotherapy (taxane or gemcitabine/carboplatin), PD-L1 expression at baseline (CPS ≥1 or <1), and previous treatment with the same class of chemotherapy in the neoadjuvant or adjuvant setting (yes or no). Dual primary efficacy end points were PFS and OS assessed in the PD-L1 CPS of 10 or more, PD-L1 CPS of 1 or more, and ITT populations. Of the 847 patients assigned to treatment, 566 patients received pembrolizumab and chemotherapy and 281 patients received placebo and chemotherapy.[56,57]
- At the second interim analysis, the median follow-up was 25.9 months in the pembrolizumab-and-chemotherapy group and 26.3 months in the placebo-and-chemotherapy group. Among patients with CPS of 10 or more, the median PFS was 9.7 months with pembrolizumab and chemotherapy and 5.6 months with placebo and chemotherapy (HRprogression or death, 0.65; 95% CI, 0.49–0.86; one-sided P = .0012 [primary objective met]). Among patients with CPS of 1 or more, the median PFS was 7.6 months with pembrolizumab and chemotherapy and 5.6 months with placebo and chemotherapy (HR, 0.74; 0.61–0.90; one-sided P = .0014 [not significant]). Among the ITT population, the median PFS was 7.5 months and 5.6 months (HR, 0.82; 0.69–0.97 [not tested]). The pembrolizumab treatment effect increased with PD-L1 enrichment.
- Based on the PFS data, the FDA approved pembrolizumab and chemotherapy for the treatment of patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥10).
- At time of final analysis, the median follow-up was 44.1 months. In the group with a CPS of at least 10, the median OS was 23 months in the pembrolizumab-and-chemotherapy group and 16.1 months in the placebo-and-chemotherapy group (HR, 0.73; 95% CI, 0.55–0.95; two-sided P = .0185 [significant]). In the group with a CPS of at least 1, the median survival was not significantly altered (17.6 vs. 16 months; P = .1125).
- Grade 3 to 5 treatment-related adverse events occurred in 68.1% of patients in the pembrolizumab group and 66.9% of patients in the placebo group. Two deaths occurred in the pembrolizumab group (acute kidney injury and pneumonia). There were no deaths in the placebo group. Grade 3 to 4 immune-mediated adverse events occurred in 5.3% of patients in the pembrolizumab group, none of which led to death.
Atezolizumab
The addition of atezolizumab, a PD-L1–positive antibody, to first-line chemotherapy for patients with HER2-negative hormone receptor–negative, advanced breast cancer was evaluated in the phase III, randomized, placebo-controlled IMpassion130 trial (NCT02425891).[58,59] Participants (N = 902) were randomly assigned 1:1 to atezolizumab plus nanoparticle albumin-bound (nab)-paclitaxel or to placebo plus nab-paclitaxel. Participants were stratified according to the presence of liver metastases (yes/no), receipt of previous taxane therapy (yes/no), and PD-L1 status (positive or negative). PD-L1 expression of 1% or greater was defined as positive. Co-primary end points included PFS and OS, both of which were evaluated in the ITT population and in the PD-L1–positive population (n = 369).
Based on the initial publication of PFS data from IMPassion130, the FDA granted accelerated approval for the use of atezolizumab in combination with protein-bound paclitaxel for patients with unresectable locally advanced or metastatic TNBC whose tumors express PD-L1. However, a 2021 final analysis revealed no OS benefit in the ITT population.[58,59]
IMpassion131 (NCT03125902) was a phase III, randomized, placebo-controlled, double-blind trial of first-line paclitaxel with or without atezolizumab for patients with unresectable locally advanced or metastatic TNBC. The study included 651 patients, 45% of whom had PD-L1–positive TNBC. This trial also did not demonstrate a benefit from the addition of atezolizumab to paclitaxel in this population.[60] Accordingly, the sponsor voluntarily withdrew this approval.
- IMpassion130 reported PFS data with a median follow-up of 12.9 months.[58]
- In the ITT population, PFS was improved with the addition of atezolizumab (median PFS, 7.2 months vs. 5.5 months; HR, 0.80; 95% CI, 0.69–0.92; P = .0025).
- In the PD-L1–positive population, PFS was improved with the addition of atezolizumab (median PFS, 7.5 months vs. 5 months; HR, 0.62; 95% CI, 0.49–0.78; P < .001).
- Adverse events occurred as expected. Adverse events that were potentially immune-related were more frequent in the atezolizumab arm.
- The primary PFS analysis from IMpassion131 revealed that adding atezolizumab to paclitaxel did not improve investigator-assessed PFS in the PD-L1–positive population. The median PFS was 6.0 months for patients who received atezolizumab and paclitaxel compared with 5.7 months for patients who received placebo and paclitaxel (HR, 0.82; 95% CI, 0.60–1.12; P = .20).[60]
- The final OS results showed no difference between the treatment arms. The median OS was 22.1 months for patients who received atezolizumab and paclitaxel compared with 28.3 months for patients who received placebo and paclitaxel in the PD-L1–positive population (HR, 1.11; 95% CI, 0.76–1.64).
- Results in the ITT population were consistent with the PD-L1–positive population. The safety profile was consistent with known effects of each study drug.
Sacituzumab govitecan
Sacituzumab govitecan is an antibody-drug conjugate that combines an a TROP2 antibody with an active metabolite of irinotecan (SN-38). TROP2 is a transmembrane calcium signal transducer highly expressed in TNBC. Internalization of TROP2–bound sacituzumab govitecan delivers SN-38 into the tumor cell through hydrolysis of the linker.[51]
Evidence (sacituzumab govitecan):
- In a phase I/II trial, 108 women with TNBC who received at least two previous chemotherapy regimens (median, three) were treated with sacituzumab govitecan at a dose of 10 mg/kg intravenously on days 1 and 8 of a 21-day cycle.[61][Level of evidence C3]
- A response rate of 33.3% (95% CI, 24.6%–43.1%) was observed.
- The median duration of response was 7.7 months (95% CI, 4.9–10.8).
- The main toxicity was neutropenia, and four deaths occurred during treatment.[61][Level of evidence C3]
- The FDA granted accelerated approval to sacituzumab govitecan for patients with metastatic TNBC after at least two previous lines of therapy.
- A phase III randomized trial (ASCENT [NCT02574455]) confirmed the efficacy of sacituzumab govitecan in patients with metastatic TNBC that was relapsed or refractory to two or more previous chemotherapy regimens. The trial randomly assigned 468 women without brain metastases to receive either sacituzumab govitecan or the physician's choice of single-agent chemotherapy (eribulin, vinorelbine, capecitabine, or gemcitabine). The primary end point was PFS. All patients had previous taxane exposure. Patients received sacituzumab govitecan at a dose of 10 mg/kg intravenously on days 1 and 8 of each 21-day cycle.[62]
- In the chemotherapy arm, 54% of patients received eribulin, 20% received vinorelbine, 13% received capecitabine, and 12% received gemcitabine.
- The median PFS was 5.6 months (95% CI, 4.3–6.3; 166 events) in the sacituzumab govitecan arm and 1.7 months (95% CI, 1.5–2.6; 150 events) in the chemotherapy arm (HRdisease progression or death, 0.41; 95% CI, 0.32–0.52; P < .001).[62][Level of evidence A1]
- The median OS was 12.1 months (95% CI, 10.7–14.0) in the sacituzumab govitecan arm and 6.7 months (95% CI, 5.8–7.7) in the chemotherapy arm (HRdeath, 0.48; 95% CI, 0.38–0.59; P < .001).
- The overall response rate was 35% in the sacituzumab govitecan arm and 5% in the chemotherapy arm.
- Key treatment-related adverse events of grade 3 or higher occurred more often in patients who received sacituzumab govitecan than patients who received chemotherapy: neutropenia (51% vs. 33%), leukopenia (10% vs. 5%), diarrhea (10% vs. <1%), anemia (8% vs. 5%), and febrile neutropenia (6% vs. 2%).
PARP inhibitor therapy
Patients with TNBC and a germline BRCA pathogenic variant are eligible for PARP inhibitor therapy. For more information, see the Germline BRCA-Mutated Metastatic Breast Cancer section.
Chemotherapy
Chemotherapy may be appropriate for patients with TNBC. For more information, see the Chemotherapy for Metastatic Breast Cancer section.
Immunotherapy monotherapy
To date, immunotherapy monotherapy has not demonstrated an OS benefit for patients with metastatic TNBC.
Evidence (pembrolizumab):
- KEYNOTE-119 (NCT02555657) was a phase III, randomized, open-label, multicenter, international trial that enrolled patients with metastatic TNBC. Patients had received one or two previous systemic treatments for metastatic disease and had previous treatment with an anthracycline or taxane. The trial randomly assigned 622 patients to receive intravenous pembrolizumab 200 mg once every 3 weeks for 35 cycles (pembrolizumab group), or to the investigator's choice of single-drug chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine). The primary end point was OS as assessed in each of the following groups: patients with a PD-L1 CPS of 10 or more, patients with a CPS of 1 or more, and all patients.[63]
- In patients with a PD-L1 CPS of 10 or more, the median OS was 12.7 months (95% CI, 9.9–16.3) in the pembrolizumab group and 11.6 months (95% CI, 8.3–13.7) in the chemotherapy group (HR, 0.78; 95% CI, 0.57–1.06; log-rank P = .057).[63][Level of evidence A1]
- In patients with a CPS of 1 or more, the median OS was 10.7 months (95% CI, 9.3–12.5) in the pembrolizumab group and 10.2 months (95% CI, 7.9–12.6) in the chemotherapy group (HR, 0.86; 95% CI, 0.69–1.06; log-rank P = .073).
- Among all patients, the median OS was 9.9 months (95% CI, 8.3–11.4) in the pembrolizumab group and 10.8 months (95% CI, 9.1–12.6) in the chemotherapy group (HR, 0.97; 95% CI, 0.82–1.15).
Metastatic HER2-Positive Breast Cancer
Antibody therapy targeting the HER2 pathway has been used since the 1990s and has revolutionized the treatment of HER2-positive metastatic breast cancer. Several HER2-targeted agents (e.g., trastuzumab, pertuzumab, trastuzumab emtansine, lapatinib) have been approved for treatment of this disease.
Monoclonal antibody therapy
Trastuzumab
Approximately 20% to 25% of patients with breast cancer have tumors that overexpress HER2.[64] Trastuzumab is a humanized monoclonal antibody that binds to the HER2 receptor.[64] In patients previously treated with cytotoxic chemotherapy whose tumors overexpress HER2, administration of trastuzumab as a single agent resulted in a response rate of 21%.[65][Level of evidence C3]
Evidence (trastuzumab):
- In a phase III trial, patients with metastatic disease
were randomly assigned to receive either chemotherapy alone (doxorubicin and
cyclophosphamide or paclitaxel) or the same chemotherapy plus trastuzumab.[66][Level of evidence A1]
- Patients treated with chemotherapy plus trastuzumab had an OS advantage over those who received chemotherapy alone (25.1 months vs. 20.3 months, P = .05).[66][Level of evidence A1]
Notably, when combined with doxorubicin, trastuzumab is associated with significant cardiac toxicity.[67]
Clinical trials comparing multiagent chemotherapy plus trastuzumab with single-agent chemotherapy have yielded conflicting results.
- In one randomized study of patients with metastatic breast cancer treated with trastuzumab, paclitaxel, and carboplatin, patients tolerated the combination well and had a longer time to disease progression, compared with those treated with trastuzumab and paclitaxel alone.[68][Level of evidence B1]
- However, no difference in OS, time to disease progression, or response rate was shown in the Breast Cancer International Research Group’s phase III trial (BCIRG-007 [NCT00047255]) that compared carboplatin and docetaxel plus trastuzumab versus docetaxel plus trastuzumab as first-line chemotherapy for metastatic HER2-overexpressing breast cancer.[69][Level of evidence A1]
Outside of a clinical trial, standard first-line treatment for metastatic HER2-overexpressing breast cancer is single-agent chemotherapy plus trastuzumab.
Pertuzumab
Pertuzumab is a humanized monoclonal antibody that binds to a different epitope at the HER2 extracellular domain than does trastuzumab. The binding of pertuzumab to HER2 prevents dimerization with other ligand-activated HER receptors, most notably HER3.
Evidence (pertuzumab):
- The phase III CLEOPATRA trial (NCT00567190) assessed the efficacy and safety of pertuzumab plus trastuzumab plus docetaxel versus placebo plus trastuzumab plus docetaxel, in the first-line HER2-positive metastatic setting.[70,71][Level of evidence A1]
- With a median follow-up of 50 months, the median OS was 40.8 months in the control group versus 56.5 months in the pertuzumab group (HR favoring pertuzumab group, 0.68; 95% CI, 0.56–0.84; P < .001). Median PFS per investigator assessment was improved by 6.3 months by the addition of pertuzumab (HR, 0.68; 95% CI, 0.58–0.80).
- Median OS was 56.5 months in the pertuzumab group compared with 40.8 months in the placebo group (HR, 0.68; 95% CI, 0.57–0.84; P < .001).[71] Eight-year landmark OS rates were 37% with the addition of pertuzumab, compared with 23% in the placebo group.[72]
- The toxicity profile was similar in both treatment groups, with no increase in cardiac toxic effects seen in the pertuzumab combination arm.
Trastuzumab emtansine
Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate that incorporates the HER2-targeted antitumor properties of trastuzumab with the cytotoxic activity of the microtubule-inhibitory agent DM1. T-DM1 allows specific intracellular drug delivery to HER2-overexpressing cells, potentially improving the therapeutic index and minimizing exposure of normal tissue.
Evidence (T-DM1):
- The phase III EMILIA or TDM4370g study (NCT00829166) was a randomized open-label trial that enrolled 991 patients with HER2-overexpressing, unresectable, locally advanced or metastatic breast cancer who were previously treated with trastuzumab and a taxane.[73][Level of evidence A1] Patients were randomly assigned to receive either T-DM1 or lapatinib plus capecitabine.
- Median PFS was 9.6 months with T-DM1 versus 6.4 months with lapatinib plus capecitabine (HR, 0.65; 95% CI, 0.55–0.77; P < .001).
- Median OS was longer with T-DM1 versus lapatinib plus capecitabine (29.9 months vs. 25.9 months; HR, 0.75 [95% CI, 0.64–0.88]).[74]
- The incidences of thrombocytopenia and increased serum aminotransferase levels were higher in patients who received T-DM1, whereas the incidences of diarrhea, nausea, vomiting, and palmar-plantar syndrome were higher in patients who received lapatinib plus capecitabine.
- Further evidence of T-DM1’s activity in metastatic HER2-overexpressed breast cancer was shown in a randomized phase II study of T-DM1 versus trastuzumab plus docetaxel.[75][Level of evidence B1] This trial randomly assigned 137 women with HER2-overexpressed breast cancer in the first-line metastatic setting.
- At median follow-up of 14 months, median PFS was 9.2 months with trastuzumab plus docetaxel and 14.2 months with T-DM1 (HR, 0.59; 95% CI, 0.36–0.97).
- Preliminary OS results were similar between treatment arms.
- T-DM1 had a favorable safety profile compared with trastuzumab plus docetaxel, with fewer grade 3 adverse events (46.4% vs. 90.9%), adverse events leading to treatment discontinuations (7.2% vs. 40.9%), and serious adverse events (20.3% vs. 25.8%).
- Evidence of activity of T-DM1 in heavily pretreated patients with metastatic, HER2-overexpressed breast cancer who had received previous trastuzumab and lapatinib was shown in the randomized phase III TH3RESA study (NCT01419197) of T-DM1 versus physician’s choice of treatment.[76][Level of evidence A1] This trial randomly assigned 602 patients in a 2:1 ratio (404 patients assigned to T-DM1 and 198 patients assigned to physician’s choice) and allowed crossover to T-DM1.
- At a median follow-up of 7.2 months in the T-DM1 group and 6.5 months in the physician’s-choice group, median PFS was 6.2 months in the T-DM1 group and 3.3 months in the physician’s-choice group (HR, 0.528; 95% CI, 0.422–0.661; P < .0001).
- OS was significantly longer with trastuzumab emtansine versus the treatment of physician’s choice (median OS, 22.7 months vs. 15.8 months; HR, 0.68; 95% CI, 0.54–0.85; P = .0007).[77]
- The role of T-DM1 as first-line treatment of metastatic HER2-overexpressed breast cancer was evaluated in the phase III MARIANNE trial (NCT01120184).[78][Level of evidence B1] This study randomly assigned 1,095 patients to receive either trastuzumab plus taxane, T-DM1 plus placebo, or T-DM1 plus pertuzumab.
- The median PFS for these treatment groups was 13.7 months for the trastuzumab-plus-taxane group, 14.1 months for the T-DM1-plus-placebo group, and 15.2 months for the T-DM1-plus-pertuzumab group.
- There was no significant difference in PFS with T-DM1 plus placebo compared with trastuzumab plus taxane (HR, 0.91; 97.5% CI, 0.73–1.13), or with T-DM1 plus pertuzumab compared with trastuzumab plus taxane (HR, 0.87; 97.5% CI, 0.69–1.08).
- Therefore, neither T-DM1 plus placebo nor T-DM1 plus pertuzumab showed PFS superiority over trastuzumab plus taxane.
Trastuzumab deruxtecan
Trastuzumab deruxtecan is an antibody-drug conjugate that combines trastuzumab with a topoisomerase inhibitor. This drug has demonstrated antitumor activity in patients with advanced HER2-positive breast cancer.
Evidence (trastuzumab deruxtecan):
- A phase III, multicenter, open-label, randomized trial (DESTINY-Breast03 [NCT03529110]) compared the efficacy and safety of trastuzumab deruxtecan with trastuzumab emtansine. The study included 524 patients with HER2-positive metastatic breast cancer previously treated with a taxane and trastuzumab. PFS was the primary end point.[79]
- At 12 months, 75.8% of patients who received trastuzumab deruxtecan were alive without disease progression, compared with 34.1% of patients who received trastuzumab emtansine (HR, 0.28; 95% CI, 0.22–0.37; P < .001).
- Secondary end points were OS, overall response rate, and safety. A total of 94.1% of patients who received trastuzumab deruxtecan were alive at 12 months, compared with 85.9% of patients who received trastuzumab emtansine (HRdeath, 0.55; 95% CI, 0.36–0.86). The overall response rate was 79.7% versus 34.2%, favoring trastuzumab deruxtecan. Grade 3 to 4 drug-related adverse events occurred in 45.1% of patients who received trastuzumab deruxtecan and 38.9% of patients who received trastuzumab emtansine. Interstitial lung disease and pneumonitis occurred in 10.5% of patients who received trastuzumab deruxtecan and 1.9% of patients who received trastuzumab emtansine; none of these events were grade 4 to 5.
This trial led to a change in the standard of care for patients with HER2-positive metastatic breast cancer, such that patients with prior taxane/trastuzumab therapy proceed to trastuzumab deruxtecan, rather than trastuzumab emtansine, for second-line therapy.
Margetuximab
Margetuximab is an Fc-engineered anti-HER2 immunoglobulin G monoclonal antibody that targets the same epitope as trastuzumab, with similar antiproliferative effects. Compared with trastuzumab, margetuximab was designed to increase binding affinity (in vitro) for the activating Fc-gamma receptor and decrease binding affinity for the inhibitory Fc-gamma receptor.
Evidence (margetuximab):
- The primary PFS analysis from the phase III SOPHIA study (NCT02492711) led to the FDA approval of margetuximab with chemotherapy in patients with HER2-positive metastatic breast cancer who have received as least two previous anti-HER2 regimens, at least one of which was for metastatic disease.
The open-label SOPHIA trial randomly assigned 536 patients in the ITT population to receive either margetuximab or trastuzumab. Both groups received investigator's choice of chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine). Eligible patients had disease progression after two or more previous anti-HER2 therapies and one to three lines of therapy for metastatic disease. All patients had received previous trastuzumab, all but one had received previous pertuzumab, and 91.2% had received prior ado-trastuzumab emtansine. The sequential primary end points were PFS by central review followed by OS.[80,81][Level of evidence B1]
- Margetuximab improved primary PFS over trastuzumab with a 24% relative risk reduction (HR, 0.76; 95% CI, 0.59–0.98; P = .03) (median PFS, 5.8 months vs. 4.9 months, favoring margetuximab).
- The median follow-up was 20.2 months. The median OS in the ITT population was not statistically different between the two treatment groups: 21.6 months in the margetuximab group and 21.9 months in the trastuzumab group (HR, 0.95; 95% CI, 0.77–1.17; P = .620).
- Grade 3 or higher adverse events that occurred in at least 5% of patients included decreased neutrophil count and anemia in both groups, fatigue in the margetuximab group, and febrile neutropenia in the trastuzumab group.
Tyrosine kinase inhibitor (TKI) therapy
The FDA has approved several TKIs for metastatic HER2-positive breast cancer.
Tucatinib
Tucatinib is an oral TKI highly selective for the kinase domain of HER2 that minimally inhibits the epidermal growth factor receptor. A phase Ib trial in pretreated patients demonstrated activity when tucatinib was combined with trastuzumab and capecitabine.
Evidence (tucatinib):
- The HER2CLIMB trial (NCT02614794) compared trastuzumab, capecitabine, and tucatinib with trastuzumab, capecitabine, and placebo in 632 patients who had previously been treated with trastuzumab, pertuzumab, and trastuzumab emtansine. Patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients with and without brain metastases were included. The primary end point was PFS.[82][Level of evidence A1]
- The median PFS was 7.8 months in the tucatinib combination group and 5.6 months in the placebo combination group (HR, 0.54; 95% CI, 0.42–0.71; P < .001)
- In an interim analysis conducted at the time of the PFS analysis, median OS was 21.9 months in the tucatinib combination group and 17.4 months in the placebo combination group (HR, 0.66; 95% CI, 0.50–0.88; P = .005)
- Patients with and without brain metastases benefited from the tucatinib combination.
- Grade 3 adverse events including diarrhea, palmar-plantar erythrodysesthesia syndrome, and elevated aminotransferase levels were more common in the tucatinib combination group but occurred in fewer than 15% of patients.
Neratinib
Neratinib is an irreversible pan-HER TKI (HER1, HER2, and HER4), which is approved in combination with capecitabine for the treatment of patients with advanced or metastatic HER2-positive breast cancer after two or more prior anti–HER2-based regimens in the metastatic setting.
Evidence (neratinib):
- In the phase III NALA trial (NCT01808573), neratinib and capecitabine were compared with lapatinib and capecitabine in 621 patients with HER2-positive metastatic breast cancer who received two or more HER2-directed regimens in the metastatic setting. PFS and OS were co-primary end points. Secondary end points were time to intervention for metastatic central nervous system (CNS) disease and duration of response. Patients with stable or asymptomatic CNS disease were included.[83][Level of evidence B1]
- Patients who received neratinib and capecitabine had significantly improved PFS (HR, 0.76; 95% CI, 0.63–0.93). OS between the two arms was not different.
- The cumulative incidence of intervention for CNS disease was 22.8% (95% CI, 15.5%–30.9%) for patients who received neratinib versus 29.2% (95% CI, 22.5%–36.1%) for patients who received lapatinib.
- Duration of response was 8.5 months for patients who received neratinib versus 5.6 months for patients who received lapatinib.
- Diarrhea, nausea, palmar-plantar erythrodysesthesia, and vomiting were the most common adverse events, with grade 3 diarrhea occurring in 24.4% of patients who received neratinib. Antidiarrheal medications were required in the neratinib arm and used by 98.3% of those patients.
- Quality-of-life scores were similar between the two groups.
Lapatinib
Lapatinib is an orally administered TKI of both HER2 and the epidermal growth factor receptor. Lapatinib plus capecitabine has shown activity in patients who have HER2-positive metastatic breast cancer that progressed after treatment with trastuzumab.
Evidence (lapatinib):
- A nonblinded randomized trial (GSK-EGF100151 [NCT00078572]) compared the combination of capecitabine and lapatinib with capecitabine alone in 324 patients with locally advanced or metastatic disease that progressed after therapies that included anthracyclines, taxanes, and trastuzumab.[84][Level of evidence A1]
- Median time-to-disease progression in the lapatinib-plus-capecitabine arm was 8.4 months compared with 4.4 months in the capecitabine-alone arm (HR, 0.49; 95% CI, 0.34–0.71; P < .001).
- There was no difference in OS (HR, 0.92; 95% CI, 0.58–1.46; P = .72).[84][Level of evidence A1]
- Patients on combination therapy were more likely to develop diarrhea, rash, and dyspepsia. For more information, see the Diarrhea section in Gastrointestinal Complications.
- No data are available on quality of life or treatment after disease progression.
mTOR inhibition
Evidence of mTOR inhibitor activity in HER2-positive breast cancer was shown in the double-blind, placebo-controlled, phase III BOLERO-3 trial (NCT01007942).[85]
- In the BOLERO-3 trial, 569 patients with HER2-positive, trastuzumab-resistant, breast cancer, who had received previous taxane therapy, were randomly assigned to receive either everolimus plus trastuzumab plus vinorelbine, or placebo plus trastuzumab plus vinorelbine.[85][Level of evidence B1]
- At median follow-up of 20.2 months, median PFS was 7.0 months in the everolimus group versus 5.78 months in the placebo group (HR, 0.78; 95% CI, 0.65–0.95; P = .0067).
- Serious adverse events were reported in 117 patients (42%) in the everolimus group and 55 patients (20%) in the placebo group.
- Final OS outcomes for this trial have not yet been reported.
Germline BRCA-Mutated Metastatic Breast Cancer
For patients with metastatic breast cancer who carry germline BRCA1 or BRCA2 pathogenic variants, oral PARP inhibitors have shown activity. BRCA1 and BRCA2 are tumor suppressor genes that encode proteins involved in DNA repair (via the homologous recombination repair pathway). PARP plays a critical role in DNA repair and has been studied as therapy for patients with breast cancer who harbor germline BRCA1 or BRCA2 variants.
Olaparib
Evidence (olaparib):
- The OlympiAD trial (NCT02000622) was a randomized, open-label, phase III trial that randomly assigned 302 patients, in a 2:1 ratio, to receive olaparib (300 mg bid) or standard therapy (either single-agent capecitabine, eribulin, or vinorelbine).[86] All patients had received anthracycline and taxane previously in either the adjuvant or metastatic setting, and those with hormone receptor–positive disease had also received endocrine therapy previously.
- Median PFS was significantly longer in the olaparib group than in the standard therapy group (7.0 months vs. 4.2. months; HRdisease progression or death, 0.58; 95% CI, 0.43–0.80; P < .001).[86][Level of evidence A1]
- OS did not differ between the two treatment groups with median time to death (HRdeath, 0.90; 95% CI, 0.63–1.29; P = .57).
- Olaparib was less toxic than standard therapy, with a rate of grade 3 or higher adverse events of 36.6% in the olaparib group and 50.5% in the standard therapy group, with anemia, nausea, vomiting, fatigue, headache, and cough occurring more frequently with olaparib; neutropenia, palmar-plantar erythrodysesthesia, and liver-function test abnormalities occurred more commonly with chemotherapy.
- Of note, subset analysis suggested that PFS improvement with olaparib appeared greater in the TNBC subgroup (HR, 0.43; 95% CI, 0.29–0.63) than in the hormone receptor–positive subgroup (HR, 0.82; 95% CI, 0.55–1.26).
Talazoparib
Evidence (talazoparib):
- The EMBRACA trial (NCT01945775) was a randomized, open label, phase III trial that assigned 431 patients with germline BRCA1 or BRCA2 pathogenic variants and locally advanced or metastatic breast cancer in a 2:1 ratio to talazoparib (1 mg PO qd) or standard single-agent chemotherapy of the physician’s choice (eribulin, capecitabine, gemcitabine, or vinorelbine).[87] All patients had received previous treatment with an anthracycline, taxane, or both. Patients had received three or fewer lines of cytotoxic chemotherapy for advanced breast cancer. Previous platinum therapy in the setting of early breast cancer was permitted if it was completed at least 6 months before progressive disease or if there was no objective progression while on platinum therapy in the advanced-disease setting. Hormone receptor–positive and hormone receptor–negative patients were enrolled.
- Median PFS was significantly longer in the talazoparib group than in the standard therapy group (8.6 months vs. 5.6 months; HRdisease progression or death, 0.54; 95% CI, 0.41–0.71; P < .001).
- Benefits were observed in all subgroups, although CIs were wide in the subgroup of patients who had received previous platinum therapy.
- Median OS did not differ between the two groups (22.3 months vs. 19.5 months; HRdeath, 0.76; 95% CI, 0.55–1.06; P = .11), although survival data are not yet mature.
- The primary toxicity observed with talazoparib was myelosuppression, especially anemia.
- Patient-reported outcome data demonstrated more favorable effects of talazoparib than standard chemotherapy on quality-of-life measures.[88]
For more information, see Genetics of Breast and Gynecologic Cancers.
High Tumor Mutational Burden Breast Cancer
An established biomarker for checkpoint inhibitor immunotherapy is high somatic tumor mutational burden (TMB). TMB is the number of mutations within the coding region of a tumor genome and is reported as mutations per megabase (Mut/Mb). TMB is high (≥10) in approximately 5% of breast cancers.[89] Highest median TMB is noted in triple-negative tumors and lowest median TMB is noted in ER-positive/HER2-negative patients.
Pembrolizumab
Evidence (pembrolizumab):
- The FDA approved pembrolizumab for the treatment of metastatic solid tumors with a TMB of at least 10 Mut/Mb, based on the KEYNOTE-158 trial.[90]
- KEYNOTE-158 (NCT02628067) was a multicohort, open-label, nonrandomized, phase II multinational study. Patients had metastatic solid malignancies, treated with at least one previous line of therapy. Participants received pembrolizumab 200 mg intravenously every 3 weeks for up to 35 cycles. TMB was assessed in formalin-fixed paraffin-embedded tumor samples using the FoundationOne CDx assay. A total of 105 of 805 evaluable patients had high TMB (≥10 Mut/Mb).
- The primary outcome was objective response rate. Objective responses were observed in 30 of 102 patients (29%) in the TMB-high group and 43 of 688 patients (6%) in the non-TMB-high group. Grade 3 to 5 treatment-related adverse events occurred in 16 patients. One patient died of pneumonia that was assessed by the investigator to be treatment related.
- The TAPUR study (NCT02693535) was a phase II basket trial done to identify the efficacy of commercially available targeted agents in patients with advanced cancers and genomic alterations known to be drug targets. TAPUR results from patients with metastatic breast cancer and high TMB have been reported. The primary end point was disease control (objective response or stable disease for ≥16 weeks).[91][Level of evidence C3]
- The study enrolled 28 patients with metastatic breast cancer and high TMB. TMB ranged from 9 to 37 Mut/Mb.
- Disease control was noted in 37% of patients, with objective response noted in 21% of patients.
- The median PFS was 10.6 weeks and the median OS was 30.6 weeks.
Chemotherapy for Metastatic Breast Cancer
Patients receiving hormone therapy whose tumors have progressed are candidates for cytotoxic chemotherapy. There are no data suggesting that combination therapy results in an OS benefit over single-agent therapy. Patients with hormone receptor–negative tumors and those with visceral metastases or symptomatic disease are also candidates for cytotoxic agents.[92]
Single agents that have shown activity in metastatic breast cancer include:
- Anthracyclines.
- Taxanes.
- Alkylating agents.
- Cyclophosphamide.
- Fluoropyrimidines.
- Antimetabolites.
- Methotrexate.
- Vinca alkaloids.
- Vinorelbine.[104]
- Vinblastine.
- Vincristine.
- Platinum.
- Carboplatin.
- Cisplatin.
- Other.
Combination regimens that have shown activity in metastatic breast cancer include:
- AC: Doxorubicin and cyclophosphamide.[109]
- EC: Epirubicin and cyclophosphamide.[110]
- Docetaxel and doxorubicin.[111]
- CAF: Cyclophosphamide, doxorubicin, and 5-FU.[112]
- CMF: Cyclophosphamide, methotrexate, and 5-FU.[113]
- Doxorubicin and paclitaxel.[114,115]
- Docetaxel and capecitabine.[116]
- Vinorelbine and epirubicin.[117]
- Capecitabine and ixabepilone.[118]
- Carboplatin and gemcitabine.[119]
- Gemcitabine and paclitaxel.[120]
There are no data suggesting that combination therapy results in an OS benefit over single-agent therapy. An ECOG intergroup study (E-1193) randomly assigned patients to receive paclitaxel and doxorubicin, given both as a combination and sequentially.[121] Although response rate and time to disease progression were both better for the combination, survival was the same in both groups.[121][Level of evidence A1]; [122,123]
The selection of therapy in individual patients is influenced by the following factors:
- Rate of disease progression.
- Presence or absence of comorbid medical conditions.
- Physician/patient preference.
Sequential use of single agents or combinations can be used for patients who relapse with metastatic disease. Combination chemotherapy is often given if there is evidence of rapidly progressive disease or visceral crisis. Combinations of chemotherapy and hormone therapy have not shown an OS advantage over the sequential use of these agents.[124,125] A systematic review of 17 randomized trials found that the addition of one or more chemotherapy drugs to a chemotherapy regimen in the attempt to intensify the treatment improved tumor response but had no effect on OS.[126][Level of evidence A1]
The following factors may be considered for decisions regarding the duration of chemotherapy:
- Patient preference and goals of treatment.
- Presence of toxicities from previous therapies.
- Availability of alternative treatment options.
The optimal time for patients with responsive or stable disease has been studied by several groups. For patients who attain a complete response to initial therapy, two randomized trials have shown a prolonged DFS after immediate treatment with a different chemotherapy regimen compared with observation and treatment upon relapse.[127,128][Level of evidence A1] Neither of these studies, however, showed an improvement in OS for patients who received immediate treatment; in one of these studies,[128] survival was actually worse in the group that was treated immediately. Similarly, no difference in survival was noted when patients with partial response or stable disease after initial therapy were randomly assigned to receive either a different chemotherapy versus observation [129] or a different chemotherapy regimen given at higher versus lower doses.[130][Level of evidence A1] However, 324 patients who achieved disease control were randomly assigned to maintenance chemotherapy or observation. Patients who received maintenance chemotherapy (paclitaxel and gemcitabine) had improved PFS at 6 months and improved OS. This was associated with an increased rate of adverse events.[131][Level of evidence A1] Because there is no standard approach for treating metastatic disease, patients requiring second-line regimens are good candidates for clinical trials.
Capecitabine and fluorouracil dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[132,133] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[132-134] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient's DPYD genotype and number of functioning DPYD alleles.[135-137] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[138] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[139]
Cardiac toxic effects with anthracyclines
The potential for anthracycline-induced cardiac toxic effects should be considered in the selection of chemotherapeutic regimens for selected patients. Recognized risk factors for cardiac toxicity include:
- Advanced age.
- Previous chest-wall radiation therapy.
- Previous anthracycline exposure.
- Hypertension and known underlying heart disease.
- Diabetes.
The cardioprotective drug dexrazoxane decreased the risk of doxorubicin-induced cardiac toxicity in patients in controlled studies. The use of this agent has permitted patients to receive higher cumulative doses of doxorubicin and has allowed patients with cardiac risk factors to receive doxorubicin.[140-143] The risk of cardiac toxicity may also be reduced by giving doxorubicin as a continuous intravenous infusion.[144] The American Society of Clinical Oncology guidelines suggest the use of dexrazoxane in patients with metastatic cancer who have received a cumulative dose of doxorubicin of 300 mg/m2 or more when further treatment with an anthracycline is likely to be of benefit.[145] Dexrazoxane has a similar protective effect in patients receiving epirubicin.[146]
Surgical Treatment for Metastatic Breast Cancer
The ECOG 2108 trial (NCT01242800) evaluated the potential benefit of breast locoregional interventions in patients with metastatic breast cancer. Women presenting with metastatic breast cancer and an intact primary tumor received systemic therapy for 4 to 8 months. If disease progression did not occur, patients were randomly assigned to receive locoregional therapy for the primary site (surgery and radiation therapy, per standards for nonmetastatic disease) or to continue systemic therapy. Of the 256 randomly assigned patients, 125 received early locoregional therapy. The primary end point was OS.[147]
- The 3-year OS was 67.9% in the systemic therapy group and 68.4% in the locoregional therapy group (HR, 1.11; 90% CI, 0.82–1.52; P = .57). The median OS was 53.1 months (95% CI, 47.9–not estimable) in the systemic therapy group and 54.9 months (95% CI, 46.7–not estimable) in the locoregional therapy group.
- Locoregional disease progression was less frequent in patients who received locoregional therapy (3-year locoregional progression rate, 16.3% vs. 39.8%; P < .001).
Surgery may be indicated for select patients. For example, patients may need surgery if the following issues occur:
- Fungating/painful breast lesions (mastectomy).
- Parenchymal brain or vertebral metastases with spinal cord compression.
- Pathological (or impending) fractures.
- Pleural or pericardial effusions.
For more information about pleural and pericardial effusions, see Cardiopulmonary Syndromes.
Radiation Therapy for Metastatic Breast Cancer
Radiation therapy has a major role in the palliation of localized symptomatic metastases.[148] Indications for external-beam radiation therapy include:
- Painful bony metastases.
- Unresectable central nervous system metastases (i.e., brain, meninges, and spinal cord).
- Bronchial obstruction.
- Fungating/painful breast or chest wall lesions.
- After surgery for decompression of intracranial or spinal cord metastases.
- After fixation of pathological fractures.
Strontium chloride Sr 89, a systemically administered radionuclide, can be given for palliation of diffuse bony metastases.[149,150]
Bone-Modifying Therapy for Metastatic Breast Cancer
The use of bone-modifying therapy to reduce skeletal morbidity in patients with bone metastases should be considered.[151] Results of randomized trials of pamidronate and clodronate in patients with bony metastatic disease show decreased skeletal morbidity.[152-154][Level of evidence A3] Zoledronate has been at least as effective as pamidronate.[155]
The optimal dosing schedule for zoledronate was studied in CALGB-70604 (Alliance; NCT00869206), which randomly assigned 1,822 patients, 855 of whom had metastatic breast cancer, to receive zoledronic acid every 4 weeks or every 12 weeks.[156] Skeletal-related events were similar in both groups, with 260 patients (29.5%) in the zoledronate every-4-week dosing group and 253 patients (28.6%) in the zoledronate every-12-week dosing group experiencing at least one skeletal-related event (risk difference of -0.3% [1-sided 95% CI, -4% to infinity]; P < .001 for noninferiority).[156][Level of evidence B1] This study suggests that the longer dosing interval of zoledronate every 12 weeks is a reasonable treatment option.
The monoclonal antibody denosumab inhibits the receptor activator of nuclear factor kappa beta ligand (RANKL). A meta-analysis of three phase III trials (NCT00321464, NCT00321620, and NCT00330759) comparing zoledronate versus denosumab for management of bone metastases suggests that denosumab is similar to zoledronate in reducing the risk of a first skeletal-related event.[157]
For more information about bisphosphonates, see Cancer Pain.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- National Cancer Institute: SEER Cancer Stat Facts: Female Breast Cancer. Bethesda, Md: National Cancer Institute. Available online. Last accessed April 9, 2025.
- Seidman AD, Bordeleau L, Fehrenbacher L, et al.: National Cancer Institute Breast Cancer Steering Committee Working Group Report on Meaningful and Appropriate End Points for Clinical Trials in Metastatic Breast Cancer. J Clin Oncol 36 (32): 3259-3268, 2018. [PUBMED Abstract]
- Rugo HS, Diéras V, Gelmon KA, et al.: Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol 29 (4): 888-894, 2018. [PUBMED Abstract]
- Rugo HS, Finn RS, Diéras V, et al.: Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat 174 (3): 719-729, 2019. [PUBMED Abstract]
- Turner NC, Ro J, André F, et al.: Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med 373 (3): 209-19, 2015. [PUBMED Abstract]
- Verma S, O'Shaughnessy J, Burris HA, et al.: Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: results from MONALEESA-2. Breast Cancer Res Treat 170 (3): 535-545, 2018. [PUBMED Abstract]
- Janni W, Alba E, Bachelot T, et al.: First-line ribociclib plus letrozole in postmenopausal women with HR+ , HER2- advanced breast cancer: Tumor response and pain reduction in the phase 3 MONALEESA-2 trial. Breast Cancer Res Treat 169 (3): 469-479, 2018. [PUBMED Abstract]
- Kaufman PA, Toi M, Neven P, et al.: Health-Related Quality of Life in MONARCH 2: Abemaciclib plus Fulvestrant in Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer After Endocrine Therapy. Oncologist 25 (2): e243-e251, 2020. [PUBMED Abstract]
- Gao JJ, Cheng J, Bloomquist E, et al.: CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol 21 (2): 250-260, 2020. [PUBMED Abstract]
- Finn RS, Crown JP, Lang I, et al.: The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16 (1): 25-35, 2015. [PUBMED Abstract]
- Finn RS, Martin M, Rugo HS, et al.: Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 375 (20): 1925-1936, 2016. [PUBMED Abstract]
- Hortobagyi GN, Stemmer SM, Burris HA, et al.: Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 375 (18): 1738-1748, 2016. [PUBMED Abstract]
- Huober J, Barrios CH, Niikura N, et al.: Atezolizumab With Neoadjuvant Anti-Human Epidermal Growth Factor Receptor 2 Therapy and Chemotherapy in Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer: Primary Results of the Randomized Phase III IMpassion050 Trial. J Clin Oncol 40 (25): 2946-2956, 2022. [PUBMED Abstract]
- Hortobagyi GN, Stemmer SM, Burris HA, et al.: Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N Engl J Med 386 (10): 942-950, 2022. [PUBMED Abstract]
- Slamon DJ, Neven P, Chia S, et al.: Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol 36 (24): 2465-2472, 2018. [PUBMED Abstract]
- Slamon DJ, Neven P, Chia S, et al.: Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N Engl J Med 382 (6): 514-524, 2020. [PUBMED Abstract]
- Tripathy D, Im SA, Colleoni M, et al.: Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 19 (7): 904-915, 2018. [PUBMED Abstract]
- Im SA, Lu YS, Bardia A, et al.: Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med 381 (4): 307-316, 2019. [PUBMED Abstract]
- Goetz MP, Toi M, Campone M, et al.: MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 35 (32): 3638-3646, 2017. [PUBMED Abstract]
- Goetz MP, Toi M, Huober J, et al.: Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2- advanced breast cancer: final overall survival results of MONARCH 3. Ann Oncol 35 (8): 718-727, 2024. [PUBMED Abstract]
- Cristofanilli M, Turner NC, Bondarenko I, et al.: Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17 (4): 425-39, 2016. [PUBMED Abstract]
- Turner NC, Slamon DJ, Ro J, et al.: Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med 379 (20): 1926-1936, 2018. [PUBMED Abstract]
- Sledge GW, Toi M, Neven P, et al.: MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 35 (25): 2875-2884, 2017. [PUBMED Abstract]
- Sledge GW, Toi M, Neven P, et al.: The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol 6 (1): 116-124, 2020. [PUBMED Abstract]
- Kalinsky K, Bianchini G, Hamilton E, et al.: Abemaciclib plus fulvestrant vs fulvestrant alone for HR+, HER2- advanced breast cancer following progression on a prior CDK4/6 inhibitor plus endocrine therapy: primary outcome of the phase 3 postMONARCH trial. [Abstract] J Clin Oncol 42 (Suppl 17): A-LBA1001, 2024.
- Dickler MN, Tolaney SM, Rugo HS, et al.: MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin Cancer Res 23 (17): 5218-5224, 2017. [PUBMED Abstract]
- Baselga J, Campone M, Piccart M, et al.: Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366 (6): 520-9, 2012. [PUBMED Abstract]
- Piccart M, Hortobagyi GN, Campone M, et al.: Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†. Ann Oncol 25 (12): 2357-62, 2014. [PUBMED Abstract]
- Bachelot T, Bourgier C, Cropet C, et al.: Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 30 (22): 2718-24, 2012. [PUBMED Abstract]
- Kornblum N, Zhao F, Manola J, et al.: Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J Clin Oncol 36 (16): 1556-1563, 2018. [PUBMED Abstract]
- Rugo HS, Seneviratne L, Beck JT, et al.: Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol 18 (5): 654-662, 2017. [PUBMED Abstract]
- Turner NC, Oliveira M, Howell SJ, et al.: Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 388 (22): 2058-2070, 2023. [PUBMED Abstract]
- André F, Ciruelos E, Rubovszky G, et al.: Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 380 (20): 1929-1940, 2019. [PUBMED Abstract]
- Bidard FC, Kaklamani VG, Neven P, et al.: Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol 40 (28): 3246-3256, 2022. [PUBMED Abstract]
- Yardley DA, Ismail-Khan RR, Melichar B, et al.: Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 31 (17): 2128-35, 2013. [PUBMED Abstract]
- Connolly RM, Zhao F, Miller KD, et al.: E2112: Randomized Phase III Trial of Endocrine Therapy Plus Entinostat or Placebo in Hormone Receptor-Positive Advanced Breast Cancer. A Trial of the ECOG-ACRIN Cancer Research Group. J Clin Oncol 39 (28): 3171-3181, 2021. [PUBMED Abstract]
- Bonneterre J, Thürlimann B, Robertson JF, et al.: Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 18 (22): 3748-57, 2000. [PUBMED Abstract]
- Nabholtz JM, Buzdar A, Pollak M, et al.: Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 18 (22): 3758-67, 2000. [PUBMED Abstract]
- Mouridsen H, Gershanovich M, Sun Y, et al.: Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 21 (11): 2101-9, 2003. [PUBMED Abstract]
- Mauri D, Pavlidis N, Polyzos NP, et al.: Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst 98 (18): 1285-91, 2006. [PUBMED Abstract]
- Robertson JFR, Bondarenko IM, Trishkina E, et al.: Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388 (10063): 2997-3005, 2016. [PUBMED Abstract]
- Osborne CK, Pippen J, Jones SE, et al.: Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 20 (16): 3386-95, 2002. [PUBMED Abstract]
- Howell A, Robertson JF, Quaresma Albano J, et al.: Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol 20 (16): 3396-403, 2002. [PUBMED Abstract]
- Henderson IC: A rose is no longer a rose. J Clin Oncol 20 (16): 3365-8, 2002. [PUBMED Abstract]
- Flemming J, Madarnas Y, Franek JA: Fulvestrant for systemic therapy of locally advanced or metastatic breast cancer in postmenopausal women: a systematic review. Breast Cancer Res Treat 115 (2): 255-68, 2009. [PUBMED Abstract]
- Chia S, Gradishar W, Mauriac L, et al.: Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 26 (10): 1664-70, 2008. [PUBMED Abstract]
- Di Leo A, Jerusalem G, Petruzelka L, et al.: Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28 (30): 4594-600, 2010. [PUBMED Abstract]
- Mehta RS, Barlow WE, Albain KS, et al.: Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367 (5): 435-44, 2012. [PUBMED Abstract]
- Bergh J, Jönsson PE, Lidbrink EK, et al.: FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 30 (16): 1919-25, 2012. [PUBMED Abstract]
- Mehta RS, Barlow WE, Albain KS, et al.: Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer. N Engl J Med 380 (13): 1226-1234, 2019. [PUBMED Abstract]
- Vidula N, Yau C, Rugo HS: Trop2 gene expression (Trop2e) in primary breast cancer (BC): Correlations with clinical and tumor characteristics. [Abstract] J Clin Oncol 35 (15 Suppl): A-1075, 2017.
- Rugo HS, Bardia A, Marmé F, et al.: Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol 40 (29): 3365-3376, 2022. [PUBMED Abstract]
- Modi S, Park H, Murthy RK, et al.: Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol 38 (17): 1887-1896, 2020. [PUBMED Abstract]
- Modi S, Jacot W, Yamashita T, et al.: Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 387 (1): 9-20, 2022. [PUBMED Abstract]
- Bardia A, Hu X, Dent R, et al.: Trastuzumab Deruxtecan after Endocrine Therapy in Metastatic Breast Cancer. N Engl J Med 391 (22): 2110-2122, 2024. [PUBMED Abstract]
- Cortes J, Cescon DW, Rugo HS, et al.: Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396 (10265): 1817-1828, 2020. [PUBMED Abstract]
- Cortes J, Rugo HS, Cescon DW, et al.: Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N Engl J Med 387 (3): 217-226, 2022. [PUBMED Abstract]
- Schmid P, Adams S, Rugo HS, et al.: Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 379 (22): 2108-2121, 2018. [PUBMED Abstract]
- Emens LA, Adams S, Barrios CH, et al.: First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol 32 (8): 983-993, 2021. [PUBMED Abstract]
- Miles D, Gligorov J, André F, et al.: Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol 32 (8): 994-1004, 2021. [PUBMED Abstract]
- Bardia A, Mayer IA, Vahdat LT, et al.: Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med 380 (8): 741-751, 2019. [PUBMED Abstract]
- Bardia A, Hurvitz SA, Tolaney SM, et al.: Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med 384 (16): 1529-1541, 2021. [PUBMED Abstract]
- Winer EP, Lipatov O, Im SA, et al.: Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol 22 (4): 499-511, 2021. [PUBMED Abstract]
- Pegram MD, Pauletti G, Slamon DJ: HER-2/neu as a predictive marker of response to breast cancer therapy. Breast Cancer Res Treat 52 (1-3): 65-77, 1998. [PUBMED Abstract]
- Cobleigh MA, Vogel CL, Tripathy D, et al.: Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17 (9): 2639-48, 1999. [PUBMED Abstract]
- Slamon DJ, Leyland-Jones B, Shak S, et al.: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344 (11): 783-92, 2001. [PUBMED Abstract]
- Seidman A, Hudis C, Pierri MK, et al.: Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20 (5): 1215-21, 2002. [PUBMED Abstract]
- Robert N, Leyland-Jones B, Asmar L, et al.: Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 24 (18): 2786-92, 2006. [PUBMED Abstract]
- Valero V, Forbes J, Pegram MD, et al.: Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol 29 (2): 149-56, 2011. [PUBMED Abstract]
- Baselga J, Cortés J, Kim SB, et al.: Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366 (2): 109-19, 2012. [PUBMED Abstract]
- Swain SM, Baselga J, Kim SB, et al.: Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372 (8): 724-34, 2015. [PUBMED Abstract]
- Swain SM, Miles D, Kim SB, et al.: Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 21 (4): 519-530, 2020. [PUBMED Abstract]
- Verma S, Miles D, Gianni L, et al.: Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367 (19): 1783-91, 2012. [PUBMED Abstract]
- Diéras V, Miles D, Verma S, et al.: Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 18 (6): 732-742, 2017. [PUBMED Abstract]
- Hurvitz SA, Dirix L, Kocsis J, et al.: Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 31 (9): 1157-63, 2013. [PUBMED Abstract]
- Krop IE, Kim SB, González-Martín A, et al.: Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 15 (7): 689-99, 2014. [PUBMED Abstract]
- Krop IE, Kim SB, Martin AG, et al.: Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 18 (6): 743-754, 2017. [PUBMED Abstract]
- Perez EA, Barrios C, Eiermann W, et al.: Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J Clin Oncol 35 (2): 141-148, 2017. [PUBMED Abstract]
- Cortés J, Kim SB, Chung WP, et al.: Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med 386 (12): 1143-1154, 2022. [PUBMED Abstract]
- Rugo HS, Im SA, Cardoso F, et al.: Margetuximab Versus Trastuzumab in Patients With Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results From a Randomized Phase 3 Trial. J Clin Oncol 41 (2): 198-205, 2023. [PUBMED Abstract]
- Rugo HS, Im SA, Cardoso F, et al.: Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 7 (4): 573-584, 2021. [PUBMED Abstract]
- Murthy RK, Loi S, Okines A, et al.: Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med 382 (7): 597-609, 2020. [PUBMED Abstract]
- Saura C, Oliveira M, Feng YH, et al.: Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J Clin Oncol 38 (27): 3138-3149, 2020. [PUBMED Abstract]
- Geyer CE, Forster J, Lindquist D, et al.: Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355 (26): 2733-43, 2006. [PUBMED Abstract]
- André F, O'Regan R, Ozguroglu M, et al.: Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 15 (6): 580-91, 2014. [PUBMED Abstract]
- Robson M, Im SA, Senkus E, et al.: Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 377 (6): 523-533, 2017. [PUBMED Abstract]
- Litton JK, Rugo HS, Ettl J, et al.: Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 379 (8): 753-763, 2018. [PUBMED Abstract]
- Ettl J, Quek RGW, Lee KH, et al.: Quality of life with talazoparib versus physician's choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol 29 (9): 1939-1947, 2018. [PUBMED Abstract]
- Barroso-Sousa R, Jain E, Cohen O, et al.: Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol 31 (3): 387-394, 2020. [PUBMED Abstract]
- Marabelle A, Fakih M, Lopez J, et al.: Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21 (10): 1353-1365, 2020. [PUBMED Abstract]
- Alva AS, Mangat PK, Garrett-Mayer E, et al.: Pembrolizumab in Patients With Metastatic Breast Cancer With High Tumor Mutational Burden: Results From the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J Clin Oncol 39 (22): 2443-2451, 2021. [PUBMED Abstract]
- Wilcken N, Dear R: Chemotherapy in metastatic breast cancer: A summary of all randomised trials reported 2000-2007. Eur J Cancer 44 (15): 2218-25, 2008. [PUBMED Abstract]
- Ranson MR, Carmichael J, O'Byrne K, et al.: Treatment of advanced breast cancer with sterically stabilized liposomal doxorubicin: results of a multicenter phase II trial. J Clin Oncol 15 (10): 3185-91, 1997. [PUBMED Abstract]
- Harris L, Batist G, Belt R, et al.: Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 94 (1): 25-36, 2002. [PUBMED Abstract]
- Keller AM, Mennel RG, Georgoulias VA, et al.: Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol 22 (19): 3893-901, 2004. [PUBMED Abstract]
- Sparano JA, Makhson AN, Semiglazov VF, et al.: Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: results from a randomized phase III study. J Clin Oncol 27 (27): 4522-9, 2009. [PUBMED Abstract]
- Seidman AD, Berry D, Cirrincione C, et al.: Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26 (10): 1642-9, 2008. [PUBMED Abstract]
- Gonzalez-Angulo AM, Hortobagyi GN: Optimal schedule of paclitaxel: weekly is better. J Clin Oncol 26 (10): 1585-7, 2008. [PUBMED Abstract]
- Gradishar WJ, Tjulandin S, Davidson N, et al.: Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23 (31): 7794-803, 2005. [PUBMED Abstract]
- Ibrahim NK, Samuels B, Page R, et al.: Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol 23 (25): 6019-26, 2005. [PUBMED Abstract]
- Blum JL, Jones SE, Buzdar AU, et al.: Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17 (2): 485-93, 1999. [PUBMED Abstract]
- Blum JL, Dieras V, Lo Russo PM, et al.: Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 92 (7): 1759-68, 2001. [PUBMED Abstract]
- Venturini M, Paridaens R, Rossner D, et al.: An open-label, multicenter study of outpatient capecitabine monotherapy in 631 patients with pretreated advanced breast cancer. Oncology 72 (1-2): 51-7, 2007. [PUBMED Abstract]
- Degardin M, Bonneterre J, Hecquet B, et al.: Vinorelbine (navelbine) as a salvage treatment for advanced breast cancer. Ann Oncol 5 (5): 423-6, 1994. [PUBMED Abstract]
- Carmichael J, Walling J: Advanced breast cancer: investigational role of gemcitabine. Eur J Cancer 33 (Suppl 1): S27-30, 1997. [PUBMED Abstract]
- Vahdat LT, Pruitt B, Fabian CJ, et al.: Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 27 (18): 2954-61, 2009. [PUBMED Abstract]
- Cortes J, O'Shaughnessy J, Loesch D, et al.: Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377 (9769): 914-23, 2011. [PUBMED Abstract]
- Smith JW, Vukelja S, Rabe A, et al.: Phase II randomized trial of weekly and every-3-week ixabepilone in metastatic breast cancer patients. Breast Cancer Res Treat 142 (2): 381-8, 2013. [PUBMED Abstract]
- Tranum BL, McDonald B, Thigpen T, et al.: Adriamycin combinations in advanced breast cancer. A Southwest Oncology Group Study. Cancer 49 (5): 835-9, 1982. [PUBMED Abstract]
- Langley RE, Carmichael J, Jones AL, et al.: Phase III trial of epirubicin plus paclitaxel compared with epirubicin plus cyclophosphamide as first-line chemotherapy for metastatic breast cancer: United Kingdom National Cancer Research Institute trial AB01. J Clin Oncol 23 (33): 8322-30, 2005. [PUBMED Abstract]
- Misset JL, Dieras V, Gruia G, et al.: Dose-finding study of docetaxel and doxorubicin in first-line treatment of patients with metastatic breast cancer. Ann Oncol 10 (5): 553-60, 1999. [PUBMED Abstract]
- Buzdar AU, Kau SW, Smith TL, et al.: Ten-year results of FAC adjuvant chemotherapy trial in breast cancer. Am J Clin Oncol 12 (2): 123-8, 1989. [PUBMED Abstract]
- Tormey DC, Gelman R, Band PR, et al.: Comparison of induction chemotherapies for metastatic breast cancer. An Eastern Cooperative Oncology Group Trial. Cancer 50 (7): 1235-44, 1982. [PUBMED Abstract]
- Jassem J, Pieńkowski T, Płuzańska A, et al.: Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first-line therapy for women with metastatic breast cancer: final results of a randomized phase III multicenter trial. J Clin Oncol 19 (6): 1707-15, 2001. [PUBMED Abstract]
- Biganzoli L, Cufer T, Bruning P, et al.: Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: The European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. J Clin Oncol 20 (14): 3114-21, 2002. [PUBMED Abstract]
- O'Shaughnessy J, Miles D, Vukelja S, et al.: Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 20 (12): 2812-23, 2002. [PUBMED Abstract]
- Serin D, Verrill M, Jones A, et al.: Vinorelbine alternating oral and intravenous plus epirubicin in first-line therapy of metastatic breast cancer: results of a multicentre phase II study. Br J Cancer 92 (11): 1989-96, 2005. [PUBMED Abstract]
- Thomas ES, Gomez HL, Li RK, et al.: Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 25 (33): 5210-7, 2007. [PUBMED Abstract]
- O'Shaughnessy J, Schwartzberg L, Danso MA, et al.: Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol 32 (34): 3840-7, 2014. [PUBMED Abstract]
- Albain KS, Nag SM, Calderillo-Ruiz G, et al.: Gemcitabine plus Paclitaxel versus Paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol 26 (24): 3950-7, 2008. [PUBMED Abstract]
- Sledge GW, Neuberg D, Bernardo P, et al.: Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol 21 (4): 588-92, 2003. [PUBMED Abstract]
- Seidman AD: Sequential single-agent chemotherapy for metastatic breast cancer: therapeutic nihilism or realism? J Clin Oncol 21 (4): 577-9, 2003. [PUBMED Abstract]
- Overmoyer B: Combination chemotherapy for metastatic breast cancer: reaching for the cure. J Clin Oncol 21 (4): 580-2, 2003. [PUBMED Abstract]
- Honig SF: Hormonal therapy and chemotherapy. In: Harris JR, Morrow M, Lippman ME, et al., eds.: Diseases of the Breast. Lippincott-Raven Publishers. 1996, pp 669-734.
- Perez EA: Current management of metastatic breast cancer. Semin Oncol 26 (4 Suppl 12): 1-10, 1999. [PUBMED Abstract]
- Jones D, Ghersi D, Wilcken N: Addition of drug/s to a chemotherapy regimen for metastatic breast cancer. Cochrane Database Syst Rev 3: CD003368, 2006. [PUBMED Abstract]
- Falkson G, Gelman RS, Pandya KJ, et al.: Eastern Cooperative Oncology Group randomized trials of observation versus maintenance therapy for patients with metastatic breast cancer in complete remission following induction treatment. J Clin Oncol 16 (5): 1669-76, 1998. [PUBMED Abstract]
- Peters WP, Jones RB, Vrendenburgh J, et al.: A large, prospective, randomized trial of high-dose combination alkylating agents (CPB) with autologous cellular support (ABMS) as consolidation for patients with metastatic breast cancer achieving complete remission after intensive doxorubicin-based induction therapy (AFM). [Abstract] Proceedings of the American Society of Clinical Oncology 15: A-149, 121, 1996.
- Muss HB, Case LD, Richards F, et al.: Interrupted versus continuous chemotherapy in patients with metastatic breast cancer. The Piedmont Oncology Association. N Engl J Med 325 (19): 1342-8, 1991. [PUBMED Abstract]
- Falkson G, Gelman RS, Glick J, et al.: Metastatic breast cancer: higher versus low dose maintenance treatment when only a partial response or a no change status is obtained following doxorubicin induction treatment. An Eastern Cooperative Oncology Group study. Ann Oncol 3 (9): 768-70, 1992. [PUBMED Abstract]
- Park YH, Jung KH, Im SA, et al.: Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol 31 (14): 1732-9, 2013. [PUBMED Abstract]
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PUBMED Abstract]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PUBMED Abstract]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PUBMED Abstract]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PUBMED Abstract]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PUBMED Abstract]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PUBMED Abstract]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PUBMED Abstract]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PUBMED Abstract]
- Swain SM, Whaley FS, Gerber MC, et al.: Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol 15 (4): 1333-40, 1997. [PUBMED Abstract]
- Swain SM, Whaley FS, Gerber MC, et al.: Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol 15 (4): 1318-32, 1997. [PUBMED Abstract]
- Hensley ML, Schuchter LM, Lindley C, et al.: American Society of Clinical Oncology clinical practice guidelines for the use of chemotherapy and radiotherapy protectants. J Clin Oncol 17 (10): 3333-55, 1999. [PUBMED Abstract]
- Marty M, Espié M, Llombart A, et al.: Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol 17 (4): 614-22, 2006. [PUBMED Abstract]
- Hortobagyi GN, Frye D, Buzdar AU, et al.: Decreased cardiac toxicity of doxorubicin administered by continuous intravenous infusion in combination chemotherapy for metastatic breast carcinoma. Cancer 63 (1): 37-45, 1989. [PUBMED Abstract]
- Hensley ML, Hagerty KL, Kewalramani T, et al.: American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol 27 (1): 127-45, 2009. [PUBMED Abstract]
- Venturini M, Michelotti A, Del Mastro L, et al.: Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. J Clin Oncol 14 (12): 3112-20, 1996. [PUBMED Abstract]
- Khan SA, Zhao F, Goldstein LJ, et al.: Early Local Therapy for the Primary Site in De Novo Stage IV Breast Cancer: Results of a Randomized Clinical Trial (EA2108). J Clin Oncol 40 (9): 978-987, 2022. [PUBMED Abstract]
- Hartsell WF, Scott CB, Bruner DW, et al.: Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 97 (11): 798-804, 2005. [PUBMED Abstract]
- Porter AT, McEwan AJ, Powe JE, et al.: Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 25 (5): 805-13, 1993. [PUBMED Abstract]
- Quilty PM, Kirk D, Bolger JJ, et al.: A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol 31 (1): 33-40, 1994. [PUBMED Abstract]
- Hillner BE, Ingle JN, Chlebowski RT, et al.: American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 21 (21): 4042-57, 2003. [PUBMED Abstract]
- Paterson AH, Powles TJ, Kanis JA, et al.: Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol 11 (1): 59-65, 1993. [PUBMED Abstract]
- Hortobagyi GN, Theriault RL, Lipton A, et al.: Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol 16 (6): 2038-44, 1998. [PUBMED Abstract]
- Powles T, Paterson A, McCloskey E, et al.: Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res 8 (2): R13, 2006. [PUBMED Abstract]
- Rosen LS, Gordon D, Kaminski M, et al.: Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 98 (8): 1735-44, 2003. [PUBMED Abstract]
- Himelstein AL, Foster JC, Khatcheressian JL, et al.: Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. JAMA 317 (1): 48-58, 2017. [PUBMED Abstract]
- Lipton A, Fizazi K, Stopeck AT, et al.: Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 48 (16): 3082-92, 2012. [PUBMED Abstract]
Treatment of Ductal Carcinoma In Situ
Introduction
Ductal carcinoma in situ (DCIS) is a noninvasive condition. DCIS can progress to invasive cancer, but estimates of the probability of this vary widely. Some reports include DCIS in breast cancer statistics. In 2025, DCIS is expected to account for about 16% of all newly diagnosed invasive plus noninvasive breast tumors in the United States.[1] For invasive and noninvasive tumors detected by screening, DCIS accounts for approximately 25% of all cases.
The frequency of a DCIS diagnosis has increased markedly in the United States since the use of screening mammography became widespread. Very few cases of DCIS present as a palpable mass, with more than 90% being diagnosed by mammography alone.[2]
DCIS comprises a heterogeneous group of histopathological lesions that have been classified into the following subtypes primarily because of architectural pattern:
- Micropapillary.
- Papillary.
- Solid.
- Cribriform.
- Comedo.
Comedo-type DCIS consists of cells that appear cytologically malignant, with the presence of high-grade nuclei, pleomorphism, and abundant central luminal necrosis. Comedo-type DCIS appears to be more aggressive, with a higher probability of associated invasive ductal carcinoma.[3]
Treatment Options for DCIS
Treatment options for DCIS include:
- Breast-conserving surgery or mastectomy plus radiation therapy with or without tamoxifen.
- Total mastectomy with or without tamoxifen.
In the past, the customary treatment for DCIS was mastectomy.[4] The rationale for mastectomy included a 30% incidence of multicentric disease, a 40% prevalence of residual tumor at mastectomy after wide excision alone, and a 25% to 50% incidence of in-breast recurrence after limited surgery for palpable tumor, with 50% of those recurrences being invasive carcinoma.[4,5] The combined local and distant recurrence rate after mastectomy is 1% to 2%. No randomized comparisons of mastectomy versus breast-conserving surgery plus breast radiation therapy are available.
Because breast-conserving surgery combined with breast radiation therapy is successful for invasive carcinoma, this conservative approach was extended to DCIS. To determine whether breast-conserving surgery plus radiation therapy was a reasonable approach to the management of DCIS, the National Surgical Adjuvant Breast and Bowel Project (NSABP) and the European Organisation for Research and Treatment of Cancer (EORTC) have each completed prospective randomized trials in which women with localized DCIS and negative surgical margins after excisional biopsy were randomly assigned to receive either breast radiation therapy (50 Gy) or no further therapy.[6-9] To date, no randomized controlled trial has found a survival benefit for DCIS treated with either adjuvant radiation therapy or adjuvant hormonal therapy, although both have been found to reduce recurrence risk.
Evidence (breast-conserving surgery plus radiation therapy to the breast):
- Of the 818 women enrolled in the NSABP-B-17 trial, 80% were diagnosed by mammography, and 70% of the patients' lesions were 1 cm or smaller. Results were reported at the 12-year actuarial follow-up interval.[7]; [9][Level of evidence B1]
- The overall rate of in-breast tumor recurrence was reduced from 31.7% to 15.7% when radiation therapy was delivered (P < .005).
- Radiation therapy reduced the occurrence of invasive cancer from 16.8% to 7.7% (P = .001) and recurrent DCIS from 14.6% to 8.0% (P = .001).
- Nine pathological features were evaluated for their ability to predict for in-breast recurrence, but only comedo necrosis was a significant predictor for recurrence.
- Similarly, of the 1,010 patients enrolled in the EORTC-10853 trial, mammography detected lesions in 71% of the women. Results were reported at a median follow-up of 10.5 years.[9][Level of evidence B1]
- The overall rate of in-breast tumor recurrence was reduced from 26% to 15% (P < .001), with a similarly effective reduction of invasive recurrence rates (13% to 8%, P = .065) and noninvasive recurrence rates (14% to 7%, P = .001).
- In this analysis, parameters associated with an increased risk of in-breast recurrence included age 40 years or younger, palpable disease, intermediate or poorly differentiated DCIS, cribriform or solid growth pattern, and indeterminate margins. Elsewhere, margins of less than 1 mm have been associated with an unacceptable local recurrence rate, even with radiation therapy.[10]
In both studies, the effect of radiation therapy was consistent across all assessed risk factors.
- The benefit of administering radiation therapy has been confirmed in a systematic review of four randomized trials (hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.41–0.58; P < .00001). In this study, the number needed to treat with radiation therapy was nine women to prevent one ipsilateral breast recurrence.[11]
- A large national
clinical trial by the Radiation Therapy Oncology Group (RTOG-9804 [NCT00003857]) comparing breast-conserving surgery and tamoxifen with or
without radiation therapy was closed because of poor accrual (636 of planned 1,790 patients accrued). Patients with good-risk DCIS (defined as mammographically detected low- or intermediate-grade DCIS, measuring <2.5 cm with margins of ≥3 mm) were enrolled.[12]
- The 15-year cumulative incidence of in-breast recurrence was 7.1% (95% CI, 4.0%–11.5%) with radiation therapy versus 15.1% (95% CI, 10.8%–20.2%) with observation (HR, 0.36; 95% CI, 0.20–0.66; P = .0007). On multivariable analysis, only radiation therapy (HR, 0.34; 95% CI, 0.19–0.64; P = .0007) and tamoxifen use (HR, 0.45; 95% CI, 0.25–0.78; P = .0047) were associated with reduced in-breast recurrence.[13]
The results of the NSABP-B-17 and EORTC-10853 trials plus two others were included in a meta-analysis that demonstrated reductions in all ipsilateral breast events (HR, 0.49; 95% CI, 0.41–0.58; P < .00001), ipsilateral invasive recurrence (HR, 0.50; 95% CI, 0.32–0.76; P = .001), and ipsilateral DCIS recurrence (HR, 0.61; 95% CI, 0.39–0.95; P = .03).[14][Level of evidence B1] However, after 10 years of follow-up, there was no significant effect on breast cancer mortality, mortality from causes other than breast cancer, or all-cause mortality.[11]
To identify a favorable group of patients for whom postoperative radiation therapy could be omitted, several pathological staging systems have been developed and tested retrospectively, but consensus recommendations have not been achieved.[15-18]
The Van Nuys Prognostic Index is one pathological staging system that combines three predictors of local recurrence (i.e., tumor size, margin width, and pathological classification). It was used to retrospectively analyze 333 patients treated with either excision alone or excision and radiation therapy.[18] Using this prognostic index, patients with favorable lesions who received surgical excision alone had a low recurrence rate (i.e., 2%, with a median follow-up of 79 months). A subsequent analysis of these data was performed to determine the influence of margin width on local control.[19] Patients whose excised lesions had margin widths of 10 mm or more in every direction had an extremely low probability of local recurrence with surgery alone (4%, with a mean follow-up of 8 years).
Both reviews are retrospective, noncontrolled, and subject to substantial selection bias. In contrast, the prospective NSABP trial did not identify any subset of patients who did not benefit from the addition of radiation therapy to breast-conserving surgery in the management of DCIS.[3,6,14,20]
To determine whether tamoxifen adds to the efficacy of local therapy in the management of DCIS, the NSABP performed a double-blind prospective trial (NSABP-B-24).
Evidence (adjuvant endocrine therapy):
- In NSABP-B-24, 1,804 women were randomly assigned to receive
breast-conserving surgery, radiation therapy (50 Gy), and placebo or breast-conserving surgery, radiation
therapy, and tamoxifen (20 mg qd for 5 years).[21] Positive or unknown
surgical margins were present in 23% of patients. Approximately 80% of the
lesions measured 1 cm or smaller, and more than 80% were detected
mammographically. Breast cancer events were defined as the presence of new
ipsilateral disease, contralateral disease, or metastases.
- Women in the tamoxifen group had fewer breast cancer events at 5 years than did those treated with a placebo (8.2% vs. 13.4%; P = .009).[21][Level of evidence B1]
- With tamoxifen, ipsilateral invasive breast cancer decreased from 4.2% to 2.1% at 5 years (P = .03).
- Tamoxifen also decreased the incidence of contralateral breast neoplasms (invasive and noninvasive) from 0.8% per year to 0.4% per year (P = .01).
- The benefit of tamoxifen extended to patients with positive or uncertain margins.[22] For more information, see Breast Cancer Prevention.
- No survival advantage was demonstrated for the use of tamoxifen.
- In the NSABP-B35 double-blind study, 3,104 postmenopausal women with DCIS who were treated with breast-conserving surgery were randomly assigned to receive either adjuvant tamoxifen or anastrozole, in addition to adjuvant radiation therapy.
- The use of anastrozole was associated with significantly fewer breast cancer events (HR, 0.73; P = .023) but no improvement in survival.[23][Level of evidence B1]
- The Second International Breast Cancer Intervention Study (IBIS II DCIS [NCT00078832]) enrolled 2,980 postmenopausal women in a double-blind comparison of tamoxifen with anastrozole as adjuvant therapy. All of the women had breast-conserving surgery, and 71% of them had radiation therapy.[24]
- No difference in the rate of breast cancer recurrence in favor of anastrozole was found (HR, 0.89; 95% CI, 0.64–1.23; P = .49), and there was no difference in survival.
The decision to prescribe endocrine therapy after a diagnosis of DCIS often involves a discussion with the patient about the potential benefits and side effects of each agent.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Siegel R, Ward E, Brawley O, et al.: Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61 (4): 212-36, 2011 Jul-Aug. [PUBMED Abstract]
- Fisher ER, Dignam J, Tan-Chiu E, et al.: Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer 86 (3): 429-38, 1999. [PUBMED Abstract]
- Fonseca R, Hartmann LC, Petersen IA, et al.: Ductal carcinoma in situ of the breast. Ann Intern Med 127 (11): 1013-22, 1997. [PUBMED Abstract]
- Lagios MD, Westdahl PR, Margolin FR, et al.: Duct carcinoma in situ. Relationship of extent of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer 50 (7): 1309-14, 1982. [PUBMED Abstract]
- Fisher B, Dignam J, Wolmark N, et al.: Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 16 (2): 441-52, 1998. [PUBMED Abstract]
- Fisher B, Land S, Mamounas E, et al.: Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the national surgical adjuvant breast and bowel project experience. Semin Oncol 28 (4): 400-18, 2001. [PUBMED Abstract]
- Julien JP, Bijker N, Fentiman IS, et al.: Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet 355 (9203): 528-33, 2000. [PUBMED Abstract]
- Bijker N, Meijnen P, Peterse JL, et al.: Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol 24 (21): 3381-7, 2006. [PUBMED Abstract]
- Chan KC, Knox WF, Sinha G, et al.: Extent of excision margin width required in breast conserving surgery for ductal carcinoma in situ. Cancer 91 (1): 9-16, 2001. [PUBMED Abstract]
- Correa C, McGale P, Taylor C, et al.: Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr 2010 (41): 162-77, 2010. [PUBMED Abstract]
- McCormick B, Winter K, Hudis C, et al.: RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol 33 (7): 709-15, 2015. [PUBMED Abstract]
- McCormick B, Winter KA, Woodward W, et al.: Randomized Phase III Trial Evaluating Radiation Following Surgical Excision for Good-Risk Ductal Carcinoma In Situ: Long-Term Report From NRG Oncology/RTOG 9804. J Clin Oncol 39 (32): 3574-3582, 2021. [PUBMED Abstract]
- Goodwin A, Parker S, Ghersi D, et al.: Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev 11: CD000563, 2013. [PUBMED Abstract]
- Page DL, Lagios MD: Pathologic analysis of the National Surgical Adjuvant Breast Project (NSABP) B-17 Trial. Unanswered questions remaining unanswered considering current concepts of ductal carcinoma in situ. Cancer 75 (6): 1219-22; discussion 1223-7, 1995. [PUBMED Abstract]
- Fisher ER, Costantino J, Fisher B, et al.: Response - blunting the counterpoint. Cancer 75 (6): 1223-1227, 1995.
- Holland R, Peterse JL, Millis RR, et al.: Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol 11 (3): 167-80, 1994. [PUBMED Abstract]
- Silverstein MJ, Lagios MD, Craig PH, et al.: A prognostic index for ductal carcinoma in situ of the breast. Cancer 77 (11): 2267-74, 1996. [PUBMED Abstract]
- Silverstein MJ, Lagios MD, Groshen S, et al.: The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med 340 (19): 1455-61, 1999. [PUBMED Abstract]
- Goodwin A, Parker S, Ghersi D, et al.: Post-operative radiotherapy for ductal carcinoma in situ of the breast--a systematic review of the randomised trials. Breast 18 (3): 143-9, 2009. [PUBMED Abstract]
- Fisher B, Dignam J, Wolmark N, et al.: Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 353 (9169): 1993-2000, 1999. [PUBMED Abstract]
- Houghton J, George WD, Cuzick J, et al.: Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet 362 (9378): 95-102, 2003. [PUBMED Abstract]
- Margolese RG, Cecchini RS, Julian TB, et al.: Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet 387 (10021): 849-56, 2016. [PUBMED Abstract]
- Forbes JF, Sestak I, Howell A, et al.: Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet 387 (10021): 866-73, 2016. [PUBMED Abstract]
Latest Updates to This Summary (04/25/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Surgical Treatment for Breast Cancer
Added text to state that, although sentinel lymph node (SLN) biopsy has been the standard for the axillary staging of patients with invasive breast cancer and a clinically negative axilla, two randomized controlled trials have identified populations for which SLN biopsy could be omitted. The SOUND trial randomly assigned 1,493 women with invasive breast cancer to undergo SLN biopsy or no axillary surgery. Patients were of any age, had tumors smaller than 2 cm, had negative preoperative axillary ultrasonography, and planned to receive breast-conserving surgery and adjuvant radiation therapy (cited Gentilini et al. as reference 34 and level of evidence A1). The INSEMA trial randomly assigned 5,502 women with clinically node-negative invasive breast cancer to undergo no axillary surgery or SLN biopsy. Tumors had to be smaller than 5 cm, and most patients had T1 disease and estrogen receptor–positive tumors. Patients were scheduled to undergo breast-conserving surgery and whole-breast radiation therapy (cited Reimer et al. as reference 35 and level of evidence A1).
Systemic Therapy for Stages I, II, and III Breast Cancer
Added text about an open-label trial in Japan that included 1,697 patients who had received either (1) 5 years of anastrozole or (2) 2 to 3 years of tamoxifen, followed by 2 to 3 years of anastrozole. Patients were randomly assigned to either discontinue anastrozole or continue it for 5 years (cited Iwase et al. as reference 88 and level of evidence B1).
Revised text about a trial that examined the effect of adding abemaciclib to standard endocrine therapy in women with HER2-negative hormone receptor–positive breast cancer who were at high risk of recurrence. Similar results were found in a prespecified subgroup analysis of patients who had received neoadjuvant chemotherapy and had residual disease after surgery (cited Martin et al. as reference 91).
Added Immunotherapy as a new subsection in the Postoperative therapy for triple-negative breast cancer section.
Revised text about the results of a single-arm trial that evaluated paclitaxel and trastuzumab in 410 women with node-negative, small, HER2-positive tumors (cited Tolaney et al. as reference 145).
Treatment of Metastatic Breast Cancer
Revised text about the results of a randomized, double-blind, phase III trial that evaluated first-line abemaciclib or placebo plus a nonsteroidal aromatase inhibitor in 493 postmenopausal women with HER2-negative hormone receptor–positive, advanced breast cancer (cited Goetz et al. as reference 20 and level of evidence B1).
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult breast cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Breast Cancer Treatment are:
- Fumiko Chino, MD (MD Anderson Cancer Center)
- Tarek Hijal, MD (McGill University Health Centre)
- Joseph L. Pater, MD (NCIC-Clinical Trials Group)
- Carol Tweed, MD (Maryland Oncology Hematology)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Breast Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/breast/hp/breast-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389406]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.