Rectal Cancer Treatment (PDQ®)–Health Professional Version
General Information About Rectal Cancer
Incidence and Mortality
It is difficult to separate epidemiological considerations of rectal cancer from those of colon cancer because studies often consider colon and rectal cancer together (i.e., colorectal cancer).
Worldwide, colorectal cancer is the third most common form of cancer. In 2022, there were an estimated 1.93 million new cases of colorectal cancer and 903,859 deaths.[1]
Estimated new cases and deaths from rectal and colon cancer in the United States in 2025:[2]
- New cases of rectal cancer: 46,950.
- New cases of colon cancer: 107,320.
- Deaths: 52,900 (rectal and colon cancers combined).
Colorectal cancer affects men and women almost equally. Among all racial groups in the United States, Black individuals have the highest sporadic colorectal cancer incidence and mortality rates.[3,4]
Anatomy
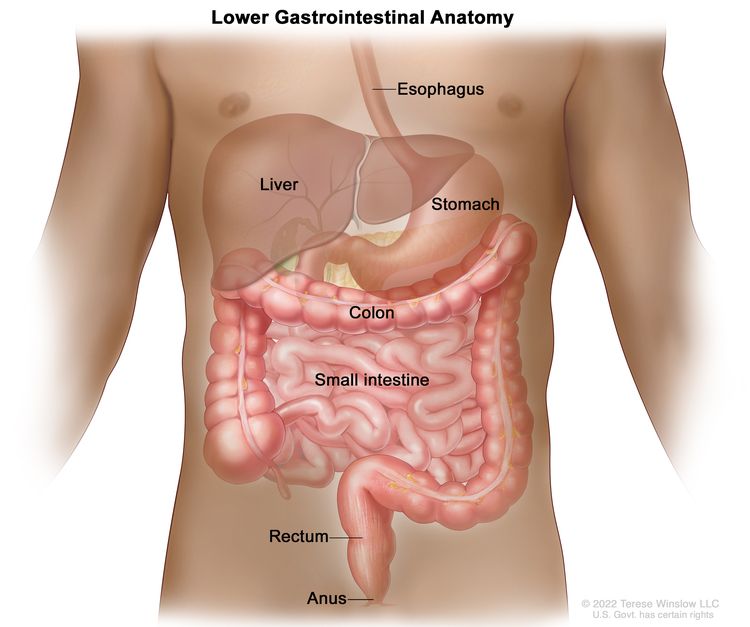
The rectum is located within the pelvis, extending from the transitional mucosa of the anal dentate line to the sigmoid colon at the peritoneal reflection. By rigid sigmoidoscopy, the rectum measures between 10 cm and 15 cm from the anal verge.[5] The location of a rectal tumor is usually indicated by the distance between the anal verge, dentate line, or anorectal ring and the lower edge of the tumor, with measurements differing depending on the use of a rigid or flexible endoscope or digital examination.[6]
The distance of the tumor from the anal sphincter musculature has implications for the ability to perform sphincter-sparing surgery. The bony constraints of the pelvis limit surgical access to the rectum, which results in a lower likelihood of attaining widely negative margins and a higher risk of local recurrence.[5]
Risk Factors
Increasing age is the most important risk factor for most cancers. Other risk factors for colorectal cancer include the following:
- Family history of colorectal cancer in a first-degree relative.[7]
- Personal history of colorectal adenomas, colorectal cancer, or ovarian cancer.[8-10]
- Hereditary conditions, including familial adenomatous polyposis (FAP) and Lynch syndrome (hereditary nonpolyposis colorectal cancer [HNPCC]).[11]
- Personal history of long-standing chronic ulcerative colitis or Crohn colitis.[12]
- Excessive alcohol use.[13]
- Cigarette smoking.[14]
- Race and ethnicity: African American.[15,16]
- Obesity.[17]
Screening
Evidence supports screening for rectal cancer as a part of routine care for all adults aged 50 years and older, especially for those with first-degree relatives with colorectal cancer. Reasons include the following:
- Incidence of the disease in adults 50 years and older.
- Ability to identify high-risk groups.
- Slow growth of primary lesions.
- Better survival of patients with early-stage lesions.
- Relative simplicity and accuracy of screening tests.
For more information, see Colorectal Cancer Screening.
Clinical Features
Similar to colon cancer, symptoms of rectal cancer may include:[18]
- Rectal bleeding.
- Change in bowel habits.
- Abdominal pain.
- Intestinal obstruction.
- Change in appetite.
- Weight loss.
- Weakness.
With the exception of obstructive symptoms, these symptoms do not necessarily correlate with the stage of disease or signify a particular diagnosis.[19]
Diagnostic Evaluation
The initial clinical evaluation may include:
- Physical exam and history.
- Digital rectal exam.
- Colonoscopy.
- Biopsy.
- Carcinoembryonic antigen (CEA) assay.
- Immunohistochemistry.
- DNA mismatch repair/microsatellite instability (MSI) testing.
Physical examination may reveal a palpable mass and bright blood in the rectum. Adenopathy, hepatomegaly, or pulmonary signs may be present with metastatic disease.[6] Laboratory examination may reveal iron-deficiency anemia and electrolyte and liver function abnormalities.
Prognostic Factors
The prognosis of patients with rectal cancer is related to several factors, including:[6,20-28]
- Tumor adherence to or invasion of adjacent organs.[20]
- Presence or absence of tumor involvement in the lymph nodes and the number of positive lymph nodes.[6,21-24]
- Presence or absence of distant metastases.[6,20]
- Perforation or obstruction of the bowel.[6,28]
- Presence or absence of high-risk pathological features, including:[26,27,29]
- Positive surgical margins.
- Lymphovascular invasion.
- Perineural invasion.
- Poorly differentiated histology.
- Circumferential resection margin (CRM) or depth of penetration of the tumor through the bowel wall.[6,25,30] Measured in millimeters, CRM is defined as the retroperitoneal or peritoneal adventitial soft-tissue margin closest to the deepest penetration of the tumor.
- Presence of MSI that results from impaired DNA mismatch repair.
Only disease stage (designated by tumor [T], nodal status [N], and distant metastasis [M]) has been validated as a prognostic factor in multi-institutional prospective studies.[20-25] A major pooled analysis evaluating the impact of T and N stage and treatment on survival and relapse in patients with rectal cancer who are treated with adjuvant therapy confirmed these findings.[31]
Mismatch repair deficiency occurs in 5% to 10% of patients with rectal adenocarcinomas. Mismatch repair–deficient tumors do not respond well to chemotherapy applied in the neoadjuvant, adjuvant, or metastatic settings.[32-34] In a population-based series of 607 patients aged 50 years or younger at the time of diagnosis, MSI-related colorectal cancer was associated with improved survival that was independent of tumor stage. MSI is also associated with Lynch syndrome.[35] In addition, gene expression profiling is useful for predicting the response of rectal adenocarcinomas to preoperative chemoradiation therapy. It can also help determine the prognosis of stages II and III rectal cancer after neoadjuvant fluorouracil-based chemoradiation therapy.[36,37]
Racial and ethnic differences in overall survival (OS) after adjuvant therapy for rectal cancer have been observed, with shorter OS for Black patients than for White patients. Factors contributing to this disparity may include tumor position, type of surgical procedure, and presence of comorbid conditions.[38]
Follow-Up After Treatment
The primary goals of postoperative surveillance programs for rectal cancer are to:[39]
- Assess the efficacy of initial therapy.
- Detect new or metachronous malignancies.
- Detect potentially curable recurrent or metastatic cancers.
Routine, periodic studies following treatment for rectal cancer may lead to earlier identification and management of recurrent disease.[39-43] A statistically significant survival benefit has been demonstrated for more intensive follow-up protocols in two clinical trials. A meta-analysis that combined these two trials with four others reported a statistically significant improvement in survival for patients who were intensively followed.[39,44,45]
Guidelines for surveillance after initial treatment with curative intent for colorectal cancer vary between leading U.S. and European oncology societies, and optimal surveillance strategies remain uncertain.[46,47] Large, well-designed, prospective, multi-institutional, randomized studies are required to establish an evidence-based consensus for follow-up evaluation.
Carcinoembryonic antigen (CEA)
Measurement of CEA, a serum glycoprotein, is frequently used in the management and follow-up of patients with rectal cancer. A review of the use of this tumor marker for rectal cancer suggests the following:[39]
- Serum CEA testing is not a valuable screening tool for rectal cancer because of its low sensitivity and low specificity.
- Postoperative CEA testing is typically restricted to patients who are potential candidates for further intervention, as follows:
- Patients with stage II or III rectal cancer (every 2–3 months for at least 2 years after diagnosis).
- Patients with rectal cancer who would be candidates for resection of liver metastases.
In one Dutch retrospective study of total mesorectal excision for the treatment of rectal cancer, investigators found that the preoperative serum CEA level was normal in most patients with rectal cancer, and yet, serum CEA levels rose by at least 50% in patients with recurrence. The authors concluded that serial, postoperative CEA testing cannot be discarded based on a normal preoperative serum CEA level in patients with rectal cancer.[48,49]
References
- Bray F, Laversanne M, Sung H, et al.: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74 (3): 229-263, 2024. [PUBMED Abstract]
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Albano JD, Ward E, Jemal A, et al.: Cancer mortality in the United States by education level and race. J Natl Cancer Inst 99 (18): 1384-94, 2007. [PUBMED Abstract]
- Kauh J, Brawley OW, Berger M: Racial disparities in colorectal cancer. Curr Probl Cancer 31 (3): 123-33, 2007 May-Jun. [PUBMED Abstract]
- Wolpin BM, Meyerhardt JA, Mamon HJ, et al.: Adjuvant treatment of colorectal cancer. CA Cancer J Clin 57 (3): 168-85, 2007 May-Jun. [PUBMED Abstract]
- Libutti SK, Willett CG, Saltz LB: Cancer of the rectum. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 1127-41.
- Johns LE, Houlston RS: A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 96 (10): 2992-3003, 2001. [PUBMED Abstract]
- Imperiale TF, Juluri R, Sherer EA, et al.: A risk index for advanced neoplasia on the second surveillance colonoscopy in patients with previous adenomatous polyps. Gastrointest Endosc 80 (3): 471-8, 2014. [PUBMED Abstract]
- Singh H, Nugent Z, Demers A, et al.: Risk of colorectal cancer after diagnosis of endometrial cancer: a population-based study. J Clin Oncol 31 (16): 2010-5, 2013. [PUBMED Abstract]
- Srinivasan R, Yang YX, Rubin SC, et al.: Risk of colorectal cancer in women with a prior diagnosis of gynecologic malignancy. J Clin Gastroenterol 41 (3): 291-6, 2007. [PUBMED Abstract]
- Mork ME, You YN, Ying J, et al.: High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J Clin Oncol 33 (31): 3544-9, 2015. [PUBMED Abstract]
- Laukoetter MG, Mennigen R, Hannig CM, et al.: Intestinal cancer risk in Crohn's disease: a meta-analysis. J Gastrointest Surg 15 (4): 576-83, 2011. [PUBMED Abstract]
- Fedirko V, Tramacere I, Bagnardi V, et al.: Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol 22 (9): 1958-72, 2011. [PUBMED Abstract]
- Liang PS, Chen TY, Giovannucci E: Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 124 (10): 2406-15, 2009. [PUBMED Abstract]
- Laiyemo AO, Doubeni C, Pinsky PF, et al.: Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst 102 (8): 538-46, 2010. [PUBMED Abstract]
- Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, et al.: Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol Biomarkers Prev 21 (5): 728-36, 2012. [PUBMED Abstract]
- Ma Y, Yang Y, Wang F, et al.: Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 8 (1): e53916, 2013. [PUBMED Abstract]
- Stein W, Farina A, Gaffney K, et al.: Characteristics of colon cancer at time of presentation. Fam Pract Res J 13 (4): 355-63, 1993. [PUBMED Abstract]
- Majumdar SR, Fletcher RH, Evans AT: How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol 94 (10): 3039-45, 1999. [PUBMED Abstract]
- Compton CC, Greene FL: The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin 54 (6): 295-308, 2004 Nov-Dec. [PUBMED Abstract]
- Swanson RS, Compton CC, Stewart AK, et al.: The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 10 (1): 65-71, 2003 Jan-Feb. [PUBMED Abstract]
- Le Voyer TE, Sigurdson ER, Hanlon AL, et al.: Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 21 (15): 2912-9, 2003. [PUBMED Abstract]
- Prandi M, Lionetto R, Bini A, et al.: Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg 235 (4): 458-63, 2002. [PUBMED Abstract]
- Tepper JE, O'Connell MJ, Niedzwiecki D, et al.: Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 19 (1): 157-63, 2001. [PUBMED Abstract]
- Balch GC, De Meo A, Guillem JG: Modern management of rectal cancer: a 2006 update. World J Gastroenterol 12 (20): 3186-95, 2006. [PUBMED Abstract]
- Weiser MR, Landmann RG, Wong WD, et al.: Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum 48 (6): 1169-75, 2005. [PUBMED Abstract]
- Fujita S, Nakanisi Y, Taniguchi H, et al.: Cancer invasion to Auerbach's plexus is an important prognostic factor in patients with pT3-pT4 colorectal cancer. Dis Colon Rectum 50 (11): 1860-6, 2007. [PUBMED Abstract]
- Griffin MR, Bergstralh EJ, Coffey RJ, et al.: Predictors of survival after curative resection of carcinoma of the colon and rectum. Cancer 60 (9): 2318-24, 1987. [PUBMED Abstract]
- DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011.
- Wieder HA, Rosenberg R, Lordick F, et al.: Rectal cancer: MR imaging before neoadjuvant chemotherapy and radiation therapy for prediction of tumor-free circumferential resection margins and long-term survival. Radiology 243 (3): 744-51, 2007. [PUBMED Abstract]
- Gunderson LL, Sargent DJ, Tepper JE, et al.: Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol 22 (10): 1785-96, 2004. [PUBMED Abstract]
- Le DT, Uram JN, Wang H, et al.: PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372 (26): 2509-20, 2015. [PUBMED Abstract]
- Overman MJ, Lonardi S, Wong KYM, et al.: Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 36 (8): 773-779, 2018. [PUBMED Abstract]
- André T, Shiu KK, Kim TW, et al.: Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 383 (23): 2207-2218, 2020. [PUBMED Abstract]
- Gryfe R, Kim H, Hsieh ET, et al.: Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 342 (2): 69-77, 2000. [PUBMED Abstract]
- Liersch T, Langer C, Ghadimi BM, et al.: Lymph node status and TS gene expression are prognostic markers in stage II/III rectal cancer after neoadjuvant fluorouracil-based chemoradiotherapy. J Clin Oncol 24 (25): 4062-8, 2006. [PUBMED Abstract]
- Ghadimi BM, Grade M, Difilippantonio MJ, et al.: Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol 23 (9): 1826-38, 2005. [PUBMED Abstract]
- Dignam JJ, Ye Y, Colangelo L, et al.: Prognosis after rectal cancer in blacks and whites participating in adjuvant therapy randomized trials. J Clin Oncol 21 (3): 413-20, 2003. [PUBMED Abstract]
- Abir F, Alva S, Longo WE, et al.: The postoperative surveillance of patients with colon cancer and rectal cancer. Am J Surg 192 (1): 100-8, 2006. [PUBMED Abstract]
- Martin EW, Minton JP, Carey LC: CEA-directed second-look surgery in the asymptomatic patient after primary resection of colorectal carcinoma. Ann Surg 202 (3): 310-7, 1985. [PUBMED Abstract]
- Bruinvels DJ, Stiggelbout AM, Kievit J, et al.: Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg 219 (2): 174-82, 1994. [PUBMED Abstract]
- Lautenbach E, Forde KA, Neugut AI: Benefits of colonoscopic surveillance after curative resection of colorectal cancer. Ann Surg 220 (2): 206-11, 1994. [PUBMED Abstract]
- Khoury DA, Opelka FG, Beck DE, et al.: Colon surveillance after colorectal cancer surgery. Dis Colon Rectum 39 (3): 252-6, 1996. [PUBMED Abstract]
- Pietra N, Sarli L, Costi R, et al.: Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis Colon Rectum 41 (9): 1127-33, 1998. [PUBMED Abstract]
- Secco GB, Fardelli R, Gianquinto D, et al.: Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg Oncol 28 (4): 418-23, 2002. [PUBMED Abstract]
- Pfister DG, Benson AB, Somerfield MR: Clinical practice. Surveillance strategies after curative treatment of colorectal cancer. N Engl J Med 350 (23): 2375-82, 2004. [PUBMED Abstract]
- Li Destri G, Di Cataldo A, Puleo S: Colorectal cancer follow-up: useful or useless? Surg Oncol 15 (1): 1-12, 2006. [PUBMED Abstract]
- Kapiteijn E, Kranenbarg EK, Steup WH, et al.: Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Prospective randomised trial with standard operative and histopathological techniques. Dutch ColoRectal Cancer Group. Eur J Surg 165 (5): 410-20, 1999. [PUBMED Abstract]
- Grossmann I, de Bock GH, Meershoek-Klein Kranenbarg WM, et al.: Carcinoembryonic antigen (CEA) measurement during follow-up for rectal carcinoma is useful even if normal levels exist before surgery. A retrospective study of CEA values in the TME trial. Eur J Surg Oncol 33 (2): 183-7, 2007. [PUBMED Abstract]
Cellular Classification and Pathology of Rectal Cancer
Adenocarcinomas account for most rectal tumors in the United States. Other histological types account for an estimated 2% to 5% of colorectal tumors.[1]
The World Health Organization classification of tumors of the colon and rectum includes:[2]
Epithelial Tumors
Adenoma
- Tubular.
- Villous.
- Tubulovillous.
- Serrated.
Carcinoma
- Adenocarcinoma.
- Mucinous adenocarcinoma.
- Signet-ring cell carcinoma.
- Small cell carcinoma.
- Adenosquamous carcinoma.
- Medullary carcinoma.
- Undifferentiated carcinoma.
Carcinoid (well-differentiated neuroendocrine neoplasm)
- Enterochromaffin-cell, serotonin-producing neoplasm.
- L-cell, glucagon-like peptide and pancreatic polypeptide/peptide YY–producing tumor.
- Others.
Intraepithelial neoplasia (dysplasia) associated with chronic inflammatory diseases
- Low-grade glandular intraepithelial neoplasia.
- High-grade glandular intraepithelial neoplasia.
Mixed carcinoma-adenocarcinoma
- Others.
Nonepithelial Tumors
- Lipoma.
- Leiomyoma.
- Gastrointestinal stromal tumor. For more information, see Gastrointestinal Stromal Tumors Treatment.
- Leiomyosarcoma.
- Angiosarcoma.
- Kaposi sarcoma. For more information, see Kaposi Sarcoma Treatment.
- Melanoma. For more information, see Melanoma Treatment.
- Others.
Malignant lymphomas
- Marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type.
- Mantle cell lymphoma.
- Diffuse large B-cell lymphoma.
- Burkitt lymphoma.
- Burkitt-like/atypical Burkitt lymphoma.
For more information, see Indolent B-Cell Non-Hodgkin Lymphoma Treatment.
References
- Kang H, O'Connell JB, Leonardi MJ, et al.: Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis 22 (2): 183-9, 2007. [PUBMED Abstract]
- Hamilton SR, Aaltonen LA: Pathology and Genetics of Tumours of the Digestive System. International Agency for Research on Cancer, 2000.
Stage Information for Rectal Cancer
Accurate staging provides crucial information about the location and size of the primary tumor in the rectum, and, if present, the size, number, and location of any metastases. Accurate initial staging can influence therapy by helping to determine the type of surgical intervention and the choice of neoadjuvant therapy to maximize the likelihood of resection with clear margins. In primary rectal cancer, pelvic imaging helps determine the following factors:[1-7]
- The depth of tumor invasion.
- The distance from the sphincter complex.
- The potential for achieving negative circumferential (radial) margins.
- The involvement of locoregional lymph nodes or adjacent organs.
Staging Evaluation
Clinical evaluation and staging procedures may include:
- Digital-rectal examination (DRE): DRE and/or rectovaginal exam and rigid proctoscopy to determine if sphincter-saving surgery is possible.[1,2,5]
- Colonoscopy: Complete colonoscopy to rule out cancers elsewhere in the bowel.[5]
- Computed tomography (CT): Pan-body CT scan to rule out metastatic disease.[5]
- Magnetic resonance imaging (MRI): MRI of the abdomen and pelvis to determine the depth of penetration and the potential for achieving negative circumferential (radial) margins and to identify locoregional nodal metastases and distant metastatic disease. MRI may be particularly helpful in determining sacral involvement in local recurrence.[1]
- Endorectal ultrasound: Endorectal ultrasound with a rigid probe or a flexible scope for stenotic lesions to determine the depth of penetration and identify locoregional nodal metastases.[2,4]
- Positron emission tomography (PET): PET to image distant metastatic disease.[1]
- Carcinoembryonic antigen (CEA): Measurement of the serum CEA level for prognostic assessment and the determination of response to therapy.[6,7]
In the tumor (T) staging of rectal carcinoma, several studies indicate that the accuracy of endorectal ultrasound ranges from 80% to 95% compared with 65% to 75% for CT and 75% to 85% for MRI. The accuracy in determining metastatic nodal involvement by endorectal ultrasound is approximately 70% to 75% compared with 55% to 65% for CT and 60% to 70% for MRI.[2] In a meta-analysis of 84 studies, none of the three imaging modalities, including endorectal ultrasound, CT, and MRI, were significantly superior to the others in staging nodal (N) status.[8] Endorectal ultrasound using a rigid probe may be similarly accurate in T and N staging when compared with endorectal ultrasound using a flexible scope. However, a technically difficult endorectal ultrasound may give an inconclusive or inaccurate result for both T stage and N stage. In this case, further assessment by MRI or flexible endorectal ultrasound may be considered.[4,9]
In patients with rectal cancer, the circumferential resection margin is an important pathological staging parameter. Measured in millimeters, it is defined as the retroperitoneal or peritoneal adventitial soft-tissue margin closest to the deepest penetration of tumor.[10]
AJCC Stage Groupings and TNM Definitions
The AJCC has designated staging by TNM (tumor, node, metastasis) classification to define rectal cancer.[11] The same classification is used for both clinical and pathological staging.[11] Treatment decisions are made with reference to the TNM classification system, rather than the older Dukes or Modified Astler-Coller classification schema.
Cancers staged using this staging system include adenocarcinomas, high-grade neuroendocrine carcinomas, and squamous carcinomas of the colon and rectum. Cancers not staged using this staging system include these histopathological types of cancer: appendiceal carcinomas, anal carcinomas, well-differentiated neuroendocrine tumors (carcinoids).[11] For more information, see Anal Cancer Treatment and Gastrointestinal Neuroendocrine Tumors Treatment.
Lymph node status
The AJCC and a National Cancer Institute-sponsored panel suggested that at least 10 to 14 lymph nodes be examined in radical colon and rectum resections in patients who did not receive neoadjuvant therapy. In cases in which a tumor is resected for palliation or in patients who have received preoperative radiation therapy, fewer lymph nodes may be present.[10-12] This takes into consideration that the number of lymph nodes examined is a reflection of both the aggressiveness of lymphovascular mesenteric dissection at the time of surgical resection and the pathological identification of nodes in the specimen.
Retrospective studies, such as Intergroup trial INT-0089 (NCT00201331), have demonstrated that the number of lymph nodes examined during colon and rectal surgery may be associated with patient outcome.[13-16]
A new tumor-metastasis staging strategy for node-positive rectal cancer has been proposed.[17]
| Stage | TNMb,c | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph nodes; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Colon and rectum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 251–74. | |||
| The explanations for superscripts b and c are at the end of Table 5. | |||
| 0 | Tis, N0, M0 | Tis = Carcinoma in situ, intramucosal carcinoma (involvement of lamina propria with no extension through muscularis mucosae). |
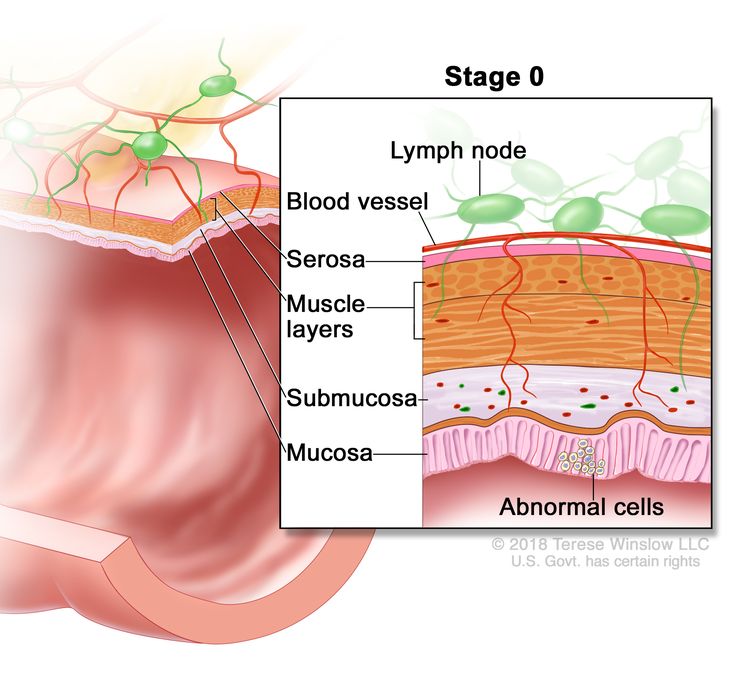 |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| Stage | TNMb,c | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph nodes; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Colon and rectum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 251–74. | |||
| The explanations for superscripts b and c are at the end of Table 5. | |||
| I | T1–T2, N0, M0 | T1 = Tumor invades the submucosa (through the muscularis mucosa but not into the muscularis propria). |
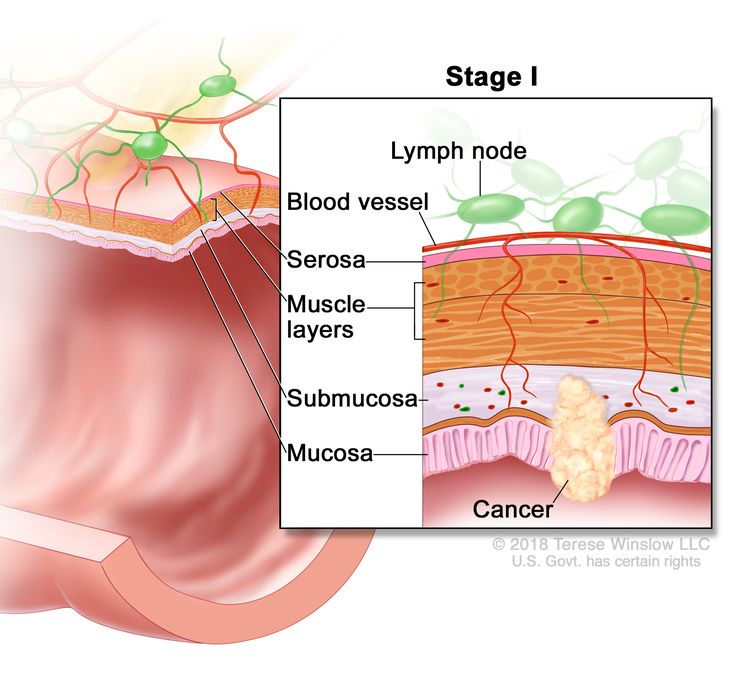 |
| T2 = Tumor invades the muscularis propria. | |||
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| Stage | TNMb,c | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph nodes; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Colon and rectum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 251–74. | |||
| The explanations for superscripts b and c are at the end of Table 5. | |||
| IIA | T3, N0, M0 | T3 = Tumor invades through the muscularis propria into pericolorectal tissues. |
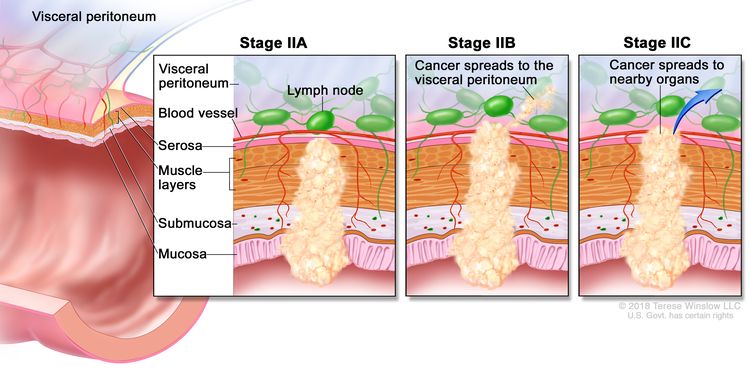 |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| IIB | T4a, N0, M0 | T4a = Tumor invades through the visceral peritoneum (including gross perforation of the bowel through tumor and continuous invasion of tumor through areas of inflammation to the surface of the visceral peritoneum). | |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| IIC | T4b, N0, M0 | T4b = Tumor directly invades or adheres to adjacent organs or structures. | |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| Stage | TNMb,c | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph nodes; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Colon and rectum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 251–74. | |||
| The explanations for superscripts b and c are at the end of Table 5. | |||
| IIIA | T1, N2a, M0 | T1 = Tumor invades the submucosa (through the muscularis mucosa but not into the muscularis propria). |
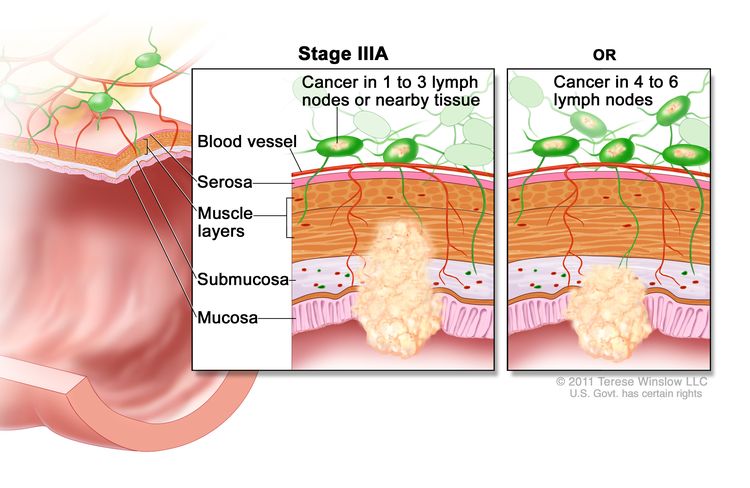 |
| N2a = Four to six regional lymph nodes are positive. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| T1–2, N1/N1c, M0 | T1 = Tumor invades the submucosa (through the muscularis mucosa but not into the muscularis propria). | ||
| T2 = Tumor invades the muscularis propria. | |||
| N1 = One to three regional lymph nodes are positive (tumor in lymph nodes measuring ≥0.2 mm), or any number of tumor deposits are present and all identifiable lymph nodes are negative. | |||
| –N1c = No regional lymph nodes are positive, but there are tumor deposits in the subserosa, mesentery, or nonperitonealized pericolic, or perirectal/mesorectal tissues. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| IIIB | T1–T2, N2b, M0 | T1 = Tumor invades the submucosa (through the muscularis mucosa but not into the muscularis propria). |
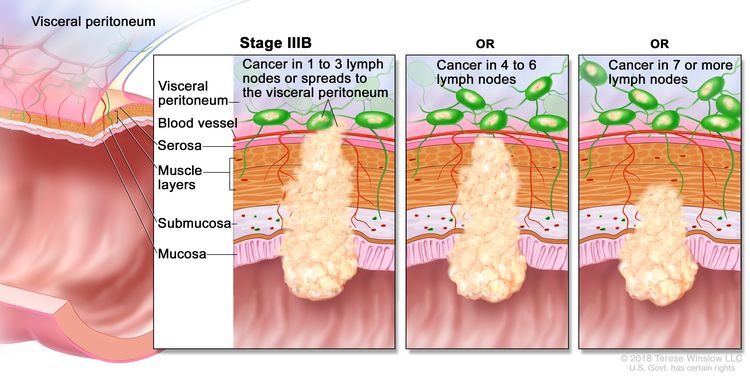 |
| T2 = Tumor invades the muscularis propria. | |||
| N2b = Seven or more regional lymph nodes are positive. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| T2–T3, N2a, M0 | T2 = Tumor invades the muscularis propria. | ||
| T3 = Tumor invades through the muscularis propria into pericolorectal tissues. | |||
| N2a = Four to six regional lymph nodes are positive. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| T3–T4a, N1/N1c, M0 | T3 = Tumor invades through the muscularis propria into pericolorectal tissues. | ||
| T4 = Tumor invades the visceral peritoneum or invades or adheres to adjacent organ or structure. | |||
| –T4a = Tumor invades through the visceral peritoneum (including gross perforation of the bowel through tumor and continuous invasion of tumor through areas of inflammation to the surface of the visceral peritoneum). | |||
| N1 = One to three regional lymph nodes are positive (tumor in lymph nodes measuring ≥0.2 mm), or any number of tumor deposits are present and all identifiable lymph nodes are negative. | |||
| –N1c = No regional lymph nodes are positive, but there are tumor deposits in the subserosa, mesentery, or nonperitonealized pericolic, or perirectal/mesorectal tissues. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| IIIC | T3–T4a, N2b, M0 | T3 = Tumor invades through the muscularis propria into pericolorectal tissues. |
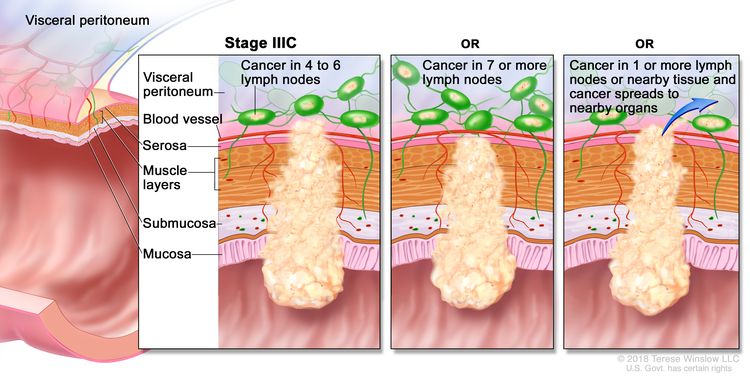 |
| T4 = Tumor invades the visceral peritoneum or invades or adheres to adjacent organ or structure. | |||
| –T4a = Tumor invades through the visceral peritoneum (including gross perforation of the bowel through tumor and continuous invasion of tumor through areas of inflammation to the surface of the visceral peritoneum). | |||
| N2b = Seven or more regional lymph nodes are positive. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| T4a, N2a, M0 | T4a = Tumor invades through the visceral peritoneum (including gross perforation of the bowel through tumor and continuous invasion of tumor through areas of inflammation to the surface of the visceral peritoneum). | ||
| N2a = Four to six regional lymph nodes are positive. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| T4b, N1–N2, M0 | T4b = Tumor directly invades or adheres to adjacent organs or structures. | ||
| N1 = One to three regional lymph nodes are positive (tumor in lymph nodes measuring ≥0.2 mm), or any number of tumor deposits are present and all identifiable lymph nodes are negative. | |||
| –N1a = One regional lymph node is positive. | |||
| –N1b = Two or three regional lymph nodes are positive. | |||
| –N1c = No regional lymph nodes are positive, but there are tumor deposits in the subserosa, mesentery, or nonperitonealized pericolic, or perirectal/mesorectal tissues. | |||
| N2 = Four or more regional nodes are positive. | |||
| –N2a = Four to six regional lymph nodes are positive. | |||
| –N2b = Seven or more regional lymph nodes are positive. | |||
| M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) | |||
| Stage | TNMb,c | Definition | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph nodes; M = distant metastasis. | |||
| aReprinted with permission from AJCC: Colon and rectum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 251–74. | |||
| b Direct invasion in T4 includes invasion of other organs or other segments of the colorectum as a result of direct extension through the serosa, as confirmed on microscopic examination (e.g., invasion of the sigmoid colon by a carcinoma of the cecum) or, for cancers in a retroperitoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond the muscularis propria (i.e., respectively, a tumor on the posterior wall of the descending colon invading the left kidney or lateral abdominal wall; or a mid or distal rectal cancer with invasion of prostate, seminal vesicles, cervix, or vagina). | |||
| cTumor that is adherent to other organs or structures, grossly, is classified cT4b. However, if no tumor is present in the adhesion, microscopically, the classification should be pT1-4a depending on the anatomical depth of wall invasion. The V and L classification should be used to identify the presence or absence of vascular or lymphatic invasion whereas the PN prognostic factor should be used for perineural invasion. | |||
| IVA | Any T, Any N, M1a | TX = Primary tumor cannot be assessed. |
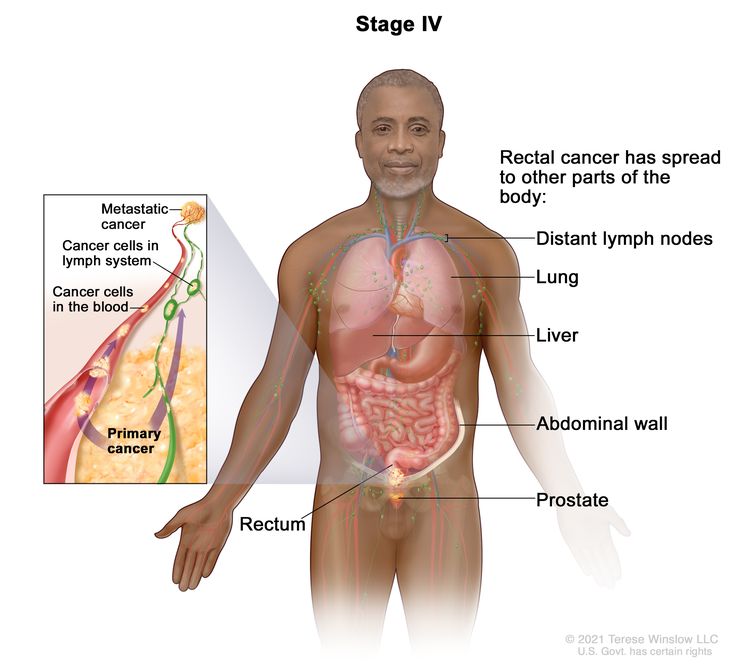 |
| T0 = No evidence of primary tumor. | |||
| Tis = Carcinoma in situ, intramucosal carcinoma (involvement of lamina propria with no extension through muscularis mucosae). | |||
| T1 = Tumor invades the submucosa (through the muscularis mucosa but not into the muscularis propria). | |||
| T2 = Tumor invades the muscularis propria. | |||
| T3 = Tumor invades through the muscularis propria into pericolorectal tissues. | |||
| T4 = Tumor invades the visceral peritoneum or invades or adheres to adjacent organ or structure. | |||
| –T4a = Tumor invades through the visceral peritoneum (including gross perforation of the bowel through tumor and continuous invasion of tumor through areas of inflammation to the surface of the visceral peritoneum). | |||
| –T4b = Tumor directly invades or adheres to adjacent organs or structures. | |||
| NX = Regional lymph nodes cannot be assessed. | |||
| N0 = No regional lymph node metastasis. | |||
| N1 = One to three regional lymph nodes are positive (tumor in lymph nodes measuring ≥0.2 mm), or any number of tumor deposits are present and all identifiable lymph nodes are negative. | |||
| –N1a = One regional lymph node is positive. | |||
| –N1b = Two or three regional lymph nodes are positive. | |||
| –N1c = No regional lymph nodes are positive, but there are tumor deposits in the subserosa, mesentery, or nonperitonealized pericolic, or perirectal/mesorectal tissues. | |||
| N2 = Four or more regional nodes are positive. | |||
| –N2a = Four to six regional lymph nodes are positive. | |||
| –N2b = Seven or more regional lymph nodes are positive. | |||
| M1a = Metastasis to one site or organ is identified without peritoneal metastasis. | |||
| IVB | Any T, Any N, M1b | Any T = See T descriptions above in Any T, Any N, M1a TNM stage group. | |
| Any N = See N descriptions above in Any T, Any N1, M1a TNM stage group. | |||
| M1b = Metastasis to two or more sites or organs is identified without peritoneal metastasis. | |||
| IVC | Any T, Any N, M1c | Any T = See T descriptions above in Any T, Any N, M1a TNM stage group. | |
| Any N = See N descriptions above in Any T, Any N1, M1a TNM stage group. | |||
| M1c = Metastasis to the peritoneal surface is identified alone or with other site or organ metastases. | |||
References
- Schmidt CR, Gollub MJ, Weiser MR: Contemporary imaging for colorectal cancer. Surg Oncol Clin N Am 16 (2): 369-88, 2007. [PUBMED Abstract]
- Siddiqui AA, Fayiga Y, Huerta S: The role of endoscopic ultrasound in the evaluation of rectal cancer. Int Semin Surg Oncol 3: 36, 2006. [PUBMED Abstract]
- Søreide K: Molecular testing for microsatellite instability and DNA mismatch repair defects in hereditary and sporadic colorectal cancers--ready for prime time? Tumour Biol 28 (5): 290-300, 2007. [PUBMED Abstract]
- Zammit M, Jenkins JT, Urie A, et al.: A technically difficult endorectal ultrasound is more likely to be inaccurate. Colorectal Dis 7 (5): 486-91, 2005. [PUBMED Abstract]
- Libutti SK, Willett CG, Saltz LB: Cancer of the rectum. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 1127-41.
- Goldstein MJ, Mitchell EP: Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest 23 (4): 338-51, 2005. [PUBMED Abstract]
- Das P, Skibber JM, Rodriguez-Bigas MA, et al.: Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer 109 (9): 1750-5, 2007. [PUBMED Abstract]
- Lahaye MJ, Engelen SM, Nelemans PJ, et al.: Imaging for predicting the risk factors--the circumferential resection margin and nodal disease--of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR 26 (4): 259-68, 2005. [PUBMED Abstract]
- Balch GC, De Meo A, Guillem JG: Modern management of rectal cancer: a 2006 update. World J Gastroenterol 12 (20): 3186-95, 2006. [PUBMED Abstract]
- Compton CC, Greene FL: The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin 54 (6): 295-308, 2004 Nov-Dec. [PUBMED Abstract]
- Jessup J, Benson A, Chen V: Colon and Rectum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 251–74.
- Nelson H, Petrelli N, Carlin A, et al.: Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 93 (8): 583-96, 2001. [PUBMED Abstract]
- Swanson RS, Compton CC, Stewart AK, et al.: The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 10 (1): 65-71, 2003 Jan-Feb. [PUBMED Abstract]
- Le Voyer TE, Sigurdson ER, Hanlon AL, et al.: Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 21 (15): 2912-9, 2003. [PUBMED Abstract]
- Prandi M, Lionetto R, Bini A, et al.: Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg 235 (4): 458-63, 2002. [PUBMED Abstract]
- Tepper JE, O'Connell MJ, Niedzwiecki D, et al.: Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 19 (1): 157-63, 2001. [PUBMED Abstract]
- Greene FL, Stewart AK, Norton HJ: New tumor-node-metastasis staging strategy for node-positive (stage III) rectal cancer: an analysis. J Clin Oncol 22 (10): 1778-84, 2004. [PUBMED Abstract]
Treatment Option Overview for Rectal Cancer
The management of rectal cancer varies somewhat from that of colon cancer because of the increased risk of local recurrence and a poorer overall prognosis. Differences include surgical technique, the use of radiation therapy, and the method of chemotherapy administration. In addition to determining the intent of rectal cancer surgery (i.e., curative or palliative), it is important to consider therapeutic issues related to the maintenance or restoration of normal anal sphincter, genitourinary function, and sexual function.[1,2]
The approach to the management of rectal cancer is multimodal and involves a multidisciplinary team of cancer specialists with expertise in gastroenterology, medical oncology, surgical oncology, radiation oncology, and radiology.
Immunotherapy
Among patients with rectal adenocarcinomas, 5% to 10% of the tumors have mismatch repair deficiency or high microsatellite instability. Immune checkpoint inhibitors are efficacious as a first-line therapy for metastatic colorectal cancers, with overall response rates of 30% to 60%.[3-5] These responses proved durable, and prolonged overall survival (OS) was demonstrated in these settings.
Evidence (immunotherapy):
- A phase II trial (NCT04165772) studied dostarlimab, an anti-programmed death-1 (PD-1) monoclonal antibody, in 12 patients with locally advanced, mismatch repair–deficient, stage II or stage III rectal adenocarcinoma.[6]
- All 12 patients had clinical complete responses of 100% (95% confidence interval [CI], 74%–100%) after a median follow-up of 12 months. Patients’ cancers did not recur when the follow-up period ranged from 6 to 25 months. At the time of follow-up, chemoradiation therapy and surgery had been avoided.[6][Level of evidence C3]
- Before this approach becomes a new standard, more patients need to be evaluated. A longer follow-up period is required to ensure durability and assess the need for future surgery or chemoradiation therapy.
Primary Surgical Therapy
The primary treatment for patients with rectal cancer is surgical resection of the primary tumor. The surgical approach to treatment varies according to:
- Tumor location.
- Stage of disease.
- Presence or absence of high-risk features (i.e., positive margins, lymphovascular invasion, perineural invasion, and poorly differentiated histology).
Types of surgical resection include:[1,2,7]
- Polypectomy for select T1 cancers.
- Transanal local excision and transanal endoscopic microsurgery for select clinically staged T1/T2, N0 rectal cancers.
- Total mesorectal excision with autonomic nerve preservation techniques via low-anterior resection.
- Total mesorectal excision via abdominoperineal resection for patients who are not candidates for sphincter-preservation, leaving patients with a permanent end-colostomy.
Polypectomy alone may be used in certain instances (T1) in which polyps with invasive cancer can be completely resected with clear margins and have favorable histological features.[8,9]
Local excision of clinical T1 tumors is an acceptable surgical technique for appropriately selected patients. For all other tumors, a mesorectal excision is the treatment of choice. Very select patients with T2 tumors may be candidates for local excision. Local failure rates in the range of 4% to 8% after rectal resection with appropriate mesorectal excision (total mesorectal excision for low/middle rectal tumors and mesorectal excision at least 5 cm below the tumor for high rectal tumors) have been reported.[10-14]
For patients with advanced cancers of the mid- to upper rectum, low-anterior resection followed by the creation of a colorectal anastomosis may be the treatment of choice. For locally advanced rectal cancers for which radical resection is indicated, however, total mesorectal excision with autonomic nerve preservation techniques via low-anterior resection is preferable to abdominoperineal resection.[1,2]
The low incidence of local relapse after meticulous mesorectal excision has led some investigators to question the routine use of adjuvant radiation therapy. Because of an increased tendency for first failure in locoregional sites only, the impact of perioperative radiation therapy is greater in rectal cancer than in colon cancer.[15]
Chemoradiation Therapy
Preoperative chemoradiation therapy
Neoadjuvant therapy for rectal cancer, using preoperative chemoradiation therapy, is the preferred treatment option for patients with stages II and III disease. However, postoperative chemoradiation therapy for patients with stage II or III rectal cancer remains an acceptable option.[16][Level of evidence A1] Total neoadjuvant therapy (chemotherapy followed by [chemo]radiation or [chemo]radiation followed by chemotherapy) is also an option.
Preoperative chemoradiation therapy has become the standard of care for patients with clinically staged T3–T4 or node-positive disease (stages II/III), based on the results of several studies:
- German Rectal Cancer Study Group trial.[17]
- National Surgical Adjuvant Breast and Bowel Project R-03 trial NSABP R-03 (NCT00410579).[18][Level of evidence A1] For more information, see the Treatment of Stages II and III Rectal Cancer section.
Multiple phase II and III studies examined the benefits of preoperative chemoradiation therapy, which include:[16]
- Tumor regression and downstaging of the tumor.
- Improved tumor resectability.
- Higher rate of local control.
- Improved toxicity profile of chemoradiation therapy.
- Higher rate of sphincter preservation.
Complete pathological response rates of 10% to 25% may be achieved with preoperative chemoradiation therapy.[19-26] However, preoperative radiation therapy is associated with increased complications compared with surgery alone. Some patients with cancers at a lower risk of local recurrence might be adequately treated with surgery and adjuvant chemotherapy.[27-30] For more information about these studies, see the Preoperative chemoradiation therapy section in the Treatment of Stages II and III Rectal Cancer section.
Postoperative chemoradiation therapy
Preoperative chemoradiation therapy is the current standard of care for stages II and III rectal cancer. However, before 1990, the following studies noted an increase in both disease-free survival (DFS) and OS with the use of postoperative combined-modality therapy:
- The Gastrointestinal Tumor Study Group trial (GITSG-7175).
- The Mayo/North Central Cancer Treatment Group trial (NCCTG-794751).
- The National Surgical Adjuvant Breast and Bowel Project trial (NSABP-R-01).
Subsequent studies have attempted to increase the survival benefit by improving radiation sensitization and by identifying the optimal chemotherapeutic agents and delivery systems.
Fluorouracil (5-FU): The following studies examined optimal delivery methods for adjuvant 5-FU:
- Intergroup protocol 86-47-51 trial (MAYO-864751).[31][Level of evidence A1]
- Intergroup 0114 trial (INT-0114 [CLB-9081]).[29][Level of evidence A1]
- Intergroup 0144.[32]
For detailed information about these study results, see the Treatment of Stages II and III Rectal Cancer section.
Acceptable postoperative chemoradiation therapy for patients with stage II or III rectal cancer not enrolled in clinical trials includes continuous-infusion 5-FU during 45 Gy to 55 Gy pelvic radiation and four cycles of adjuvant maintenance chemotherapy with bolus 5-FU with or without modulation with leucovorin (LV).
Findings from the NSABP-R-01 trial compared surgery alone with surgery followed by chemotherapy or radiation therapy.[33] Subsequently, the NSABP-R-02 study (NCT00410579), addressed whether adding postoperative radiation therapy to chemotherapy would enhance the survival advantage reported in R-01.[34][Level of evidence A1]
In the NSABP-R-02 study, the addition of radiation therapy significantly reduced local recurrence at 5 years (8% for chemotherapy and radiation vs. 13% for chemotherapy alone, P = .02) but failed to demonstrate a significant survival benefit. Radiation therapy appeared to improve survival among patients younger than 60 years and among patients who underwent abdominoperineal resection.
While this trial has initiated discussion in the oncologic community about the proper role of postoperative radiation therapy, omission of radiation therapy seems premature because of the serious complications of locoregional recurrence.
Chemotherapy regimens
Table 7 describes the chemotherapy regimens used to treat rectal cancer.
| Regimen Name | Drug Combination | Dose |
|---|---|---|
| 5-FU = fluorouracil; AIO = Arbeitsgemeinschaft Internistische Onkologie; bid = twice a day; IV = intravenous; LV = leucovorin. | ||
| AIO or German AIO | LV, 5-FU, and irinotecan | Irinotecan (100 mg/m2) and LV (500 mg/m2) administered as 2-h infusions on d 1, followed by 5-FU (2,000 mg/m2) IV bolus administered via ambulatory pump weekly over 24 h, 4 times a y (52 wk). |
| CAPOX | Capecitabine and oxaliplatin | Capecitabine (1,000 mg/m2) bid on d 1–14, plus oxaliplatin (70 mg/m2) on d 1 and 8 every 3 wk. |
| Douillard | LV, 5-FU, and irinotecan | Irinotecan (180 mg/m2) administered as a 2-h infusion on d 1, LV (200 mg/m2) administered as a 2-h infusion on d 1 and 2, followed by a loading dose of 5-FU (400 mg/m2) IV bolus, then 5-FU (600 mg/m2) administered via ambulatory pump over 22 h every 2 wk on d 1 and 2. |
| FOLFIRI | LV, 5-FU, and irinotecan | Irinotecan (180 mg/m2) and LV (400 mg/m2) administered as 2-h infusions on d 1, followed by a loading dose of 5-FU (400 mg/m2) IV bolus administered on d 1, then 5-FU (2,400–3,000 mg/m2) administered via ambulatory pump over 46 h every 2 wk. |
| FOLFOX4 | Oxaliplatin, LV, and 5-FU | Oxaliplatin (85 mg/m2) administered as a 2-h infusion on day 1, LV (200 mg/m2) administered as a 2-h infusion on d 1 and 2, followed by a loading dose of 5-FU (400 mg/m2) IV bolus, then 5-FU (600 mg/m2) administered via ambulatory pump over 22 h every 2 wk on d 1 and 2. |
| FOLFOX6 | Oxaliplatin, LV, and 5-FU | Oxaliplatin (85–100 mg/m2) and LV (400 mg/m2) administered as 2-h infusions on d 1, followed by a loading dose of 5-FU (400 mg/m2) IV bolus on d 1, then 5-FU (2,400–3,000 mg/m2) administered via ambulatory pump over 46 h every 2 wk. |
| FOLFOXIRI | Irinotecan, oxaliplatin, LV, 5-FU | Irinotecan (165 mg/m2) administered as a 60-min infusion, then concomitant infusion of oxaliplatin (85 mg/m2) and LV (200 mg/m2) over 120 min, followed by 5-FU (3,200 mg/m2) administered as a 48-h continuous infusion. |
| FUFOX | 5-FU, LV, and oxaliplatin | Oxaliplatin (50 mg/m2) plus LV (500 mg/m2) plus 5-FU (2,000 mg/m2) administered as a 22-h continuous infusion on d 1, 8, 22, and 29 every 36 d. |
| FUOX | 5-FU plus oxaliplatin | 5-FU (2,250 mg/m2) administered as a continuous infusion over 48 h on d 1, 8, 15, 22, 29, and 36 plus oxaliplatin (85 mg/m2) on d 1, 15, and 29 every 6 wk. |
| IFL (or Saltz) | Irinotecan, 5-FU, and LV | Irinotecan (125 mg/m2) plus 5-FU (500 mg/m2) IV bolus and LV (20 mg/m2) IV bolus administered weekly for 4 out of 6 wk. |
| XELOX | Capecitabine plus oxaliplatin | Oral capecitabine (1,000 mg/m2) administered bid for 14 d plus oxaliplatin (130 mg/m2) on d 1 every 3 wk. |
Total neoadjuvant therapy
Data support giving all radiation therapy and chemotherapy neoadjuvantly.
The RAPIDO trial (NCT01558921) randomly assigned 920 patients to receive either short-course radiation therapy followed by six cycles of CAPOX (capecitabine and oxaliplatin) or nine cycles of FOLFOX (LV, 5-FU, and oxaliplatin) followed by surgery, or long-course chemoradiation therapy followed by surgery with the option to add adjuvant chemotherapy. The primary end point was 3-year disease-related treatment failure (defined as first occurrence of locoregional failure, distant metastasis, new primary colorectal tumor, or treatment-related death). The 3-year disease-related treatment failure rate was 23.7% (95% CI, 19.8%–27.6%) in the short-course radiation therapy group and 30.4% (95% CI, 26.1%–34.6%) in the long-course chemoradiation therapy group (hazard ratio [HR], 0.75; 95% CI, 0.60–0.95; P = .019).[35][Level of evidence B1]
In the randomized, phase III, French UNICANCER-PRODIGE 23 study (NCT01804790), 461 patients were randomly assigned to receive either six cycles of FOLFIRINOX (LV, 5-FU, irinotecan, and oxaliplatin) followed by chemoradiation therapy (experimental group) or chemoradiation therapy (standard-of-care group). Patients in both groups underwent total mesorectal excision. This was not fully a total neoadjuvant therapy trial as both groups also received adjuvant chemotherapy with modified FOLFOX or capecitabine for 3 months (experimental group) or 6 months (standard-of-care group). The 3-year DFS rate was 76% (95% CI, 69%–81%) in the experimental group and 69% (95% CI, 62%–74%) in the standard-of-care group (stratified HR, 0.69; 95% CI, 0.49–0.97; P = .034).[36][Level of evidence B1]
The total neoadjuvant approach was studied in clinical trials because data showed that many patients do not receive all of the recommended chemotherapy when given after surgery. For example, in the OPRA trial (NCT02008656), which used a total neoadjuvant therapy approach, approximately 85% of patients received all of the recommended chemotherapy, an improvement in adherence over trials that used adjuvant chemotherapy. Another potential benefit of this approach is that it allows more patients to receive nonoperative management (also known as the watch-and-wait approach), which is described in more detail below. This approach may interest patients who would otherwise require an abdominoperineal resection, which results in the need for lifelong stoma.[37,38][Level of evidence B1]
Select patients with locally advanced rectal cancer may omit radiation therapy if they receive escalated chemotherapy, but they would still need a total mesorectal excision. In the PROSPECT trial (NCT01515787), 1,194 patients were randomly assigned to receive either neoadjuvant FOLFOX chemotherapy (with chemoradiation therapy only given if the primary tumor decreased in size by <20% or if FOLFOX was discontinued because of side effects) or standard neoadjuvant chemoradiation therapy. All patients then underwent surgery and had the option to receive adjuvant FOLFOX (four or six cycles for the neoadjuvant chemotherapy group and eight cycles for the neoadjuvant chemoradiation therapy group). The study population included patients with T2, node-positive; T3, N0; or T3, node-positive disease who were eligible for sphincter-sparing surgery (thus, excluding most patients with low-rectal tumors). This study found that the omission of radiation therapy was possible in select patients without compromising oncologic outcomes based on a noninferiority study design. It should be noted that in Europe, many patients with T3, N0 disease do not undergo any neoadjuvant therapy prior to resection. Omission of radiation is beneficial for patients desiring to preserve fertility.[39]
Total neoadjuvant therapy is currently the preferred approach for most patients with locally advanced rectal cancer without distant metastases.
Treatment toxicity
The acute side effects of pelvic radiation therapy for rectal cancer are mainly the result of gastrointestinal toxicity, are self-limiting, and usually resolve within 4 to 6 weeks of completing treatment.
Of greater concern is the potential for late morbidity after rectal cancer treatment. Patients who undergo aggressive surgical procedures for rectal cancer can have chronic symptoms, particularly if there is impairment of the anal sphincter.[40] Patients treated with radiation therapy appear to have increased chronic bowel dysfunction, anorectal sphincter dysfunction (if the sphincter was surgically preserved), and sexual dysfunction than do patients who undergo surgical resection alone.[28,41-46]
An analysis of patients treated with postoperative chemotherapy and radiation therapy suggests that these patients may have more chronic bowel dysfunction than do patients who undergo surgical resection alone.[47] A Cochrane review highlights the risks of increased surgical morbidity as well as late rectal and sexual function in association with radiation therapy.[40]
Improved radiation therapy planning and techniques may minimize these acute and late treatment-related complications. These techniques include:[48-52]
- The use of high-energy radiation machines.
- The use of multiple pelvic radiation fields.
- Prone patient positioning.
- Customized patient molds (belly boards) to exclude as much small bowel as possible from the radiation fields and immobilize patients during treatment.
- Bladder distention during radiation therapy to exclude as much small bowel as possible from the radiation fields.
- Visualization of the small bowel through oral contrast during treatment planning so that, when possible, the small bowel can be excluded from the radiation field.
- The use of 3-dimensional or other advanced radiation planning techniques.
Long-course versus short-course radiation therapy
There are two approaches commonly used for radiation therapy:
- Long-course chemoradiation therapy (generally to doses of 50.4–54 Gy), commonly given with concurrent capecitabine or 5-FU/LV.
- Short-course radiation therapy (25 Gy in five fractions), generally given without chemotherapy.
In Europe, preoperative radiation therapy is commonly delivered alone in 1 week (5 Gy × five daily treatments) followed by surgery one week later, rather than the long-course chemoradiation therapy approach used in the United States. One reason for this difference is the concern in the United States for heightened late effects when high radiation doses per fraction are given.
A Polish study randomly assigned 316 patients to receive either preoperative long-course chemoradiation therapy (50.4 Gy in 28 daily fractions with 5-FU/LV) or short-course preoperative radiation therapy (25 Gy in five fractions).[46] Although the primary end point was sphincter preservation, late toxicity was not statistically significantly different between the two treatment approaches (7% for the long-course group vs. 10% for the short-course group). Of note, data on anal sphincter and sexual function were not reported, and toxicity was determined by the physician, not patient reported.
The choice of long-course versus short-course radiation therapy for rectal cancer is an area of active study, and it is not known which is superior. Generally, long-course chemoradiation therapy results in a higher biologically equivalent dose being delivered to the patient (along with chemosensitization, most commonly with capecitabine or 5-FU), which would theoretically result in improved local control. This is supported by the RAPIDO trial, where a higher local recurrence rate was seen in the patients who received short-course radiation therapy rather than those who received long-course chemoradiation therapy.[35]
Alternatively, short-course radiation therapy requires a shorter break from stronger systemic therapy. Therefore, if a patient is at a relatively higher risk of local recurrence than distant recurrence, long-course chemoradiation therapy may be preferred, but if the patient is at a higher risk of distant recurrence, short-course therapy may be preferred to allow a quicker return to chemotherapy. Many physicians also do not offer short-course chemoradiation therapy when a nonoperative management approach is used, as it has not been studied, and given the potentially lower local control rates due to the lower biologically equivalent dose as compared with long-course chemoradiation therapy. The optimal sequencing of radiation therapy and chemotherapy when given as a part of total neoadjuvant therapy is still being evaluated. There are also some clinical situations where short-course radiation therapy may not be preferred, such as when a rectal stent is present (which may result in greater rectal toxicity).
Capecitabine and fluorouracil dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[53,54] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[53-55] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient's DPYD genotype and number of functioning DPYD alleles.[56-58] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[59] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[60]
References
- Balch GC, De Meo A, Guillem JG: Modern management of rectal cancer: a 2006 update. World J Gastroenterol 12 (20): 3186-95, 2006. [PUBMED Abstract]
- Baxter NN, Garcia-Aguilar J: Organ preservation for rectal cancer. J Clin Oncol 25 (8): 1014-20, 2007. [PUBMED Abstract]
- Le DT, Uram JN, Wang H, et al.: PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372 (26): 2509-20, 2015. [PUBMED Abstract]
- Overman MJ, Lonardi S, Wong KYM, et al.: Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 36 (8): 773-779, 2018. [PUBMED Abstract]
- André T, Shiu KK, Kim TW, et al.: Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 383 (23): 2207-2218, 2020. [PUBMED Abstract]
- Cercek A, Lumish M, Sinopoli J, et al.: PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med 386 (25): 2363-2376, 2022. [PUBMED Abstract]
- Guillem JG, Cohen AM: Current issues in colorectal cancer surgery. Semin Oncol 26 (5): 505-13, 1999. [PUBMED Abstract]
- Cooper HS, Deppisch LM, Gourley WK, et al.: Endoscopically removed malignant colorectal polyps: clinicopathologic correlations. Gastroenterology 108 (6): 1657-65, 1995. [PUBMED Abstract]
- Seitz U, Bohnacker S, Seewald S, et al.: Is endoscopic polypectomy an adequate therapy for malignant colorectal adenomas? Presentation of 114 patients and review of the literature. Dis Colon Rectum 47 (11): 1789-96; discussion 1796-7, 2004. [PUBMED Abstract]
- MacFarlane JK, Ryall RD, Heald RJ: Mesorectal excision for rectal cancer. Lancet 341 (8843): 457-60, 1993. [PUBMED Abstract]
- Enker WE, Thaler HT, Cranor ML, et al.: Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 181 (4): 335-46, 1995. [PUBMED Abstract]
- Zaheer S, Pemberton JH, Farouk R, et al.: Surgical treatment of adenocarcinoma of the rectum. Ann Surg 227 (6): 800-11, 1998. [PUBMED Abstract]
- Heald RJ, Smedh RK, Kald A, et al.: Abdominoperineal excision of the rectum--an endangered operation. Norman Nigro Lectureship. Dis Colon Rectum 40 (7): 747-51, 1997. [PUBMED Abstract]
- Lopez-Kostner F, Lavery IC, Hool GR, et al.: Total mesorectal excision is not necessary for cancers of the upper rectum. Surgery 124 (4): 612-7; discussion 617-8, 1998. [PUBMED Abstract]
- Gunderson LL, Sosin H: Areas of failure found at reoperation (second or symptomatic look) following "curative surgery" for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer 34 (4): 1278-92, 1974. [PUBMED Abstract]
- Sauer R, Becker H, Hohenberger W, et al.: Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351 (17): 1731-40, 2004. [PUBMED Abstract]
- Sauer R, Liersch T, Merkel S, et al.: Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30 (16): 1926-33, 2012. [PUBMED Abstract]
- Roh MS, Colangelo LH, O'Connell MJ, et al.: Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27 (31): 5124-30, 2009. [PUBMED Abstract]
- Janjan NA, Khoo VS, Abbruzzese J, et al.: Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys 44 (5): 1027-38, 1999. [PUBMED Abstract]
- Crane CH, Skibber JM, Birnbaum EH, et al.: The addition of continuous infusion 5-FU to preoperative radiation therapy increases tumor response, leading to increased sphincter preservation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 57 (1): 84-9, 2003. [PUBMED Abstract]
- Grann A, Minsky BD, Cohen AM, et al.: Preliminary results of preoperative 5-fluorouracil, low-dose leucovorin, and concurrent radiation therapy for clinically resectable T3 rectal cancer. Dis Colon Rectum 40 (5): 515-22, 1997. [PUBMED Abstract]
- Rich TA, Skibber JM, Ajani JA, et al.: Preoperative infusional chemoradiation therapy for stage T3 rectal cancer. Int J Radiat Oncol Biol Phys 32 (4): 1025-9, 1995. [PUBMED Abstract]
- Chari RS, Tyler DS, Anscher MS, et al.: Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 221 (6): 778-86; discussion 786-7, 1995. [PUBMED Abstract]
- Hyams DM, Mamounas EP, Petrelli N, et al.: A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum: a progress report of National Surgical Breast and Bowel Project Protocol R-03. Dis Colon Rectum 40 (2): 131-9, 1997. [PUBMED Abstract]
- Bosset JF, Magnin V, Maingon P, et al.: Preoperative radiochemotherapy in rectal cancer: long-term results of a phase II trial. Int J Radiat Oncol Biol Phys 46 (2): 323-7, 2000. [PUBMED Abstract]
- Hiotis SP, Weber SM, Cohen AM, et al.: Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg 194 (2): 131-5; discussion 135-6, 2002. [PUBMED Abstract]
- Lai LL, Fuller CD, Kachnic LA, et al.: Can pelvic radiotherapy be omitted in select patients with rectal cancer? Semin Oncol 33 (6 Suppl 11): S70-4, 2006. [PUBMED Abstract]
- Peeters KC, van de Velde CJ, Leer JW, et al.: Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol 23 (25): 6199-206, 2005. [PUBMED Abstract]
- Tepper JE, O'Connell M, Niedzwiecki D, et al.: Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control--final report of intergroup 0114. J Clin Oncol 20 (7): 1744-50, 2002. [PUBMED Abstract]
- Gunderson LL, Sargent DJ, Tepper JE, et al.: Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol 22 (10): 1785-96, 2004. [PUBMED Abstract]
- O'Connell MJ, Martenson JA, Wieand HS, et al.: Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 331 (8): 502-7, 1994. [PUBMED Abstract]
- Smalley SR, Benedetti JK, Williamson SK, et al.: Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol 24 (22): 3542-7, 2006. [PUBMED Abstract]
- Fisher B, Wolmark N, Rockette H, et al.: Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 80 (1): 21-9, 1988. [PUBMED Abstract]
- Wolmark N, Wieand HS, Hyams DM, et al.: Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 92 (5): 388-96, 2000. [PUBMED Abstract]
- Bahadoer RR, Dijkstra EA, van Etten B, et al.: Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 22 (1): 29-42, 2021. [PUBMED Abstract]
- Conroy T, Bosset JF, Etienne PL, et al.: Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 22 (5): 702-715, 2021. [PUBMED Abstract]
- Verheij FS, Omer DM, Williams H, et al.: Long-Term Results of Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J Clin Oncol 42 (5): 500-506, 2024. [PUBMED Abstract]
- Garcia-Aguilar J, Patil S, Gollub MJ, et al.: Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol 40 (23): 2546-2556, 2022. [PUBMED Abstract]
- Schrag D, Weiser M, Saltz L, et al.: Challenges and solutions in the design and execution of the PROSPECT Phase II/III neoadjuvant rectal cancer trial (NCCTG N1048/Alliance). Clin Trials 16 (2): 165-175, 2019. [PUBMED Abstract]
- Wong RK, Tandan V, De Silva S, et al.: Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev (2): CD002102, 2007. [PUBMED Abstract]
- Randomised trial of surgery alone versus surgery followed by radiotherapy for mobile cancer of the rectum. Medical Research Council Rectal Cancer Working Party. Lancet 348 (9042): 1610-4, 1996. [PUBMED Abstract]
- Initial report from a Swedish multicentre study examining the role of preoperative irradiation in the treatment of patients with resectable rectal carcinoma. Swedish Rectal Cancer Trial. Br J Surg 80 (10): 1333-6, 1993. [PUBMED Abstract]
- Dahlberg M, Glimelius B, Graf W, et al.: Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study. Dis Colon Rectum 41 (5): 543-9; discussion 549-51, 1998. [PUBMED Abstract]
- Birgisson H, Påhlman L, Gunnarsson U, et al.: Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol 23 (34): 8697-705, 2005. [PUBMED Abstract]
- Marijnen CA, van de Velde CJ, Putter H, et al.: Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol 23 (9): 1847-58, 2005. [PUBMED Abstract]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al.: Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 93 (10): 1215-23, 2006. [PUBMED Abstract]
- Kollmorgen CF, Meagher AP, Wolff BG, et al.: The long-term effect of adjuvant postoperative chemoradiotherapy for rectal carcinoma on bowel function. Ann Surg 220 (5): 676-82, 1994. [PUBMED Abstract]
- Martling A, Holm T, Johansson H, et al.: The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer 92 (4): 896-902, 2001. [PUBMED Abstract]
- Dahlberg M, Glimelius B, Påhlman L: Improved survival and reduction in local failure rates after preoperative radiotherapy: evidence for the generalizability of the results of Swedish Rectal Cancer Trial. Ann Surg 229 (4): 493-7, 1999. [PUBMED Abstract]
- Guerrero Urbano MT, Henrys AJ, Adams EJ, et al.: Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys 65 (3): 907-16, 2006. [PUBMED Abstract]
- Koelbl O, Richter S, Flentje M: Influence of patient positioning on dose-volume histogram and normal tissue complication probability for small bowel and bladder in patients receiving pelvic irradiation: a prospective study using a 3D planning system and a radiobiological model. Int J Radiat Oncol Biol Phys 45 (5): 1193-8, 1999. [PUBMED Abstract]
- Gunderson LL, Russell AH, Llewellyn HJ, et al.: Treatment planning for colorectal cancer: radiation and surgical techniques and value of small-bowel films. Int J Radiat Oncol Biol Phys 11 (7): 1379-93, 1985. [PUBMED Abstract]
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PUBMED Abstract]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PUBMED Abstract]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PUBMED Abstract]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PUBMED Abstract]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PUBMED Abstract]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PUBMED Abstract]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PUBMED Abstract]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PUBMED Abstract]
Treatment of Stage 0 Rectal Cancer
Treatment Options for Stage 0 Rectal Cancer
Stage 0 rectal cancer or carcinoma in situ is the most superficial of all rectal lesions and is limited to the mucosa without invasion of the lamina propria.
Treatment options for stage 0 rectal cancer include:
Polypectomy or surgery
Local excision or simple polypectomy may be indicated for stage 0 rectal cancer tumors.[1] Because of its localized nature at presentation, stage 0 rectal cancer has a high cure rate. For large lesions not amenable to local excision, full-thickness rectal resection by the transanal or transcoccygeal route may be performed.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Bailey HR, Huval WV, Max E, et al.: Local excision of carcinoma of the rectum for cure. Surgery 111 (5): 555-61, 1992. [PUBMED Abstract]
Treatment of Stage I Rectal Cancer
Treatment Options for Stage I Rectal Cancer
Stage I tumors extend beneath the mucosa into the submucosa (T1) or into, but not through, the bowel muscle wall (T2). Because of its localized nature at presentation, stage I rectal cancer has a high cure rate.
Treatment options for stage I rectal cancer include:
Surgery with or without chemoradiation therapy
There are three potential options for surgical resection in stage I rectal cancer:
- Local excision. Local excision is restricted to tumors that are confined to the rectal wall and that do not, on rectal ultrasound or magnetic resonance imaging, involve the full thickness of the rectum (i.e., are not T3 tumors). The ideal candidate for local excision has a T1 tumor with well-to-moderate differentiation that occupies less than one-third of the circumference of the bowel wall. Local excision is associated with a higher risk of local and systemic failure and is applicable only to select patients with T2 tumors. Local transanal or other resection [1,2] with or without perioperative external-beam radiation therapy (EBRT) plus fluorouracil (5-FU) may be indicated.
- Low-anterior resection. Wide surgical resection and anastomosis are options when an adequate low-anterior resection can be performed with sufficient distal rectum to allow a conventional anastomosis or coloanal anastomosis.
- Abdominoperineal resection. Wide surgical resection with abdominoperineal resection is used for lesions too distal to permit low-anterior resection.
Patients with tumors that are pathologically T1 may not need postoperative therapy. Patients with tumors that are T2 or greater have lymph node involvement about 20% of the time. Patients may want to consider additional therapy, such as radiation therapy and chemotherapy, or wide surgical resection of the rectum.[3] Patients with poor histological features or positive margins after local excision may consider low-anterior resection or abdominoperineal resection and postoperative treatment as dictated by full surgical staging.
For patients with T1 and T2 tumors, no randomized trials are available to compare local excision with or without postoperative chemoradiation therapy to wide surgical resection (low-anterior resection and abdominoperineal resection).
Evidence (surgery):
- Investigators with the Cancer and Leukemia Group B enrolled patients with T1 and T2 rectal adenocarcinomas that were within 10 cm of the dentate line and not more than 4 cm in diameter, and involving not more than 40% of the rectal circumference, onto a prospective protocol, CLB-8984. Patients with T1 tumors received no additional treatment after surgery, whereas patients with T2 tumors were treated with EBRT (54 Gy in 30 fractions, 5 days/week) and 5-FU (500 mg/m2 on days 1 through 2 and days 29 through 31 of radiation therapy).[4]
- For patients with T1 tumors, at 48 months median follow-up, the 6-year failure-free survival (FFS) rate was 83%, and the overall survival (OS) rate was 87%.
- For patients with T2 tumors, the 6-year FFS rate was 71%, and the OS rate was 85%.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Bailey HR, Huval WV, Max E, et al.: Local excision of carcinoma of the rectum for cure. Surgery 111 (5): 555-61, 1992. [PUBMED Abstract]
- Benson R, Wong CS, Cummings BJ, et al.: Local excision and postoperative radiotherapy for distal rectal cancer. Int J Radiat Oncol Biol Phys 50 (5): 1309-16, 2001. [PUBMED Abstract]
- Sitzler PJ, Seow-Choen F, Ho YH, et al.: Lymph node involvement and tumor depth in rectal cancers: an analysis of 805 patients. Dis Colon Rectum 40 (12): 1472-6, 1997. [PUBMED Abstract]
- Steele GD, Herndon JE, Bleday R, et al.: Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol 6 (5): 433-41, 1999 Jul-Aug. [PUBMED Abstract]
Treatment of Stages II and III Rectal Cancer
Treatment Options for Stages II and III Rectal Cancer
Treatment options for stages II and III rectal cancer include:
- Preoperative chemoradiation therapy.
- Neoadjuvant chemotherapy with FOLFOX without preoperative chemoradiation therapy (for select patients with lower-risk disease).
- Short-course preoperative radiation therapy followed by surgery and chemotherapy.
- Postoperative chemoradiation therapy.
- Surgery.
- Primary chemoradiation therapy followed by intensive surveillance for complete clinical responders.
- Immunotherapy (for patients with mismatch repair deficiency or high microsatellite instability [MSI]).
Preoperative chemoradiation therapy
Preoperative chemoradiation therapy has become the standard of care for patients with clinically staged T3 or T4 or node-positive disease, based on the results of several studies. The results of one study affirm neoadjuvant FOLFOX (leucovorin [LV], fluorouracil [5-FU], and oxaliplatin) as an alternative to chemoradiation therapy for select patients with lower-risk disease.[1]
Evidence (preoperative chemoradiation therapy):
- The German Rectal Cancer Study Group (CAO/ARO/AIO-94 [Working Group of Surgical Oncology/Working Group of Radiation Oncology/Working Group of Medical Oncology of the Germany Cancer Society]) randomly assigned 823 patients with ultrasound-staged T3 or T4 or lymph node-positive rectal cancer to either preoperative chemoradiation therapy or postoperative chemoradiation therapy (50.4 Gy in 28 daily fractions to the tumor and pelvic lymph nodes concurrent with infusional 5-FU 1,000 mg/m2 daily for 5 days during the first and fifth weeks of radiation therapy).[2][Level of evidence A1] All patients underwent total mesorectal excision and received four additional cycles of 5-FU–based chemotherapy.
- The 5-year overall survival (OS) rates were 76% for preoperative chemoradiation therapy and 74% for postoperative chemoradiation therapy (P = .80). The 5-year cumulative incidence of local relapse was 6% for patients assigned to the preoperative chemoradiation therapy group and 13% for patients in the postoperative chemoradiation therapy group (P = .006).
- Grade 3 or 4 acute toxic effects occurred in 27% of patients in the preoperative-treatment group and in 40% of patients in the postoperative-treatment group (P = .001). The corresponding rates of long-term toxic effects were 14% and 24%, respectively (P = .01).
- The same number of patients underwent abdominoperineal resection in each arm. However, among the 194 patients with tumors that were determined by the surgeon before randomization to require an abdominoperineal excision, a statistically significant increase in sphincter preservation was achieved among patients who received preoperative chemoradiation therapy (P = .004). These results have now been updated with a median follow-up of 11 years.[3]
- The 10-year OS was equivalent in both arms, (59.6% in the preoperative group vs. 59.9% in the postoperative group; P = .85). However, a local control benefit persists among patients treated with preoperative chemoradiation therapy compared with patients treated with postoperative chemoradiation therapy (10-year cumulative incidence of local relapse: 7.1% in the preoperative group vs. 10.1% in the postoperative group; P = .048). There were no significant differences detected for 10-year cumulative incidence of distant metastases or disease-free survival (DFS).[3]
- Among the patients assigned to the postoperative chemoradiation therapy treatment arm, 18% actually had pathologically determined stage I disease and were overestimated by endorectal ultrasound to have T3 or T4 or N1 disease. A similar number of patients were possibly overtreated in the preoperative treatment group.
- The NSABP R-03 trial (NCT00410579) similarly compared preoperative versus postoperative chemoradiation therapy for patients with clinically staged T3 or T4 or lymph node-positive rectal cancer. Chemotherapy consisted of 5-FU/LV with 45 Gy in 25 fractions with a 5.4 Gy boost. Although the intended sample size was 900 patients, the study with 267 patients closed early because of poor accrual.[4][Level of evidence A1]
- With a median follow-up of 8.4 years, preoperative chemoradiation therapy was found to confer a significant improvement in 5-year DFS (64.7% vs. 53.4% for postoperative patients; P = .011).
- Similar to the German Rectal Cancer Study, there was no significant difference in OS between treatment arms (74.5% for preoperative chemoradiation therapy vs. 65.6% for postoperative chemoradiation therapy; P =. 065).
Neoadjuvant chemotherapy with FOLFOX without preoperative chemoradiation therapy (for select patients with lower-risk disease)
Evidence (neoadjuvant chemotherapy with FOLFOX without preoperative chemoradiation therapy [for select patients with lower-risk disease]):
- The PROSPECT trial (NCT01515787) included 1,194 patients with clinical T2, node-positive; T3, N0; or T3, node-positive rectal cancer who were candidates for sphincter-sparing surgery. A total of 1,128 patients were randomly assigned to receive either neoadjuvant FOLFOX (six cycles) or chemoradiation therapy.[1][Level of evidence B1]
- Patients in the neoadjuvant FOLFOX group who had less than 20% clinical response upon restaging, or who were unable to tolerate at least five cycles of FOLFOX were selected to receive chemoradiation therapy. Neoadjuvant FOLFOX with only selective use of pelvic chemoradiation therapy was noninferior to up-front neoadjuvant pelvic chemoradiation therapy for DFS (hazard ratio [HR]disease recurrence or death, 0.92; 90.2% confidence interval [CI], 0.74–1.14; P = .005 for noninferiority).[1]
These results show that using six cycles of FOLFOX instead of neoadjuvant chemoradiation therapy is an acceptable option for this patient population, which is considered to represent potentially over one-half of all patients with locally advanced rectal cancer in the United States. Avoidance of chemoradiation therapy could potentially spare patients from long-term side effects, such as impairment in bowel, bladder and sexual function, increased risk of pelvic fractures and secondary malignancies, decreased bone marrow reserve, and fertility impacts.
Short-course preoperative radiation therapy followed by surgery and chemotherapy
The use of short-course radiation therapy before surgery has been a standard approach in parts of Europe and Australia.
Evidence (short-course preoperative radiation therapy):
- The use of short-course radiation therapy was evaluated in a randomized study in the Swedish Rectal Cancer Trial (NCT00337545).[5][Level of evidence A1] In the trial, 1,168 patients younger than 80 years with stage I to stage III resectable rectal adenocarcinoma were randomly assigned to receive preoperative radiation therapy (25 Gy in five fractions) or to undergo immediate surgery. Patients did not receive adjuvant chemotherapy.
- The 5-year OS rate was 58% in the radiation therapy group and 48% in the surgery group (P = .005).
- The rate of local control was 11% in the radiation therapy group and 27% in the surgery group (P < .001).
Subsequently, the Polish Rectal Trial and the Trans-Tasman Radiation Oncology Group (TROG) compared short-course preoperative radiation therapy with the standard long-course preoperative chemoradiation therapy administered with 5-FU.
- In the Polish Rectal Trial, 312 patients with clinical stage T3 or T4 rectal cancer were randomly assigned to receive preoperative radiation therapy (25 Gy in five fractions) followed by total mesorectal excision within 7 days, 6 months of adjuvant 5-FU/LV or preoperative chemoradiation therapy (50.4 Gy in 28 fractions with concurrent bolus 5-FU/LV), total mesorectal excision in 4 to 6 weeks after completion of radiation therapy, and 4 months of adjuvant 5-FU/LV.[6] The primary end point of the study was to detect a difference of at least 15% in sphincter preservation with a power of 80%.
- The rates of sphincter preservation were 61.2% in the short-course group and 58% in the chemoradiation therapy group (P = .570).
- The actuarial 4-year survival rate was 67.2% in the short-course group and 66.2% in the chemoradiation therapy group (HR, 1.01; 95% CI, 0.69–1.48; P = .960).
- The HR for local recurrence in the short-course group compared with the chemoradiation therapy group was 0.65 (95% CI, 0.32–1.28; P = .210).
- There was no difference in late toxicity between the short-course group and the chemoradiation therapy group.
- In the TROG trial (TROG 01.04 [NCT00145769]), 326 patients with ultrasound-staged or magnetic resonance imaging (MRI)–staged T3, N0 to N2, M0 rectal adenocarcinoma within 12 cm from the anal verge were randomly assigned to receive short-course radiation therapy (25 Gy in five fractions) followed by surgery 3 to 7 days later or long-course chemoradiation therapy (50.4 Gy in 28 fractions with concurrent continuous infusional 5-FU) followed by surgery in 4 to 6 weeks. All patients received adjuvant chemotherapy (5-FU/LV) after surgery. The trial was designed to have 80% power to detect a 10% difference in local recurrence at 3 years with a two-sided test at the 5% level of significance.[7]
- Cumulative incidence of local recurrence at 3 years was 7.5% for the short-course group and 4.4% for the long-course group (P = .24).
- The OS rate at 5 years was 74% for the short-course group and 70% for the long-course group (HR, 1.12; 95% CI, 0.76–1.67; P = .62).
- The Medical Research Council of the United Kingdom and the National Cancer Institute of Canada built on the short-course experience and conducted a randomized study (MRC CR07 and NCIC-CTG C016 [NCT00003422]) that compared short-course preoperative radiation therapy with selective postoperative chemoradiation therapy.[8] In the trial, 1,350 patients from 80 centers who had resectable rectal adenocarcinomas that were less than 15 cm from the anal verge were randomly assigned. Of note, pelvic MRI or ultrasound was not mandated. Patients randomly assigned to short-course radiation therapy received 25 Gy in five fractions followed by total mesorectal excision and then adjuvant chemotherapy according to the local center policy about nodal and margin status. Patients randomly assigned to selective postoperative chemoradiation therapy received immediate surgery followed by postoperative chemoradiation (45 Gy in 25 fractions with concurrent 5-FU) if their circumferential resection margin was 1 mm or smaller. Adjuvant chemotherapy for the group that received selective chemoradiation therapy was again given based on local standards regarding nodal and margin status.[8]
- The risk of local recurrence at 3 years was 4.4% in the preoperative short-course group and 10.6% in the selective chemoradiation therapy group (HR, 0.39; 95% CI, 0.27–0.58; P < .0001).
- OS did not differ between the groups.
Taken together, these studies demonstrate that short-course preoperative radiation therapy and long-course preoperative chemoradiation therapy are both reasonable treatment strategies for patients with stage II or III rectal adenocarcinoma.
Postoperative chemoradiation therapy
Progress in the development of postoperative treatment regimens relates to the integration of systemic chemotherapy and radiation therapy, as well as redefining the techniques for both modalities. The efficacy of postoperative radiation therapy and 5-FU-based chemotherapy for stages II and III rectal cancer was established by a series of prospective, randomized clinical trials, including:[9-11][Level of evidence A1]
- Gastrointestinal Tumor Study Group (GITSG-7175).
- Mayo/North Central Cancer Treatment Group (NCCTG-794751).
- National Surgical Adjuvant Breast and Bowel Project (NSABP-R-01).
These studies demonstrated an increase in DFS interval and OS when radiation therapy was combined with chemotherapy after surgical resection. After the publication in 1990 of the results of these trials, experts at a National Cancer Institute-sponsored Consensus Development Conference recommended postoperative combined-modality treatment for patients with stages II and III rectal carcinoma.[12] Since that time, preoperative chemoradiation therapy has become the standard of care, although postoperative chemoradiation therapy is still an acceptable alternative. For more information, see the Preoperative chemoradiation therapy section.
Additional evidence (postoperative chemoradiation therapy):
- Intergroup protocol 86-47-51 (MAYO-864751) compared continuous-infusion 5-FU (225 mg/m2/day throughout the entire course of radiation therapy) with bolus 5-FU (500 mg/m2/day for 3 consecutive days during the first and fifth weeks of radiation therapy).[13][Level of evidence A1]
- A 10% improvement in OS was demonstrated with the use of continuous-infusion 5-FU.
- A three-arm randomized trial determined whether continuous-infusion 5-FU given throughout the entire standard six-cycle course of adjuvant chemotherapy was more effective than continuous infusion 5-FU given only during pelvic radiation therapy. Median follow-up was 5.7 years.[14]
- Arm 1 received bolus 5-FU in two 5-day cycles before (500 mg/m2/day) and after (450 mg/m2/day) radiation therapy, with protracted venous infusion 5-FU (225 mg/m2/day) during radiation therapy.
- Arm 2 received continuous infusion 5-FU before (300 mg/m2/day for 42 days), after (300 mg/m2/day for 56 days), and during (225 mg/m2/day) radiation therapy.
- Arm 3 received bolus 5-FU/LV in two 5-day cycles before (5-FU, 425 mg/m2/day; LV, 20 mg/m2/day) and after (5-FU, 380 mg/m2/day; LV, 20 mg/m2/day) radiation therapy, and bolus 5-FU/LV (5-FU, 400 mg/m2/day; LV, 20 mg/m2/day; days 1 to 4, every 28 days) during radiation therapy. Levamisole (150 mg/day) was administered in 3-day cycles every 14 days before and after radiation therapy.
- No DFS, OS, or locoregional failure difference was detected (across all arms: 3-year DFS rate, 67%–69%; 3-year OS rate, 81%–83%; locoregional failure rate, 4.6%–8%).
- Lethal toxicity was less than 1%, with grades 3 to 4 hematologic toxicity in 55% of patients in arm 1 and in 49% of the patients in arm 3, versus in 4% of patients in the continuous-infusion arm.[14][Level of evidence A1]
- The final study results of Intergroup trial 0114 (INT-0114) showed no survival or local-control benefit with the addition of LV, levamisole, or both to 5-FU administered postoperatively for patients with stages II and III rectal cancers at a median follow-up of 7.4 years.[15][Level of evidence A1]
- A pooled analysis of 3,791 patients enrolled in clinical trials demonstrated that, for patients with T3, N0 disease, the 5-year OS rate with surgery plus chemotherapy (OS, 84%) compared favorably with the survival rates of patients treated with surgery plus radiation therapy and bolus chemotherapy (OS rate, 76%) or surgery plus radiation therapy and protracted-infusion chemotherapy (OS rate, 80%).[16]
Surgery
Total mesorectal excision with either low anterior resection or abdominoperineal resection is usually performed for stages II and III rectal cancer before or after chemoradiation therapy.
Retrospective studies have demonstrated that some patients with pathological T3, N0 disease treated with surgery and no additional therapy have a very low risk of local and systemic recurrence.[17]
Primary chemoradiation therapy followed by intensive surveillance for complete clinical responders
Since the advent of preoperative chemoradiation therapy in rectal cancer, the standard approach has been to recommend definitive surgical resection by either abdominoperineal resection or laparoscopic-assisted resection. In most series, after long-course chemoradiation therapy, 10% to 20% of patients will have a complete clinical response in which there is no sign of persistent cancer by imaging, rectal exam, or direct visualization during sigmoidoscopy. It was a long-held belief that most patients who did not undergo surgery for personal or medical reasons would experience a local and/or systemic recurrence. However, it became clear that patients with a pathological complete response to preoperative chemoradiation therapy followed by definitive surgery had a better DFS than did patients who did not have a pathological clinical response.[18]
Several single-institution studies have challenged this standard of care by demonstrating that most patients with complete clinical response will be cured of rectal cancer without surgery and that many patients who experience a local recurrence can be treated with surgical resection (abdominoperineal resection or laparoscopic-assisted resection) at the time of their recurrence.[19-22] These institutional series were hampered by their small size and inherent selection bias.
Evidence (primary chemoradiation therapy followed by intensive surveillance for complete clinical responders):
- Investigators in England performed the Oncological Outcomes after Clinical Complete Response in Patients with Rectal Cancer trial.[23] This was a propensity-score−matched cohort analysis. At a tertiary medical center in Manchester, 228 patients who chose watchful waiting from 2011 to 2013 after a complete clinical response to preoperative chemoradiation therapy were combined with 98 patients from a registry of three neighboring medical centers who chose watchful waiting after chemoradiation therapy beginning in 2005. A clinical complete response was considered in the absence of residual ulceration, stenosis, or mass within the rectum during digital rectal examination and endoscopic examination 8 weeks or more after completion of concurrent chemoradiation therapy. The only positive findings consistent with a complete clinical response during clinical or endoscopic examination were whitening of the mucosa and telangiectasia. Classification of complete clinical response required normal radiological imaging of the mesorectum and pelvis. Complete clinical responders (n = 129) were compared with a cohort of patients treated similarly who underwent surgery for complete resection (n = 228). Compared with all patients who underwent surgery, patients who chose watch and wait had tumors with an earlier T stage and N stage and that were less likely to be poorly differentiated.
- After a median follow-up of 33 months, 44 (34%) of the 129 patients who chose watchful waiting had a local recurrence, and 36 patients had a salvage resection.
- In the paired-cohort analysis, the 3-year non-regrowth DFS rate for all patients was 83% (95% CI, 76%–88%): 88% (95% CI, 75%–94%) for the watch-and-wait group and 78% (95% CI, 63%–87%) for the surgical resection group (log-rank P = .022).
- The 3-year OS rate was 96% (95% CI, 88%–98%) in the watch-and-wait group versus 87% (95% CI, 77%–93%) for the surgical resection group (log-rank P = .015).
- The 3-year colostomy-free survival rate was 74% (95% CI, 64%–82%) for the watch-and-wait group and 47% (95% CI, 37%–57%; log-rank P < .0001) for the surgical group.
Patients managed by watch and wait underwent a more intensive follow-up protocol consisting of outpatient digital rectal examination; MRI (every 4–6 months in the first 2 years); examination under anesthesia or endoscopy; computed tomography scan of the chest, abdomen, and pelvis; and at least two carcinoembryonic antigen measurements in the first 2 years. The optimal follow-up has not been determined.
For patients who have a complete clinical response to therapy, it is reasonable to consider a watch-and-wait approach with intensive surveillance instead of immediate surgical resection.
- In the OPRA study (NCT02008656), 324 patients with stage II/III rectal cancer were randomly assigned to receive either induction chemotherapy followed by chemoradiation therapy or chemoradiation therapy followed by consolidation chemotherapy. Patients had the potential to omit surgery based on response assessment.[24,25]
- The 5-year DFS rate was 71% (95% CI, 64%–79%) and the total mesorectal excision–free survival rate was 39% (95% CI, 32%–48%) in the patients who received induction chemotherapy followed by chemoradiation therapy. The 5-year DFS rate was 69% (95% CI, 62%–77%) and the total mesorectal excision–free survival rate was 54% (95% CI, 46%–62%) in the patients who received chemoradiation therapy followed by consolidation chemotherapy.[24][Level of evidence B1]
While the optimal surveillance regimen for patients undergoing nonoperative management is still under active study, the regimen in the OPRA trial involved periodic surveillance with digital rectal examination, sigmoidoscopy, and MRI. Digital rectal examination and flexible sigmoidoscopy were performed every 4 months for the first 2 years from the time of assessment of response, and every 6 months for the following 3 years. Rectal MRI was performed every 6 months for the first 2 years and yearly for the following 3 years. Patients could have additional assessments if clinically indicated.
The optimal follow-up for these patients has not been determined. For patients who have a complete clinical response to therapy, nonoperative management with intensive surveillance instead of immediate surgical resection is a standard-of-care approach.
Immunotherapy
Among patients with rectal adenocarcinomas, 5% to 10% of the tumors have mismatch repair deficiency or high MSI. Immune checkpoint inhibitors are efficacious as a first-line therapy for metastatic colorectal cancers, with overall response rates of 30% to 60%.[26-28] These responses proved durable, and prolonged OS was demonstrated in these settings.
Evidence (immunotherapy):
- A phase II trial (NCT04165772) studied dostarlimab, an anti-programmed death-1 (PD-1) monoclonal antibody, in 12 patients with locally advanced, mismatch repair–deficient, stage II or stage III rectal adenocarcinoma.[29]
- All 12 patients had clinical complete responses of 100% (95% CI, 74%–100%) after a median follow-up of 12 months. Patients’ cancers did not recur when the follow-up period ranged from 6 to 25 months. At the time of follow-up, chemoradiation therapy and surgery had been avoided.[29][Level of evidence C3]
- Before this approach becomes a new standard, more patients need to be evaluated. A longer follow-up period is required to ensure durability and assess the need for future surgery or chemoradiation therapy.
Chemotherapy regimens
Many academic oncologists suggest that FOLFOX be considered the standard for adjuvant chemotherapy in rectal cancer. However, there are no data about rectal cancer to support this consideration. FOLFOX has become the standard arm in the latest Intergroup study evaluating adjuvant chemotherapy in rectal cancer. An Eastern Cooperative Oncology Group trial (ECOG-E5202 [NCT00217737]) randomly assigned patients with stage II or III rectal cancer who received preoperative or postoperative chemoradiation therapy to receive 6 months of FOLFOX with or without bevacizumab, but this trial closed because of poor accrual. No efficacy data are available.
Preoperative oxaliplatin with chemoradiation therapy
Oxaliplatin has also shown radiosensitizing properties in preclinical models.[30] Phase II studies that combined oxaliplatin with fluoropyrimidine-based chemoradiation therapy have reported pathological complete response rates ranging from 14% to 30%.[31-35] Data from multiple studies have demonstrated a correlation between rates of pathological complete response and end points including distant metastasis-free survival, DFS, and OS.[36-38]
There is no current role for off-trial use of concurrent oxaliplatin and radiation therapy in the treatment of patients with rectal cancer.
Evidence (preoperative oxaliplatin with chemoradiation therapy):
- The ACCORD 12/0405-Prodige 2 trial (NCT00227747), which randomly assigned 598 patients with clinically staged T2 or T3 or resectable T4 rectal cancer accessible by digital rectal examination to either preoperative radiation therapy (45 Gy in 25 fractions over 5 weeks) with capecitabine (800 mg/m2 twice daily 5 of every 7 days) or to a higher dose of radiation (50 Gy in 25 fractions over 5 weeks) with the same dose of capecitabine and oxaliplatin (50 mg/m2 weekly). Total mesorectal excision was performed in 98% of both groups at a median interval of 6 weeks after chemoradiation therapy was completed.[39]
- Pathological complete response was the primary end point (albeit never validated as a true surrogate of OS). A higher percentage of patients achieved a pathological complete response in the oxaliplatin-treated group (19.2% vs. 13.9%). However, the difference did not reach statistical significance (P = .09).
- The rate of grade 3 or 4 toxicity was significantly higher in the oxaliplatin-treated group (25% vs. 11%; P < .001), and there was no difference in the rate of sphincter-sparing surgery (75% vs. 78%).
- Similarly, the STAR-01 trial investigated the role of oxaliplatin combined with 5-FU chemoradiation therapy for locally advanced rectal cancer.[40][Level of evidence A1] This Italian study randomly assigned 747 patients with resectable, locally advanced, clinically staged T3 or T4 and/or clinical N1 to N2 adenocarcinoma of the mid- to low-rectum to receive either continuous-infusion 5-FU with radiation therapy or to receive the same regimen in combination with oxaliplatin (60 mg/m2). Although the primary end point was OS, a protocol-planned analysis of response to preoperative therapy has been preliminarily reported.
- The rate of pathological complete response was equivalent at 16% in both arms (odds ratio, 0.98; 95% CI, 0.66–1.44; P = .904).
- There was no difference noted in the rate of pathologically positive lymph nodes, tumor infiltration beyond the muscularis propria, or the rate of circumferential margin positivity.
- An increase in grades 3 to 4 treatment-related acute toxicity was noted with the addition of oxaliplatin (24% vs. 8%; P <.001). Longer-term outcomes including OS have not yet been reported.
- The NSABP-R-04 trial (NCT00058474) randomly assigned 1,608 patients with clinically staged T3 or T4 or clinical node-positive adenocarcinoma within 12 cm of the anal verge in a 2 × 2 factorial design to one of the following four treatment groups:
- Intravenous (IV) continuous infusion 5-FU with radiation therapy.
- Capecitabine with radiation therapy.
- IV continuous infusion 5-FU plus weekly oxaliplatin with radiation therapy.
- Capecitabine plus weekly oxaliplatin with radiation therapy.
The primary objective of this study is locoregional disease control.[41][Level of evidence B1] Preliminary results, reported in abstract form at the 2011 American Society of Clinical Oncology annual meeting, demonstrated the following results:
- There was no significant difference in the rates of pathological complete response, sphincter-sparing surgery, or surgical downstaging between the 5-FU and capecitabine regimens or between the regimens with and without oxaliplatin.
- Patients treated with oxaliplatin had significantly higher rates of grade 3 and grade 4 acute toxicity (15.4% vs. 6.6%; P < .001).
- The German CAO/ARO/AIO-04 trial randomly assigned 1,236 patients with clinically staged T3 to T4 or clinical lymph node-positive adenocarcinoma within 12 cm from the anal verge to receive either concurrent chemoradiation therapy with 5-FU (week 1 and week 5) or concurrent chemoradiation therapy with 5-FU daily (250 mg/m2) and oxaliplatin (50 mg/m2).[42][Level of evidence B1]
- In contrast to the previous studies, a significantly higher rate of pathological complete response was achieved in patients who received oxaliplatin (17% vs. 13%; P = .038).
- There was no significant difference in rates of overall grades 3 and 4 toxicity; however, diarrhea and nausea and vomiting were more common among those treated with oxaliplatin.
- The 5-FU schedules in this study differed between the two arms, which may have contributed to the difference in outcomes noted. Longer follow-up will be necessary to determine the effect on the primary end point of the study, DFS.
Postoperative oxaliplatin-containing regimens
Based on results of several studies, oxaliplatin as a radiation sensitizer does not appear to add any benefit in terms of primary tumor response, and it has been associated with increased acute treatment-related toxicity. The question of whether oxaliplatin should be added to adjuvant 5-FU/LV for postoperative management of stages II and III rectal cancer is an ongoing debate. There are no randomized phase III studies to support the use of oxaliplatin for the adjuvant treatment of rectal cancer. However, the addition of oxaliplatin to 5-FU/LV for the adjuvant treatment of colon cancer is now considered standard care.
Evidence (postoperative oxaliplatin):
- In the randomized Multicenter International Study of Oxaliplatin/5-FU/LV in the Adjuvant Treatment of Colon Cancer (MOSAIC) study, the toxic effects and efficacy of FOLFOX4 (a 2-hour infusion of 200 mg/m2 LV, followed by a bolus of 400 mg/m2 5-FU, and then a 22-hour infusion of 600 mg/m2 5-FU on 2 consecutive days every 14 days for 12 cycles, plus a 2-hour infusion of 85 mg/m2 oxaliplatin on day 1, given simultaneously with LV) were compared with the same 5-FU/LV regimen without oxaliplatin when administered for 6 months. Each arm of the trial included 1,123 patients.[43]
- Preliminary results of the study, with 37 months of follow-up, demonstrated a significant improvement in DFS at 3 years in favor of FOLFOX4 (77.8% vs. 72.9%; P = .01). When initially reported, there was no difference in OS.[44][Level of evidence B1]
- Further follow-up at 6 years demonstrated that the OS for all patients (both stage II and stage III) who entered the study was not significantly different (OS rate, 78.5% FOLFOX4 vs. 76.0% 5-FU/LV group; HR, 0.84; 95% CI, 0.71–1.00).
- On subset analysis, the 6-year OS rate in patients with stage III colon cancer was 72.9% in the patients who received FOLFOX4 and 68.9% in the patients who received 5-FU/LV (HR, 0.80; 95% CI, 0.65–0.97; P = .023).[44][Level of evidence A1]
- Patients treated with FOLFOX4 experienced more frequent toxic effects, consisting mainly of neutropenia (41% > grade 3) and reversible peripheral sensory neuropathy (12.4% > grade 3).
- The results of the completed NSABP-C-07 study confirmed and extended the results of the MOSAIC trial.[45] In NSABP C-07, 2,492 patients with stage II or III colon or rectal cancer were randomly assigned to receive either FLOX (2-hour IV infusion of 85 mg/m2 oxaliplatin on days 1, 15, and 29 of each 8-week treatment cycle, followed by a 2-hour IV infusion of 500 mg/m2 LV plus bolus 500 mg/m2 5-FU 1 hour after the start of the LV infusion on days 1, 8, 15, 22, 29, and 36, followed by a 2-week rest period, for a total of three cycles [24 weeks]) or the same chemotherapy without oxaliplatin (Roswell Park regimen).
- The 3- and 4-year DFS rates were 71.8% and 67% for the Roswell Park regimen and 76.1% and 73.2% for FLOX, respectively.
- The HR was 0.80 (95% CI, 0.69–0.93), a 20% risk reduction in favor of FLOX (P < .004).
It is unclear whether the results of these colon cancer trials can be applied to the management of patients with rectal cancer. There are no randomized phase III studies to support the routine practice of administering FOLFOX as adjuvant therapy to patients with rectal cancer.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Schrag D, Shi Q, Weiser MR, et al.: Preoperative Treatment of Locally Advanced Rectal Cancer. N Engl J Med 389 (4): 322-334, 2023. [PUBMED Abstract]
- Sauer R, Becker H, Hohenberger W, et al.: Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351 (17): 1731-40, 2004. [PUBMED Abstract]
- Sauer R, Liersch T, Merkel S, et al.: Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30 (16): 1926-33, 2012. [PUBMED Abstract]
- Roh MS, Colangelo LH, O'Connell MJ, et al.: Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27 (31): 5124-30, 2009. [PUBMED Abstract]
- Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 336 (14): 980-7, 1997. [PUBMED Abstract]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al.: Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 93 (10): 1215-23, 2006. [PUBMED Abstract]
- Ngan SY, Burmeister B, Fisher RJ, et al.: Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 30 (31): 3827-33, 2012. [PUBMED Abstract]
- Sebag-Montefiore D, Stephens RJ, Steele R, et al.: Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 373 (9666): 811-20, 2009. [PUBMED Abstract]
- Thomas PR, Lindblad AS: Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: a review of the Gastrointestinal Tumor Study Group experience. Radiother Oncol 13 (4): 245-52, 1988. [PUBMED Abstract]
- Krook JE, Moertel CG, Gunderson LL, et al.: Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 324 (11): 709-15, 1991. [PUBMED Abstract]
- Fisher B, Wolmark N, Rockette H, et al.: Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 80 (1): 21-9, 1988. [PUBMED Abstract]
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 264 (11): 1444-50, 1990. [PUBMED Abstract]
- O'Connell MJ, Martenson JA, Wieand HS, et al.: Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 331 (8): 502-7, 1994. [PUBMED Abstract]
- Smalley SR, Benedetti JK, Williamson SK, et al.: Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol 24 (22): 3542-7, 2006. [PUBMED Abstract]
- Tepper JE, O'Connell M, Niedzwiecki D, et al.: Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control--final report of intergroup 0114. J Clin Oncol 20 (7): 1744-50, 2002. [PUBMED Abstract]
- Gunderson LL, Sargent DJ, Tepper JE, et al.: Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol 22 (10): 1785-96, 2004. [PUBMED Abstract]
- Willett CG, Badizadegan K, Ancukiewicz M, et al.: Prognostic factors in stage T3N0 rectal cancer: do all patients require postoperative pelvic irradiation and chemotherapy? Dis Colon Rectum 42 (2): 167-73, 1999. [PUBMED Abstract]
- Maas M, Nelemans PJ, Valentini V, et al.: Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11 (9): 835-44, 2010. [PUBMED Abstract]
- Maas M, Beets-Tan RG, Lambregts DM, et al.: Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 29 (35): 4633-40, 2011. [PUBMED Abstract]
- Lambregts DM, Maas M, Bakers FC, et al.: Long-term follow-up features on rectal MRI during a wait-and-see approach after a clinical complete response in patients with rectal cancer treated with chemoradiotherapy. Dis Colon Rectum 54 (12): 1521-8, 2011. [PUBMED Abstract]
- Smith JD, Ruby JA, Goodman KA, et al.: Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 256 (6): 965-72, 2012. [PUBMED Abstract]
- Dalton RS, Velineni R, Osborne ME, et al.: A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis 14 (5): 567-71, 2012. [PUBMED Abstract]
- Renehan AG, Malcomson L, Emsley R, et al.: Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 17 (2): 174-83, 2016. [PUBMED Abstract]
- Verheij FS, Omer DM, Williams H, et al.: Long-Term Results of Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J Clin Oncol 42 (5): 500-506, 2024. [PUBMED Abstract]
- Garcia-Aguilar J, Patil S, Gollub MJ, et al.: Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol 40 (23): 2546-2556, 2022. [PUBMED Abstract]
- Le DT, Uram JN, Wang H, et al.: PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372 (26): 2509-20, 2015. [PUBMED Abstract]
- Overman MJ, Lonardi S, Wong KYM, et al.: Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 36 (8): 773-779, 2018. [PUBMED Abstract]
- André T, Shiu KK, Kim TW, et al.: Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 383 (23): 2207-2218, 2020. [PUBMED Abstract]
- Cercek A, Lumish M, Sinopoli J, et al.: PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med 386 (25): 2363-2376, 2022. [PUBMED Abstract]
- Cividalli A, Ceciarelli F, Livdi E, et al.: Radiosensitization by oxaliplatin in a mouse adenocarcinoma: influence of treatment schedule. Int J Radiat Oncol Biol Phys 52 (4): 1092-8, 2002. [PUBMED Abstract]
- Gérard JP, Chapet O, Nemoz C, et al.: Preoperative concurrent chemoradiotherapy in locally advanced rectal cancer with high-dose radiation and oxaliplatin-containing regimen: the Lyon R0-04 phase II trial. J Clin Oncol 21 (6): 1119-24, 2003. [PUBMED Abstract]
- Machiels JP, Duck L, Honhon B, et al.: Phase II study of preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: the RadiOxCape study. Ann Oncol 16 (12): 1898-905, 2005. [PUBMED Abstract]
- Rödel C, Liersch T, Hermann RM, et al.: Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol 25 (1): 110-7, 2007. [PUBMED Abstract]
- Ryan DP, Niedzwiecki D, Hollis D, et al.: Phase I/II study of preoperative oxaliplatin, fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer: Cancer and Leukemia Group B 89901. J Clin Oncol 24 (16): 2557-62, 2006. [PUBMED Abstract]
- Valentini V, Coco C, Minsky BD, et al.: Randomized, multicenter, phase IIb study of preoperative chemoradiotherapy in T3 mid-distal rectal cancer: raltitrexed + oxaliplatin + radiotherapy versus cisplatin + 5-fluorouracil + radiotherapy. Int J Radiat Oncol Biol Phys 70 (2): 403-12, 2008. [PUBMED Abstract]
- García-Aguilar J, Hernandez de Anda E, Sirivongs P, et al.: A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum 46 (3): 298-304, 2003. [PUBMED Abstract]
- Guillem JG, Chessin DB, Cohen AM, et al.: Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 241 (5): 829-36; discussion 836-8, 2005. [PUBMED Abstract]
- Rödel C, Martus P, Papadoupolos T, et al.: Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 23 (34): 8688-96, 2005. [PUBMED Abstract]
- Gérard JP, Azria D, Gourgou-Bourgade S, et al.: Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 28 (10): 1638-44, 2010. [PUBMED Abstract]
- Aschele C, Cionini L, Lonardi S, et al.: Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 29 (20): 2773-80, 2011. [PUBMED Abstract]
- Roh MS, Yothers GA, O'Connell MJ, et al.: The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. [Abstract] J Clin Oncol 29 (Suppl 15): A-3503, 2011.
- Rödel C, Liersch T, Becker H, et al.: Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 13 (7): 679-87, 2012. [PUBMED Abstract]
- André T, Boni C, Mounedji-Boudiaf L, et al.: Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350 (23): 2343-51, 2004. [PUBMED Abstract]
- André T, Boni C, Navarro M, et al.: Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27 (19): 3109-16, 2009. [PUBMED Abstract]
- de Gramont A, Boni C, Navarro M, et al.: Oxaliplatin/5FU/LV in the adjuvant treatment of stage II and stage III colon cancer: efficacy results with a median follow-up of 4 years. [Abstract] J Clin Oncol 23 (Suppl 16): A-3501, 246s, 2005.
Treatment of Stage IV and Recurrent Rectal Cancer
Treatment of patients with advanced or recurrent rectal cancer depends on the location of the disease.
Treatment Options for Stage IV and Recurrent Rectal Cancer
Treatment options for stage IV and recurrent rectal cancer include:
Surgery with or without chemotherapy or radiation therapy
For patients with locally recurrent, liver-only, or lung-only metastatic disease, surgical resection, if feasible, is the only potentially curative treatment.[1] Patients with limited pulmonary metastasis, and patients with both pulmonary and hepatic metastasis, may also be considered for surgical resection, with 5-year survival possible in highly selected patients.[2-5] The presence of hydronephrosis associated with recurrence appears to be a contraindication to surgery with curative intent.[6]
Locally recurrent rectal cancer may be resectable, particularly if an inadequate prior operation was performed. For patients with local recurrence alone after an initial, attempted curative resection, aggressive local therapy with repeat low anterior resection and coloanal anastomosis, abdominoperineal resection, or posterior or total pelvic exenteration can lead to long-term disease-free survival.[7,8]
The use of induction chemoradiation therapy for previously nonirradiated patients with locally advanced pelvic recurrence (pelvic side-wall, sacral, and/or adjacent organ involvement) may increase resectability and allow for sphincter preservation.[9,10] Intraoperative radiation therapy in patients who underwent previous external-beam radiation therapy may improve local control in patients with locally recurrent disease, with acceptable morbidity.[11]
Systemic therapy
The following drugs are used alone and in combination with other drugs for patients with metastatic colorectal cancer:
- Fluorouracil (5-FU).
- Irinotecan.
- Oxaliplatin.
- Capecitabine.
- Bevacizumab.
- FOLFOXIRI (irinotecan, oxaliplatin, leucovorin [LV], and 5-FU).
- Cetuximab.
- Ziv-aflibercept.
- Ramucirumab.
- Panitumumab.
- Anti-epidermal growth factor receptor (EGFR) antibody versus anti-vascular endothelial growth factor (VEGF) antibody with first-line chemotherapy.
- Regorafenib.
- Fruquintinib.
- Trifluridine-tipiracil.
- Encorafenib with cetuximab for patients with BRAF V600E variants.
- Sotorasib with panitumumab for patients with KRAS G12C variants.
5-FU
When 5-FU was the only active chemotherapy drug, trials in patients with locally advanced, unresectable, or metastatic disease demonstrated partial responses and prolongation of the time-to-progression (TTP) of disease,[12,13] and improved survival and quality of life in patients who received chemotherapy versus best supportive care.[14-16] Several trials have analyzed the activity and toxic effects of various 5-FU/LV regimens using different doses and administration schedules and showed essentially equivalent results with a median survival time in the approximately 12-month range.[17]
Irinotecan and oxaliplatin
Three randomized studies in patients with metastatic colorectal cancer demonstrated improved response rates, progression-free survival (PFS), and overall survival (OS) when irinotecan or oxaliplatin was combined with 5-FU/LV.[18-20]
Evidence (irinotecan vs. oxaliplatin):
- An intergroup study (NCCTG-N9741 [NCT00003594]) compared irinotecan/5-FU/LV (IFL) with oxaliplatin/LV/5-FU (FOLFOX4) in first-line treatment for patients with metastatic colorectal cancer.[21][Level of evidence A1]
- Patients assigned to FOLFOX4 experienced an improved PFS compared with patients randomly assigned to IFL (median, 8.7 months vs. 6.9 months; P = .014; hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.61–0.89) and OS (19.5 months vs. 15.0 months; P = .001; HR, 0.66; 95% CI, 0.54–0.82).
- Subsequently, two studies compared FOLFOX with LV/5-FU/irinotecan (FOLFIRI), and patients were allowed to cross over after progression on first-line therapy.[22,23][Level of evidence B1]
- PFS and OS were identical between the treatment arms in both studies.
- The Bolus, Infusional, or Capecitabine with Camptosar-Celecoxib (BICC-C [NCT00094965]) trial evaluated several different irinotecan-based regimens in patients with previously untreated metastatic colorectal cancer: FOLFIRI, irinotecan plus bolus 5-FU/LV (mIFL), and capecitabine/irinotecan (CAPIRI).[24] The study randomly assigned 430 patients and was closed early due to poor accrual.
- The patients who received FOLFIRI had a better PFS than the patients who received either mIFL (7.6 months vs. 5.9 months; P = .004) or CAPIRI (7.6 months vs. 5.8 months; P = .015).
- Patients who received CAPIRI had the highest (grade 3 or higher) rates of nausea, vomiting, diarrhea, dehydration, and hand-foot syndrome.
Since the publication of these studies, the use of either FOLFOX or FOLFIRI is considered acceptable for first-line treatment of patients with metastatic colorectal cancer. However, when using an irinotecan-based regimen as first-line treatment of metastatic colorectal cancer, FOLFIRI is preferred.[24][Level of evidence B1]
Capecitabine
Before the advent of multiagent chemotherapy, two randomized studies demonstrated that capecitabine was associated with equivalent efficacy when compared with the Mayo Clinic regimen of 5-FU/LV.[25,26][Level of evidence A1]
Randomized phase III trials have addressed the equivalence of substituting capecitabine for infusional 5-FU. Two phase III studies have evaluated capecitabine/oxaliplatin (CAPOX) versus 5-FU/oxaliplatin regimens (FUOX or FUFOX).[27,28]
Evidence (oxaliplatin vs. capecitabine):
- The Arbeitsgemeinschaft Internische Onkologie (AIO) Colorectal Study Group randomly assigned 474 patients to either CAPOX or FUFOX.
- The median PFS was 7.1 months for the CAPOX arm and 8.0 months for the FUFOX arm (HR, 1.17; 95% CI, 0.96–1.43; P = .117), and the HR was in the prespecified equivalence range.[28]
- The Spanish Cooperative Group randomly assigned 348 patients to CAPOX or FUOX.[27][Level of evidence B1]
- The TTP was 8.9 months for CAPOX versus 9.5 months for FUOX (P = .153) and met the prespecified range for noninferiority.
When using an oxaliplatin-based regimen as first-line treatment of metastatic colorectal cancer, a CAPOX regimen is not inferior to a 5-FU/oxaliplatin regimen.
Bevacizumab
Bevacizumab can reasonably be added to either FOLFIRI or FOLFOX for patients undergoing first-line treatment of metastatic colorectal cancer. There are currently no completed randomized controlled studies evaluating whether continued use of bevacizumab in second-line or third-line treatment after progressing on a first-line bevacizumab regimen extends survival.
Evidence (bevacizumab):
- After bevacizumab was approved, the BICC-C trial was amended, and an additional 117 patients were randomly assigned to receive FOLFIRI/bevacizumab or mIFL/bevacizumab.[24]
- Although the primary end point of PFS was not significantly different, patients who received FOLFIRI/bevacizumab had a significantly better OS (28.0 months vs. 19.2 months; P = .037; HR for death, 1.79; 95% CI, 1.12–2.88).
- In the Hurwitz study, patients with previously untreated metastatic colorectal cancer were randomly assigned to either IFL or IFL/bevacizumab.[29]
- The patients randomly assigned to the IFL/bevacizumab arm experienced a significantly better PFS (10.6 months in the IFL/bevacizumab arm compared with 6.2 months in the IFL/placebo arm; HRdisease progression, 0.54; P < .001) and OS (20.3 months in the IFL/bevacizumab arm compared with 15.6 months in the IFL/placebo arm; HRdeath, 0.66; P < .001).[29]
- Despite the lack of direct data, in standard practice bevacizumab was added to FOLFOX as a standard first-line regimen based on the results of NCCTG-N9741.[21] Subsequently, in a randomized phase III study, 1,401 patients with untreated, stage IV colorectal cancer were randomly assigned in a 2 × 2 factorial design to CAPOX versus FOLFOX4, then to bevacizumab versus placebo. PFS was the primary end point.[30][Level of evidence B1]
- The median PFS was 9.4 months for patients who received bevacizumab and 8.0 months for the patients who received placebo (HR, 0.83; 97.5% CI, 0.72–0.95; P = .0023).
- Median OS was 21.3 months for patients who received bevacizumab and 19.9 months for patients who received placebo (HR, 0.89; 97.5% CI, 0.76–1.03; P = .077).
- The median PFS (intention-to-treat analysis) was 8.0 months in the pooled CAPOX-containing arms versus 8.5 months in the FOLFOX4-containing arms (HR, 1.04; 97.5% CI, 0.93–1.16), with the upper limit of the 97.5% CI being below the predefined noninferiority margin of 1.23.[30,31]
- The effect of bevacizumab on OS is likely to be less than what was seen in the original Hurwitz study.
- Investigators from the Eastern Cooperative Oncology Group randomly assigned patients who had progressed on 5-FU/LV and irinotecan to either FOLFOX or FOLFOX/bevacizumab.
- Patients randomly assigned to FOLFOX/bevacizumab experienced a statistically significant improvement in PFS compared with patients assigned to FOLFOX alone (7.43 months vs. 4.7 months; HR, 0.61; P < .0001) and OS (12.9 months vs. 10.8 months; HR, 0.75; P = .0011).[32][Level of evidence A1]
FOLFOXIRI
Evidence (FOLFOXIRI):
- The combination of FOLFOXIRI with bevacizumab was compared with FOLFIRI with bevacizumab in a randomized, phase III study of 508 patients with untreated metastatic colorectal cancer.[33]
- The median PFS was 12.1 months in the FOLFOXIRI group, compared with 9.7 months in the FOLFIRI group (HR for progression, 0.75; 95% CI, 0.62–0.90; P = .003). OS was not significantly different between the groups (31.0 vs. 25.8 months; HRdeath, 0.79; 95% CI, 0.63–1.00; P = .054).[33][Level of evidence B1]
- Patients who received FOLFOXIRI had significantly more grade 3 and 4 toxicities, including neutropenia, stomatitis, and peripheral neuropathy.
Cetuximab
Cetuximab is a partially humanized monoclonal antibody against EGFR. Importantly, patients with KRAS-altered tumors may experience worse outcome when cetuximab is added to multiagent chemotherapy regimens containing bevacizumab.
Evidence (cetuximab):
- For patients who had disease progression while receiving irinotecan-containing regimens, a randomized phase II study was performed that used either cetuximab or irinotecan/cetuximab.[34][Level of evidence C3]
- The median TTP for patients who received cetuximab was 1.5 months, compared with median TTP of 4.2 months for patients who received irinotecan and cetuximab. Based on this study, cetuximab was approved for use in patients with metastatic colorectal cancer refractory to 5-FU and irinotecan.
- The Crystal Study (EMR 62202-013 [NCT00154102]) randomly assigned 1,198 patients with stage IV colorectal cancer to FOLFIRI with or without cetuximab.[35][Level of evidence B1]
- The addition of cetuximab was associated with an improved PFS (HR, 0.85; 95% CI, 0.72–0.99; stratified log-rank P = .048) but not OS.
- Retrospective studies of patients with metastatic colorectal cancer have suggested that responses to anti-EGFR antibody therapy are confined to patients with tumors that harbor wild types of KRAS (i.e., lack activating variants at codon 12 or 13 of the KRAS gene).
- A subset analysis evaluating efficacy in relation to KRAS status was done in patients enrolled in the Crystal Study. There was a significant interaction for KRAS variant status and treatment for tumor response (P = .03) but not for PFS (P = .07). Among patients with KRAS wild-type tumors, the HR favored the FOLFIRI/cetuximab group (HR, 0.68; 95% CI, 0.50–0.94).
- In a randomized trial, patients with metastatic colorectal cancer received capecitabine/oxaliplatin/bevacizumab with or without cetuximab.[36][Level of evidence B1]
- The median PFS was 9.4 months in the group who received cetuximab and 10.7 months in the group who did not receive cetuximab (P = .01).
- In a subset analysis, patients with KRAS-altered tumors who received cetuximab had significantly decreased PFS compared with patients with wild-type KRAS tumors who received cetuximab (8.1 months vs. 10.5 months; P = .04).
- Among patients with KRAS-altered tumors, PFS was significantly shorter in those who received cetuximab than those did not receive cetuximab (8.1 months vs. 12.5 months; P = .003). OS was also significantly shorter (17.2 months vs. 24.9 months, respectively; P = .03).
- The Medical Research Council (MRC) COIN trial (NCT00182715) sought to determine if adding cetuximab to combination chemotherapy with a fluoropyrimidine and oxaliplatin in first-line treatment for patients with KRAS wild-type tumors was beneficial.[37,38] In addition, the MRC sought to evaluate the effect of intermittent chemotherapy versus continuous chemotherapy. The 1,630 patients were randomly assigned to three treatment groups:
- Arm A: fluoropyrimidine/oxaliplatin.
- Arm B: fluoropyrimidine/oxaliplatin/cetuximab.
- Arm C: intermittent fluoropyrimidine/oxaliplatin.
The comparisons between arms A and B and arms A and C were analyzed and published separately.[37,38]
- In patients with KRAS wild-type tumors (arm A, n = 367; arm B, n = 362), OS did not differ between treatment groups (median survival, 17.9 months [interquartile range (IQR), 10.3–29.2] in the control group vs. 17.0 months [IQR, 9.4–30.1] in the cetuximab group; HR, 1.04; 95% CI, 0.87–1.23; P = .67). Similarly, there was no effect on PFS (8.6 months [IQR, 5.0–12.5] in the control group vs. 8.6 months [IQR, 5.1–13.8] in the cetuximab group; HR, 0.96; 95% CI, 0.82–1.12, P = .60).[37,38][Level of evidence A1]
- The reasons for lack of benefit in adding cetuximab are unclear. Subset analyses suggest that the use of capecitabine was associated with an inferior outcome, and the use of second-line therapy was less in patients treated with cetuximab.
- There was no difference between the continuously treated patients (arm A) and the intermittently treated patients (arm C).
- Median survival in the intent-to-treat population (n = 815 in both groups) was 15.8 months (IQR, 9.4–26.1) in arm A and 14.4 months (IQR, 8.0–24.7) in arm C (HR, 1.084; 80% CI, 1.008–1.165).
- In the per-protocol population, which included only those patients who were free from progression at 12 weeks and randomly assigned to continue treatment or go on a chemotherapy holiday (arm A, n = 467; arm C, n = 511), median survival was 19.6 months (IQR, 13.0–28.1) in arm A and 18.0 months (IQR, 12.1–29.3) in arm C (HR, 1.087, 95% CI, 0.986–1.198).
- The upper limits of CIs for HRs in both analyses were greater than the predefined noninferiority boundary. While intermittent chemotherapy was not deemed noninferior, there appeared to be clinically insignificant differences in patient outcomes.
Ziv-aflibercept
Ziv-aflibercept is an anti-VEGF molecule and has been evaluated as a component of second-line therapy in patients with metastatic colorectal cancer.
Evidence (ziv-aflibercept):
- In one trial, 1,226 patients were randomly assigned to receive ziv-aflibercept (4 mg/kg intravenously) or placebo every 2 weeks in combination with FOLFIRI.[39][Level of evidence A2]
- Patients who received ziv-aflibercept plus FOLFIRI had significantly improved OS rates, with median survival times of 13.50 months compared with patients who received placebo plus FOLFIRI, with median survival times of 12.06 months (HR, 0.817; 95.34% CI, 0.713–0.937; P = .0032).
- Patients who received ziv-aflibercept plus FOLFIRI also had significantly improved PFS rates, with median PFS rates of 6.90 months compared with patients who received placebo plus FOLFIRI, with median PFS rates of 4.67 months (HR, 0.758; 95% CI, 0.661–0.869; P < .0001).
- Based on these results, the use of FOLFIRI plus ziv-aflibercept is an acceptable second-line regimen for patients previously treated with FOLFOX-based chemotherapy. Whether to continue bevacizumab or initiate ziv-aflibercept in second-line therapy has not been addressed as yet in any clinical trial, and there are no data available.
Ramucirumab
Ramucirumab is a fully humanized monoclonal antibody that binds to vascular endothelial growth factor receptor-2 (VEGFR-2).
Evidence (ramucirumab):
- In the randomized, unblinded, phase III RAISE study (NCT01183780), 1,072 patients with stage IV colorectal cancer who had progressed on first-line chemotherapy were randomly assigned to FOLFIRI with or without ramucirumab (8 mg/kg).[40][Level of evidence A1]
- Patients assigned to FOLFIRI plus ramucirumab had a significant improvement in median OS (13.3 months vs. 11.7 months; HR, 0.84; P = .0219) and PFS (5.7 months vs. 4.5 months; HR, 0.793; P = .0005).
- Grade 3 adverse events were more common in the ramucirumab group, including grade 3 neutropenia.
- Based on this data, FOLFIRI plus ramucirumab is an acceptable second-line regimen for patients previously treated with FOLFOX-bevacizumab. Whether to continue bevacizumab in second-line chemotherapy or use ramucirumab in second-line chemotherapy has not yet been addressed in a clinical trial.
Panitumumab
Panitumumab is a fully humanized antibody against the EGFR. The U.S. Food and Drug Administration (FDA) approved panitumumab for use in patients with metastatic colorectal cancer refractory to chemotherapy.[41] In clinical trials, panitumumab demonstrated efficacy as a single agent or in combination therapy, which was consistent with the effects on PFS and OS with cetuximab. There appears to be a consistent class effect.
Evidence (panitumumab):
- In a phase III trial, patients with chemotherapy-refractory colorectal cancer were randomly assigned to panitumumab or best supportive care.[41][Level of evidence B1]
- Patients who received panitumumab experienced an improved PFS (8 weeks vs. 7.3 weeks; HR, 0.54; 95% CI, 0.44–0.66; P < .0001).
- There was no difference in OS, which could be because 76% of patients on best supportive care crossed over to panitumumab.
- In the Panitumumab Randomized Trial in Combination With Chemotherapy for
Metastatic Colorectal Cancer to Determine Efficacy (PRIME [NCT00364013]) study, 1,183 patients were randomly assigned to FOLFOX4 with or without panitumumab as first-line therapy for metastatic colorectal cancer. The study was amended to enlarge the sample size to address patients with KRAS wild-type tumors and patients withKRAS-altered tumors separately.[42][Level of evidence B1]
- For patients with KRAS wild-type tumors, a statistically significant improvement in PFS was observed in those who received panitumumab/FOLFOX4 compared with those who received only FOLFOX4 (HR, 0.80; 95% CI, 0.66–0.97; stratified log-rank P = .02).
- Median PFS was 9.6 months (95% CI, 9.2–11.1 months) for patients who received panitumumab/FOLFOX4 and 8.0 months (95% CI, 7.5–9.3 months) for patients who received FOLFOX4. OS was not significantly different between the groups (HR, 0.83; 95% CI, 0.67–1.02; P = .072).
- For patients with KRAS-altered tumors, PFS was worse with the addition of panitumumab (HR, 1.29; 95% CI, 1.04–1.62; stratified log-rank P = .02).
- Median PFS was 7.3 months (95% CI, 6.3–8.0 months) for panitumumab/FOLFOX4 and 8.8 months (95% CI, 7.7–9.4 months) for FOLFOX4 alone.
- Subsequently, a retrospective analysis evaluated patients with wild-type KRAS exon 2 wild-type status for other KRAS and BRAF variants.[43][Level of evidence C1]
- Of the 620 patients who were initially identified as not having a variant in exon 2 of KRAS, 108 patients (17%) were found to have additional RAS variants and 53 patients (8%) were found to have BRAF variants. In a retrospective analysis, patients without any RAS or BRAF variants had a longer PFS (10.8 months vs. 9.2 months, P = .002) and OS (28.3 months vs. 20.9 months, P = .02) when assigned to the FOLFOX-4/panitumumab arm than the patients assigned to the FOLFOX-4 arm.
- Similarly, the addition of panitumumab to a regimen of FOLFOX/bevacizumab resulted in a worse PFS and worse toxicity compared with a regimen of FOLFOX/bevacizumab alone in patients not selected for KRAS variant in metastatic rectal cancer (11.4 months vs. 10.0 months; HR, 1.27; 95% CI, 1.06–1.52).[44][Level of evidence B1]
- In another study (NCT00339183), patients with metastatic colorectal cancer who had already received a fluoropyrimidine regimen were randomly assigned to either FOLFIRI or FOLFIRI/panitumumab.[45][Level of evidence B1]
- In a post hoc analysis, patients with KRAS wild-type tumors experienced a statistically significant PFS advantage (HR, 0.73; 95% CI, 0.59–0.90; stratified log-rank P = .004).
- Median PFS was 5.9 months (95% CI, 5.5–6.7 months) for FOLFIRI/panitumumab and 3.9 months (95% CI, 3.7–5.3 months) for FOLFIRI alone.
- OS was not significantly different. Median OS was 14.5 months for the FOLFIRI/panitumumab group versus 12.5 months for the FOLFIRI alone group.
- Patients with KRAS-altered tumors experienced no benefit from the addition of panitumumab.
- In a post hoc analysis, patients with KRAS wild-type tumors experienced a statistically significant PFS advantage (HR, 0.73; 95% CI, 0.59–0.90; stratified log-rank P = .004).
Anti-EGFR antibody versus anti-VEGF antibody with first-line chemotherapy
In the management of patients with stage IV colorectal cancer, it is unknown whether patients with KRAS wild-type cancer should receive an anti-EGFR antibody with chemotherapy or an anti-VEGF antibody with chemotherapy. Two studies attempted to answer this question.[46,47]
Evidence (anti-EGFR antibody vs. anti-VEGF antibody with first-line chemotherapy):
- The FIRE-3 study (NCT00433927) randomly assigned 592 patients with KRAS exon 2 wild-type tumors who were previously untreated to FOLFIRI plus cetuximab (297 patients) or FOLFIRI plus bevacizumab (295 patients). The primary end point of the study was objective response rate.[46][Level of evidence A1]
- The objective response rate was not significantly different between the groups (objective response rate, 62.0%; 95% CI, 56.2–67.5 vs. objective response rate, 58.0%; 95% CI, 52.1–63.7; odds ratio, 1.18; 95% CI, 0.85–1.64; P = .18).
- Median PFS was 10.0 months (95% CI, 8.8–10.8) in the cetuximab group and 10.3 months (95% CI, 9.8–11.3) in the bevacizumab group (HR, 1.06; 95% CI, 0.88–1.26; P = .55).
- Median OS was 28.7 months (95% CI, 24.0–36.6) in the cetuximab group compared with 25.0 months (range, 22.7–27.6 months) in the bevacizumab group (HR, 0.77; 95% CI, 0.62–0.96; P = .017).
- In a post hoc analysis of patients with expanded RAS wild-type tumors (sequencing for mutational hot spots within KRAS and NRAS genes, including exon 2 codons 12 and 13; exon 3 codons 59 and 61; and exon 4 codons 117 and 146), the median OS was 33.1 months (95% CI, 24.5–39.4) in the cetuximab group compared with 25.0 months (95% CI, 23.0–28.1) in the bevacizumab group (HR, 0.70; 95% CI, 0.54–0.90; P = .0059).[48]
- Of note, only 52% of patients assigned to the bevacizumab arm subsequently received cetuximab or panitumumab.[49]
- The Cancer and Leukemia Group B Intergroup study 80405 (NCT00265850) was presented at the American Society of Clinical Oncology meeting in 2014. This study randomly assigned 2,334 previously untreated patients with KRAS wild-type cancer to chemotherapy (FOLFOX or FOLFIRI) plus bevacizumab or chemotherapy plus cetuximab. OS was the primary end point.[47][Level of evidence B1]
- There was no statistically significant difference in OS among the patients assigned to bevacizumab or cetuximab (for OS differences, chemotherapy/bevacizumab = 29.04 months [range, 25.66–31.21 months] vs. chemotherapy/cetuximab = 29.93 months [range, 27.56–31.21 months]; HR, 0.92 [0.78–1.09]; P = .34).
Based on these two studies, no apparent significant difference is evident about starting treatment with chemotherapy/bevacizumab or chemotherapy/cetuximab in patients with KRAS wild-type metastatic colorectal cancer. However, in patients with KRAS wild-type cancer, administration of an anti-EGFR antibody at some point during management improves OS.
Regorafenib
Regorafenib is an inhibitor of multiple tyrosine kinase pathways including VEGF. In 2012, the FDA granted approval for the use of regorafenib in patients who had progressed on previous therapy.
Evidence (regorafenib):
- The safety and effectiveness of regorafenib were evaluated in a single, clinical study of 760 patients with previously treated metastatic colorectal cancer. Patients were randomly assigned in a 2:1 fashion to receive regorafenib or a placebo in addition to the best supportive care.[50,51]
- Patients treated with regorafenib had a statistically significant improvement in OS (6.4 months in the regorafenib group vs. 5.0 months in the placebo group; HR, 0.77; 95% CI, 0.64–0.94; one-sided P = .0052).
Fruquintinib
Fruquintinib is an inhibitor of VEGF receptors 1, 2 and 3. In 2023, the FDA approved fruquintinib for adults with metastatic colorectal cancer who had previously received fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and an anti-VEGF therapy. If patients had RAS wild-type disease and were medically appropriate, an anti-EGFR therapy was also required
Evidence (fruquintinib):
- The phase III, international, double-blind, placebo-controlled FRESCO-2 trial (NCT04322539) included 691 patients with metastatic colorectal adenocarcinoma who had received all standard approved cytotoxic and targeted therapies. Patients had disease progression during, or were intolerant of, trifluridine-tipiracil, regorafenib, or both. Patients had received a median of four lines of prior systemic therapy. Patients were randomly assigned in a 2:1 fashion to receive one of the following regimens:
- Fruquintinib (5 mg orally once daily on days 1–21 of a 28-day cycle).
- Placebo (orally once daily on days 1–21 of a 28-day cycle).
All patients received best supportive care. A total of 461 patients received fruquintinib, and 230 received placebo. The primary end point was OS.[52] The results were as follows:
- The median OS was significantly longer in the fruquintinib group (7.4 months; 95% CI, 6.7–8.2) than in the placebo group (4.8 months; 95% CI, 4.0–5.8) (HR, 0.66; 95% CI, 0.55–0.80; P < .0001).[52][Level of evidence A1]
- The median PFS was 3.7 months (95% CI. 3.5–3.8) in the fruquintinib group and 1.8 months (95% CI, 1.8–1.9) in the placebo group (HR, 0.26; 95% CI, 0.21–0.34; P < .001).
- Subgroup analyses demonstrated the benefit of fruquintinib as opposed to placebo in most subgroups. Notably, this benefit was seen in patients with both RAS variants and RAS wild-type disease, patients with liver metastases, and patients pretreated with trifluridine-tipiracil with or without regorafenib.
- The disease control rate was significantly longer in the fruquintinib group (56%) than in the placebo group (16%), with an adjusted difference of 39% (95% CI, 32.8%–46.0%; P < .0001).
- Grade 3 or higher adverse events occurred in 65% of patients in the fruquintinib group. The most common adverse events were hypertension (14%), asthenia (8%), abnormal hepatic function (8%), dermatologic toxicity (7%), and hand-foot syndrome (6%). Grade 3 or higher adverse events occurred in 50% of patients in the placebo group. The most common were abnormal hepatic function (9%), infection (6%), and asthenia (4%).
Trifluridine-tipiracil
Trifluridine-tipiracil (Lonsurf; also called TAS-102) is an orally administered combination of a thymidine-based nucleic acid analogue, trifluridine, and a thymidine phosphorylase inhibitor, tipiracil hydrochloride. Trifluridine, in its triphosphate form, inhibits thymidylate synthase; therefore, trifluridine, in this form, has an anti-tumor effect. Tipiracil hydrochloride is a potent inhibitor of thymidine phosphorylase, which actively degrades trifluridine. The combination of trifluridine and tipiracil allows for adequate plasma levels of trifluridine.
Evidence (trifluridine-tipiracil):
- A phase III double-blind study (RECOURSE [NCT01607957]) randomly assigned 800 patients with stage IV colorectal cancer whose cancer had been refractory to two previous therapies. Patients were required to have received 5-FU, oxaliplatin, irinotecan, bevacizumab and, if the patients had KRAS wild-type cancer, cetuximab or panitumumab. Patients were randomly assigned in a 2:1 ratio to receive best supportive care plus trifluridine-tipiracil (n = 534) or placebo (n = 266). The median age of patients was 63 years, and most patients (60%–63%) received four or more previous lines of therapy. All patients had formerly received a fluoropyrimidine, irinotecan, oxaliplatin, and bevacizumab, and 52% of them had received an EGFR inhibitor. Approximately 20% of the patients had received previous treatment with regorafenib.[53][Level of evidence A1]
- Trifluridine-tipiracil was administered at 35 mg/m2 twice daily with meals for 5 days, with 2 days of rest for 2 weeks, followed by a 14-day rest period.
- The primary end point of the study was OS. The median OS for patients with metastatic colorectal cancer who received trifluridine-tipiracil was 7.1 months compared with 5.3 months for those who received a placebo (HR, 0.68; P < .0001).
- The median PFS in the trifluridine-tipiracil arm was 2 months versus 1.7 months with a placebo (HR, 0.48; P < .0001).
- Secondary end points focused on PFS, overall response rate, and disease control rate.
- The overall response rate was 1.6% with trifluridine-tipiracil, which consisted of a complete response in one patient and partial responses in other patients. The overall response rate with a placebo was 0.4% (P = .29).
The FDA approved trifluridine-tipiracil for the treatment of patients with metastatic colorectal cancer, based on the results of the RECOURSE trial.
Evidence (combination of trifluridine-tipiracil and bevacizumab):
- A phase III, international, multi-institutional trial (SUNLIGHT [NCT04737187]) included 492 patients with stage IV colorectal cancer whose cancer was refractory to up to two prior chemotherapy regimens. Patients were required to have received 5-FU, oxaliplatin, irinotecan, an anti-VEGF monoclonal antibody, or an anti-EGFR monoclonal antibody (for patients with RAS wild-type disease). Patients were randomly assigned 1:1 to receive either trifluridine-tipiracil monotherapy (n = 246) or trifluridine-tipiracil combined with bevacizumab (n = 246). The median patient age was 62 years for the combination arm, and 64 years for the monotherapy arm, and most patients (93% and 91%, respectively) had received two prior lines of therapy. More than 98% of patients in both arms had received 5-FU, irinotecan, and oxaliplatin, and more than 93% of patients with RAS wild-type variants in both arms had received anti-EGFR monoclonal antibody therapy. The median follow-up was 14.2 months. Trifluridine-tipiracil was given at 35 mg/m2 twice daily on days 1 to 5 and 8 to 12 of a 28-day cycle. Bevacizumab was given on days 1 and 15 of each cycle at 5 mg/kg. The primary end point of the study was OS.[54]
- The median OS was 10.8 months for patients who received trifluridine-tipiracil and bevacizumab and 7.5 months for patients who received trifluridine-tipiracil monotherapy (HR, 0.61; 95% CI, 0.49–0.77; P < .001).[54][Level of evidence A1]
- OS was significant across subgroups, including patients with RAS variants and wild-type variants, patients with microsatellite instability-high (MSI-H) and microsatellite stability cancers, and both for patients previously treated with bevacizumab and bevacizumab-naïve patients.
- The median PFS was 5.6 months in the combination arm and 2.4 months in the trifluridine-tipiracil monotherapy arm (HR, 0.44; 95% CI, 0.36–0.54; P < .001).
- Secondary end points focused on PFS, overall response rate, and safety.
- The overall response rate was 6.1% for the combination arm and 1.2% for the monotherapy arm.
- Adverse events leading to therapy discontinuation were observed in 12.6% of patients in both arms. Dose reductions occurred in 16.3% of patients in the combination group and 12.2% of patients in the trifluridine-tipiracil monotherapy group. The most common adverse event was neutropenia, with grade 3 or 4 neutropenia observed in 43% of patients in the combination arm and 32% of patients in the monotherapy arm.
The FDA approved the combination of trifluridine-tipiracil and bevacizumab for the treatment of patients with previously treated metastatic colorectal cancer based on the results of the SUNLIGHT trial.
Encorafenib with cetuximab for patients with BRAF V600E variants
BRAF V600E variants occur in about 10% of metastatic colorectal cancers and are an indicator of poor prognosis. Unlike in melanoma, BRAF inhibitor monotherapy has not shown a benefit in colorectal cancer, and multiple studies have evaluated concurrent targeting of the EGFR-MAPK pathway.
Evidence (encorafenib with cetuximab for patients with BRAF V600E variants):
- Encorafenib (BRAF inhibitor), binimetinib (MEK inhibitor), and cetuximab (EGFR inhibitor):
In the international, open-label, randomized, phase III BEACON trial, patients with metastatic colorectal cancer and BRAF V600E variants who previously received one or two treatment regimens were enrolled.[55] The trial randomly assigned 665 patients in a 1:1:1 ratio to receive one of the following regimens:
The primary end points were OS and objective response in the triplet-therapy group when compared with the control group.
- Triplet therapy: encorafenib (300 mg PO daily), binimetinib (45 mg PO twice daily), and cetuximab (400 mg/m2 IV loading dose followed by 250 mg/m2 IV weekly) (n = 224).
- Doublet therapy: encorafenib and cetuximab (as per triplet therapy dosing) (n = 220).
- Control group: FOLFIRI or irinotecan (every 2 weeks) with cetuximab (400 mg/m2 IV loading dose followed by 250 mg/m2 IV weekly) (n = 221).
Updated data were presented in abstract form in 2020:[56]- The OS was 9.0 months in the triplet-therapy arm and 5.4 months in the control group (HR, 0.52; 95% CI, 0.39–0.70, P < .0001).[55][Level of evidence A1]
- Grade 3 or higher side effects occurred in 58% of patients in the triplet-therapy arm, with 10% of patients experiencing diarrhea and 11% of patients experiencing anemia. Grade 3 or higher side effects occurred in 50% of patients in the doublet-therapy arm and 61% of patients in the control arm. Fourteen percent of patients who received the doublet regimen developed melanocytic nevi.
- The median OS was 9.3 months in both the triplet-therapy and doublet-therapy arms and 5.9 months in the control arm (HR, 0.60 for triplet therapy vs. control; 95% CI, 0.47–0.75; HR, 0.61 for doublet therapy vs. control; 95% CI, 0.48–0.77).
- The objective response rate was 26.8% for patients who received triplet therapy (95% CI, 21.1%–33.1%) and 19.5% for patients who received doublet therapy (95% CI, 14.5%–
Based on these data, the FDA approved the combination of encorafenib with cetuximab for patients with previously treated metastatic colorectal cancer and BRAF V600E variants.
Sotorasib with panitumumab for patients with KRAS G12C variants
KRAS G12C variants are found in approximately 4% of patients with colorectal cancer and are associated with poor prognosis.[57-60] Sotorasib and adagrasib are two of the first KRAS G12C–specific inhibitors to show benefit in patients with KRAS G12C–mutated cancers.[61,62] Given that EGFR reactivation is a well-described resistance mechanism to KRAS G12C inhibition, sotorasib was combined with the anti-EGFR antibody panitumumab in patients with colorectal cancer and KRAS G12C variants.
- The phase III, multicenter, open-label CodeBreaK 300 trial (NCT05198934) included patients with metastatic colorectal cancer and KRAS G12C variants who previously received treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.[61] The trial randomly assigned 160 patients 1:1:1 to receive one of the following regimens:
- Doublet therapy with sotorasib 960 mg once daily plus panitumumab (6 mg/kg IV every 2 weeks) (n = 53).
- Doublet therapy with sotorasib 240 mg once daily plus panitumumab (6 mg/kg IV every 2 weeks) (n = 53).
- Investigator's choice standard-of-care therapy with trifluridine-tipiracil (35 mg/m2) or regorafenib (160 mg once daily) (control group).
The primary end point was PFS assessed by blinded independent central review according to RECIST 1.1. Secondary end points included OS and objective response rate.
- The median PFS was 5.6 months (95% CI, 4.2–6.3) in the 960 mg-sotorasib/panitumumab group, 3.9 months (95% CI, 3.7–5.8) in the 240 mg-sotorasib/panitumumab group, and 2.2 months (95% CI, 1.9–3.9) in the standard-of-care group.[61][Level of evidence B1]
- The HR for progression of disease or death was 0.49 (95% CI, 0.3–0.8; P = .006) for the 960 mg-sotorasib/panitumumab group and 0.58 (95% CI, 0.36–0.98; P = .03) for the 240 mg-sotorasib/panitumumab group.
- The objective response rate was 26.4% (95% CI, 15.3%–40.3%) in the 960 mg-sotorasib/panitumumab group, 5.7% (95% CI, 1.2%–15.7%) in the 240 mg-sotorasib/panitumumab group, and 0% (95% CI, 0.0%–6.6%) in the standard-of-care group. OS data are still not mature. However, at data cutoff the HRs were 0.77 (95% CI, 0.4–1.45) for the 960 mg-sotorasib/panitumumab group and 0.91 (95% CI, 0.48–1.71) for the 240 mg-sotorasib/panitumumab group when compared with standard-of-care therapy.
- Grade 3 or higher side effects occurred in 35.8% of patients who received 960 mg sotorasib/panitumumab, 30.2% of patients who received 240 mg sotorasib/panitumumab, and 43.1% of patients who received the standard of care. The most common adverse effects with combined sotorasib and panitumumab therapy were skin-related toxicities and hypomagnesemia.
Second-line chemotherapy
Second-line chemotherapy with irinotecan in patients treated with 5-FU/LV as first-line therapy demonstrated improved OS when compared with either infusional 5-FU or supportive care.[63-66]
Similarly, a phase III trial randomly assigned patients who progressed on irinotecan and 5-FU/LV to bolus and infusional 5-FU/LV, single-agent oxaliplatin, or FOLFOX4. The median TTP for FOLFOX4 versus 5-FU/LV was 4.6 months versus 2.7 months (stratified log-rank test, 2-sided P < .001).[67][Level of evidence B1]
Immunotherapy
Approximately 4% of patients with stage IV colorectal cancer have tumors that are mismatch repair deficient (dMMR) or microsatellite unstable/MSI-H. The MSI-H phenotype is associated with germline defects in the MLH1, MSH2, MSH6, and PMS2 genes and is the primary phenotype observed in tumors from patients with hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome. Patients can also have the MSI-H phenotype because one of these genes was silenced via DNA methylation. Testing for microsatellite instability can be done with molecular genetic tests, which look for microsatellite instability in the tumor tissue, or with immunohistochemistry, which looks for the loss of mismatch repair proteins. MSI-H status has historically been prognostic of increased survival for patients with earlier-stage disease and since 2015, has also been found to predict tumor response to checkpoint inhibition.
The FDA approved pembrolizumab for use in patients with treatment-naïve, metastatic, dMMR/MSI-H colorectal cancer in 2020. Studies regarding first-line treatment with dual checkpoint inhibitors are ongoing. The FDA approved the anti-programmed cell death protein 1 (PD-1) antibodies pembrolizumab in 2017 and nivolumab in 2017 for the treatment of patients with microsatellite-unstable tumors who had previously received 5-FU, oxaliplatin, and irinotecan-based therapy. In 2018, the FDA granted accelerated approval for the combination of nivolumab with ipilimumab (a CTLA-4 inhibitor) to treat MSI-H colorectal cancers that progressed after prior 5-FU, oxaliplatin, and irinotecan-based therapies.
First-line immunotherapy
Pembrolizumab monotherapy
Evidence (pembrolizumab monotherapy):
- In the phase III, open-label, international, randomized KEYNOTE-177 trial (NCT02563002), 307 patients with treatment-naïve MSI-H or dMMR metastatic colorectal cancer were randomly assigned in a 1:1 ratio to receive either pembrolizumab (200 mg every 3 weeks) or chemotherapy (FOLFIRI or modified FOLFOX-6 with or without bevacizumab or cetuximab).[68]
- The median PFS was 16.5 months for patients who received pembrolizumab and 8.2 months for patients who received chemotherapy (HR, 0.60; 95% CI, 0.45–0.80; P = .0002).[68][Level of evidence A3]
- The PFS in prespecified subgroups showed HRs that favored the pembrolizumab arm, except in patients with KRAS or NRAS variants.
- The objective response rate was 43.8% in the pembrolizumab arm and 33.3% in the chemotherapy arm. The median duration of response was not reached in the pembrolizumab arm (range, 2.3–41.4 months) and was 10.6 months in the chemotherapy arm (range, 2.8–37.5 months).
- Grade 3 or higher adverse events occurred in 56% of patients who received pembrolizumab (with 9% experiencing grade 3 or higher infusion-related adverse events), compared with 78% of patients who received chemotherapy.
- A final review of OS, presented in abstract form, showed that median OS was not reached in the pembrolizumab arm and was 36.7 months in the chemotherapy arm (HR, 0.74; 95% CI, 0.53–1.03; P = .0359).[69]
Nivolumab and ipilimumab
Evidence (nivolumab and ipilimumab):
- In a single-arm cohort of the phase II, multicenter CheckMate-142 study (NCT02060188) presented in abstract form, 45 treatment-naïve patients with MSI-H/dMMR metastatic colorectal cancer received nivolumab (3 mg/kg every 2 weeks) with ipilimumab (1 mg/kg every 6 weeks). The primary end point was objective response rate.[70]
- The objective response rate was 69% among all enrolled patients and 80% for patients with KRAS variants (n = 10).[70][Level of evidence C2]
- At a 2-year clinical follow-up, the median PFS and OS had not been reached.
- In the CheckMate 8HW trial (NCT04008030), published in abstract form, 303 patients who had received various lines of treatment were randomly assigned to receive either nivolumab and ipilimumab (n = 202) or chemotherapy alone (n = 101). Some patients were also randomly assigned to receive nivolumab, but results from these patients were not presented in the abstract. Treatments were continued until progression or unacceptable toxicity (all arms), or for up to 2 years (nivolumab-ipilimumab arm). A total of 171 patients who received nivolumab and ipilimumab and 84 patients who received chemotherapy alone were centrally confirmed to have dMMR/MSI-H metastatic colorectal cancer.[71]
- At a median follow-up of 31.5 months, the PFS was superior for patients who received nivolumab and ipilimumab compared with those who received chemotherapy alone (HR, 0.21; 97.91% CI, 0.13–0.35; P < .0001). Of note, in the chemotherapy arm, 67% of patients received subsequent immunotherapy.
- Two grade 5 deaths occurred in the nivolumab-ipilimumab arm. Grade 3 to 4 events occurred in 23% of patients in the nivolumab-ipilimumab arm and 48% of patients in the chemotherapy-alone arm.
Second-line immunotherapy
Pembrolizumab monotherapy
Evidence (pembrolizumab monotherapy):
- The FDA approval of pembrolizumab monotherapy was based on data from 149 patients with MSI-H or dMMR cancers enrolled across five uncontrolled, multicohort, multicenter, single-arm clinical trials. Ninety patients had colorectal cancer, and 59 patients were diagnosed with 1 of 14 other cancer types. Patients received either 200 mg of pembrolizumab every 3 weeks or 10 mg/kg of pembrolizumab every 2 weeks. Treatment continued until unacceptable toxicity or disease progression occurred. The major efficacy outcome measures were objective response rate (assessed by blinded independent central radiologists’ review in accordance with Response Evaluation Criteria in Solid Tumors [RECIST] 1.1) and response duration.
- The objective response rate was 39.6% (95% CI, 31.7%–47.9%).
- Responses lasted 6 months or longer for 78% of patients who responded to pembrolizumab. There were 11 complete responses and 48 partial responses.
- The objective response rate was similar whether patients were diagnosed with colorectal cancer (36%) or a different cancer (46% across the 14 other cancer types).
Nivolumab monotherapy
Evidence (nivolumab monotherapy):
- In the CheckMate-142 trial (NCT02060188), 74 patients with previously treated dMMR/MSI-H colorectal cancer were enrolled in an open-label, single-arm, phase II study to receive nivolumab (3 mg/kg every 2 weeks). The primary end point was objective response as per RECIST 1.1.[72]
- The objective response rate was 31.1% (95% CI, 20.8%–42.9%).
- Grade 3 to 4 treatment-related adverse events occurred in 21% of patients.
Nivolumab and ipilimumab
Evidence (nivolumab and ipilimumab):
- CheckMate-142 (NCT02060188) was a multicenter, open-label, phase II trial with a cohort for patients with recurrent or metastatic dMMR and/or MSI-H colorectal cancer who had progressed on, were intolerant of, or declined at least one line of chemotherapy (including 5-FU and oxaliplatin and/or irinotecan). The trial enrolled 119 patients who received four doses of nivolumab (3 mg/kg) and ipilimumab (1 mg/kg) every 3 weeks (induction), then nivolumab (3 mg/kg IV) every 2 weeks (maintenance). The primary end point was objective response rate.[72]
- The objective response rate was 55% (95% CI, 45.2%–63.8%).
- Among patients experiencing a response, 83% had responses lasting more than 6 months.
- Grade 3 to 4 treatment-related adverse events occurred in 32% of patients.
Palliative therapy
Palliative radiation therapy,[11,66] chemotherapy,[13,73-78] and chemoradiation therapy [79,80] may be indicated. Palliative, endoscopically-placed stents may be used to relieve obstruction.[81]
Treatment of Liver Metastasis
Approximately 15% to 25% of patients with colorectal cancer will present with liver metastases at diagnosis, and another 25% to 50% will develop metachronous hepatic metastasis after resection of the primary tumor.[82-84] Although only a small proportion of patients with liver metastasis are candidates for surgical resection, advances in tumor ablation techniques and in both regional and systemic chemotherapy administration provide a number of treatment options. These include:
Surgery
Hepatic metastasis may be considered resectable on the basis of the following factors:[65,85-97]
- Limited number of lesions.
- Intrahepatic locations of lesions.
- Lack of major vascular involvement.
- Absent or limited extrahepatic disease.
- Sufficient functional hepatic reserve.
For patients with resectable hepatic metastasis, a negative margin resection has been associated with 5-year survival rates of 25% to 40% in mostly nonrandomized studies, such as the North Central Cancer Treatment Group trial NCCTG-934653 (NCT00002575).[98-102][Level of evidence C3] Improved surgical techniques and advances in preoperative imaging have improved patient selection for resection. In addition, multiple studies with multiagent chemotherapy have demonstrated that patients with metastatic disease isolated to the liver, which historically would be considered unresectable, can occasionally be made resectable after the administration of neoadjuvant chemotherapy.[103]
For patients with unresectable liver metastases, excellent outcomes have been achieved with liver transplant. The optimal patient cohort for this therapy is still being determined, but in general, the goal is to achieve good initial systemic control with chemotherapy, followed by transplant. In one study of 91 patients, 11% underwent live donor liver transplant. At a median follow-up of 1.5 years after transplant, the recurrence-free survival rate was 62%, and the OS rate was 100%.[104][Level of evidence C3]
In the TRANSMET study (NCT02597348), published in abstract form, 94 patients were randomly assigned to receive either chemotherapy and liver transplant (n = 47) or chemotherapy alone (n = 47). In an intent-to-treat analysis, the 5-year OS rate was 57% in the chemotherapy and liver transplant arm and 13% in the chemotherapy-alone arm. In a per-protocol analysis, the 5-year OS rate was 73% in the chemotherapy and liver transplant arm and 9% in the chemotherapy-alone arm.[105][Level of evidence A1]
Neoadjuvant chemotherapy for unresectable liver metastases
Patients with hepatic metastases that are deemed unresectable will occasionally become candidates for resection if they have a good response to chemotherapy. These patients have 5-year survival rates similar to patients who initially had resectable disease.[103]
Local ablation for unresectable liver metastases
Radiofrequency ablation has emerged as a safe technique (2% major morbidity and <1% mortality rate) that may provide long-term tumor control.[106-112] Radiofrequency ablation and cryosurgical ablation remain options for patients with tumors that cannot be resected and for patients who are not candidates for liver resection.
Adjuvant chemotherapy
The role of adjuvant chemotherapy after potentially curative resection of liver metastases is uncertain.
Evidence (adjuvant chemotherapy):
- A trial of hepatic arterial floxuridine and dexamethasone plus systemic 5-FU/LV compared with systemic 5-FU/LV alone showed improved 2-year PFS (57% vs. 42%; P =.07) and OS (86% vs. 72%; P = .03) for patients in the combined therapy arm but did not show a significant statistical difference in
median survival when compared with systemic 5-FU therapy alone.[113][Level of evidence A1]
- Median survival in the combined therapy arm was 72.2 months versus 59.3 months in the monotherapy arm (P = .21).
- A second trial preoperatively randomly assigned patients with one to three potentially resectable colorectal hepatic metastases to either no further therapy or postoperative hepatic arterial floxuridine plus systemic 5-FU.[114] Among those randomly assigned patients, 27% were deemed ineligible at the time of surgery, leaving only 75 patients evaluable for recurrence and survival.
- While liver recurrence was decreased, median or 4-year survival was not significantly different between the patient groups.
Additional studies are required to evaluate this treatment approach and to determine whether more effective systemic combination chemotherapy alone would provide results similar to hepatic intra-arterial therapy plus systemic treatment.
Intra-arterial chemotherapy after liver resection
Hepatic intra-arterial chemotherapy with floxuridine for liver metastases has produced higher overall response rates but no consistent improvement in survival when compared with systemic chemotherapy.[93,115-119] Controversy regarding the efficacy of regional chemotherapy was the basis of a large, multicenter, phase III trial (Leuk-9481 [NCT00002716]) of hepatic arterial infusion versus systemic chemotherapy. The use of combination intra-arterial chemotherapy with hepatic radiation therapy, especially employing focal radiation of metastatic lesions, is under evaluation.[120]
Several studies show increased local toxic effects after hepatic infusional therapy, including liver function abnormalities and fatal biliary sclerosis.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Wanebo HJ, Koness RJ, Vezeridis MP, et al.: Pelvic resection of recurrent rectal cancer. Ann Surg 220 (4): 586-95; discussion 595-7, 1994. [PUBMED Abstract]
- Girard P, Ducreux M, Baldeyrou P, et al.: Surgery for lung metastases from colorectal cancer: analysis of prognostic factors. J Clin Oncol 14 (7): 2047-53, 1996. [PUBMED Abstract]
- McAfee MK, Allen MS, Trastek VF, et al.: Colorectal lung metastases: results of surgical excision. Ann Thorac Surg 53 (5): 780-5; discussion 785-6, 1992. [PUBMED Abstract]
- Headrick JR, Miller DL, Nagorney DM, et al.: Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg 71 (3): 975-9; discussion 979-80, 2001. [PUBMED Abstract]
- Tepper JE, O'Connell M, Donna H, et al.: Analysis of surgical salvage after failure of primary therapy in rectal cancer: results from INT 0114. [Abstract] Proceedings of the American Society of Clinical Oncology 21: A-507, 2002.
- Rodriguez-Bigas MA, Herrera L, Petrelli NJ: Surgery for recurrent rectal adenocarcinoma in the presence of hydronephrosis. Am J Surg 164 (1): 18-21, 1992. [PUBMED Abstract]
- Ogunbiyi OA, McKenna K, Birnbaum EH, et al.: Aggressive surgical management of recurrent rectal cancer--is it worthwhile? Dis Colon Rectum 40 (2): 150-5, 1997. [PUBMED Abstract]
- Vermaas M, Ferenschild FT, Verhoef C, et al.: Total pelvic exenteration for primary locally advanced and locally recurrent rectal cancer. Eur J Surg Oncol 33 (4): 452-8, 2007. [PUBMED Abstract]
- Lowy AM, Rich TA, Skibber JM, et al.: Preoperative infusional chemoradiation, selective intraoperative radiation, and resection for locally advanced pelvic recurrence of colorectal adenocarcinoma. Ann Surg 223 (2): 177-85, 1996. [PUBMED Abstract]
- Valentini V, Morganti AG, De Franco A, et al.: Chemoradiation with or without intraoperative radiation therapy in patients with locally recurrent rectal carcinoma: prognostic factors and long term outcome. Cancer 86 (12): 2612-24, 1999. [PUBMED Abstract]
- Haddock MG, Gunderson LL, Nelson H, et al.: Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys 49 (5): 1267-74, 2001. [PUBMED Abstract]
- Petrelli N, Herrera L, Rustum Y, et al.: A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol 5 (10): 1559-65, 1987. [PUBMED Abstract]
- Petrelli N, Douglass HO, Herrera L, et al.: The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol 7 (10): 1419-26, 1989. [PUBMED Abstract]
- Scheithauer W, Rosen H, Kornek GV, et al.: Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 306 (6880): 752-5, 1993. [PUBMED Abstract]
- Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. Nordic Gastrointestinal Tumor Adjuvant Therapy Group. J Clin Oncol 10 (6): 904-11, 1992. [PUBMED Abstract]
- Buyse M, Thirion P, Carlson RW, et al.: Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: a meta-analysis. Meta-Analysis Group in Cancer. Lancet 356 (9227): 373-8, 2000. [PUBMED Abstract]
- Leichman CG, Fleming TR, Muggia FM, et al.: Phase II study of fluorouracil and its modulation in advanced colorectal cancer: a Southwest Oncology Group study. J Clin Oncol 13 (6): 1303-11, 1995. [PUBMED Abstract]
- Saltz LB, Cox JV, Blanke C, et al.: Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343 (13): 905-14, 2000. [PUBMED Abstract]
- de Gramont A, Figer A, Seymour M, et al.: Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18 (16): 2938-47, 2000. [PUBMED Abstract]
- Douillard JY, Cunningham D, Roth AD, et al.: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355 (9209): 1041-7, 2000. [PUBMED Abstract]
- Sanoff HK, Sargent DJ, Campbell ME, et al.: Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 26 (35): 5721-7, 2008. [PUBMED Abstract]
- Tournigand C, André T, Achille E, et al.: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22 (2): 229-37, 2004. [PUBMED Abstract]
- Colucci G, Gebbia V, Paoletti G, et al.: Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol 23 (22): 4866-75, 2005. [PUBMED Abstract]
- Fuchs CS, Marshall J, Mitchell E, et al.: Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25 (30): 4779-86, 2007. [PUBMED Abstract]
- Van Cutsem E, Twelves C, Cassidy J, et al.: Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 19 (21): 4097-106, 2001. [PUBMED Abstract]
- Hoff PM, Ansari R, Batist G, et al.: Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 19 (8): 2282-92, 2001. [PUBMED Abstract]
- Díaz-Rubio E, Tabernero J, Gómez-España A, et al.: Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol 25 (27): 4224-30, 2007. [PUBMED Abstract]
- Porschen R, Arkenau HT, Kubicka S, et al.: Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol 25 (27): 4217-23, 2007. [PUBMED Abstract]
- Hurwitz H, Fehrenbacher L, Novotny W, et al.: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350 (23): 2335-42, 2004. [PUBMED Abstract]
- Saltz LB, Clarke S, Díaz-Rubio E, et al.: Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26 (12): 2013-9, 2008. [PUBMED Abstract]
- Cassidy J, Clarke S, Díaz-Rubio E, et al.: Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26 (12): 2006-12, 2008. [PUBMED Abstract]
- Giantonio BJ, Catalano PJ, Meropol NJ, et al.: High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: results from the Eastern Cooperative Oncology Group (ECOG) study E3200. [Abstract] J Clin Oncol 23 (Suppl 16): A-2, 1s, 2005.
- Loupakis F, Cremolini C, Masi G, et al.: Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 371 (17): 1609-18, 2014. [PUBMED Abstract]
- Cunningham D, Humblet Y, Siena S, et al.: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351 (4): 337-45, 2004. [PUBMED Abstract]
- Van Cutsem E, Köhne CH, Hitre E, et al.: Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360 (14): 1408-17, 2009. [PUBMED Abstract]
- Tol J, Koopman M, Cats A, et al.: Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 360 (6): 563-72, 2009. [PUBMED Abstract]
- Maughan TS, Adams RA, Smith CG, et al.: Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 377 (9783): 2103-14, 2011. [PUBMED Abstract]
- Adams RA, Meade AM, Seymour MT, et al.: Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol 12 (7): 642-53, 2011. [PUBMED Abstract]
- Van Cutsem E, Tabernero J, Lakomy R, et al.: Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30 (28): 3499-506, 2012. [PUBMED Abstract]
- Tabernero J, Yoshino T, Cohn AL, et al.: Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 16 (5): 499-508, 2015. [PUBMED Abstract]
- Van Cutsem E, Peeters M, Siena S, et al.: Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25 (13): 1658-64, 2007. [PUBMED Abstract]
- Douillard JY, Siena S, Cassidy J, et al.: Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28 (31): 4697-705, 2010. [PUBMED Abstract]
- Douillard JY, Oliner KS, Siena S, et al.: Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369 (11): 1023-34, 2013. [PUBMED Abstract]
- Hecht JR, Mitchell E, Chidiac T, et al.: A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 27 (5): 672-80, 2009. [PUBMED Abstract]
- Peeters M, Price TJ, Cervantes A, et al.: Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28 (31): 4706-13, 2010. [PUBMED Abstract]
- Heinemann V, von Weikersthal LF, Decker T, et al.: FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15 (10): 1065-75, 2014. [PUBMED Abstract]
- Venook AP, Niedzwiecki D, Lenz HJ, et al.: CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). [Abstract] J Clin Oncol 32 (Suppl 5): A-LBA3, 2014.
- Stintzing S, Modest DP, Rossius L, et al.: FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 17 (10): 1426-1434, 2016. [PUBMED Abstract]
- Modest DP, Stintzing S, von Weikersthal LF, et al.: Impact of Subsequent Therapies on Outcome of the FIRE-3/AIO KRK0306 Trial: First-Line Therapy With FOLFIRI Plus Cetuximab or Bevacizumab in Patients With KRAS Wild-Type Tumors in Metastatic Colorectal Cancer. J Clin Oncol 33 (32): 3718-26, 2015. [PUBMED Abstract]
- Grothey A, Sobrero AF, Siena S, et al.: Results of a phase III randomized, double-blind, placebo-controlled, multicenter trial (CORRECT) of regorafenib plus best supportive care (BSC) versus placebo plus BSC in patients (pts) with metastatic colorectal cancer (mCRC) who have progressed after standard therapies. [Abstract] J Clin Oncol 30 (Suppl 4): A-LBA385, 2012.
- Grothey A, Van Cutsem E, Sobrero A, et al.: Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381 (9863): 303-12, 2013. [PUBMED Abstract]
- Dasari A, Lonardi S, Garcia-Carbonero R, et al.: Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet 402 (10395): 41-53, 2023. [PUBMED Abstract]
- Mayer RJ, Van Cutsem E, Falcone A, et al.: Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372 (20): 1909-19, 2015. [PUBMED Abstract]
- Prager GW, Taieb J, Fakih M, et al.: Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N Engl J Med 388 (18): 1657-1667, 2023. [PUBMED Abstract]
- Kopetz S, Grothey A, Yaeger R, et al.: Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med 381 (17): 1632-1643, 2019. [PUBMED Abstract]
- Kopetz S, Grothey A, Van Cutsem E, et al.: Encorafenib plus cetuximab with or without binimetinib for BRAF V600E metastatic colorectal cancer: updated survival results from a randomized, three-arm, phase III study versus choice of either irinotecan or FOLFIRI plus cetuximab (BEACON CRC). [Abstract] J Clin Oncol 38 (15) (suppl): A-4001, 2020. Available online. Last accessed January 30, 2025.
- Fakih M, Tu H, Hsu H, et al.: Real-World Study of Characteristics and Treatment Outcomes Among Patients with KRAS p.G12C-Mutated or Other KRAS Mutated Metastatic Colorectal Cancer. Oncologist 27 (8): 663-674, 2022. [PUBMED Abstract]
- Lee JK, Sivakumar S, Schrock AB, et al.: Comprehensive pan-cancer genomic landscape of KRAS altered cancers and real-world outcomes in solid tumors. NPJ Precis Oncol 6 (1): 91, 2022. [PUBMED Abstract]
- Neumann J, Zeindl-Eberhart E, Kirchner T, et al.: Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract 205 (12): 858-62, 2009. [PUBMED Abstract]
- Henry JT, Coker O, Chowdhury S, et al.: Comprehensive Clinical and Molecular Characterization of KRAS G12C-Mutant Colorectal Cancer. JCO Precis Oncol 5: , 2021. [PUBMED Abstract]
- Fakih MG, Salvatore L, Esaki T, et al.: Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N Engl J Med 389 (23): 2125-2139, 2023. [PUBMED Abstract]
- Yaeger R, Weiss J, Pelster MS, et al.: Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N Engl J Med 388 (1): 44-54, 2023. [PUBMED Abstract]
- Rothenberg ML, Eckardt JR, Kuhn JG, et al.: Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. J Clin Oncol 14 (4): 1128-35, 1996. [PUBMED Abstract]
- Conti JA, Kemeny NE, Saltz LB, et al.: Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol 14 (3): 709-15, 1996. [PUBMED Abstract]
- Rougier P, Van Cutsem E, Bajetta E, et al.: Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 352 (9138): 1407-12, 1998. [PUBMED Abstract]
- Cunningham D, Pyrhönen S, James RD, et al.: Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352 (9138): 1413-8, 1998. [PUBMED Abstract]
- Rothenberg ML, Oza AM, Bigelow RH, et al.: Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol 21 (11): 2059-69, 2003. [PUBMED Abstract]
- André T, Shiu KK, Kim TW, et al.: Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 383 (23): 2207-2218, 2020. [PUBMED Abstract]
- Andre T, Shiu K, Kim TW, et al.: Final overall survival for the phase III KN177 study: pembrolizumab versus chemotherapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). [Abstract] J Clin Oncol 39 (15) (suppl): A-3500, 2021. Available online. Last accessed January 30, 2025.
- Lenz H, Lonardi S, Zagonel V, et al.: Subgroup analyses of patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC) treated with nivolumab (NIVO) plus low-dose ipilimumab (IPI) as first-line (1L) therapy: two-year clinical update. [Abstract] J Clin Oncol 39 (3) (suppl): A-58, 2021. Available online. Last accessed January 30, 2025.
- Lenz HJ, Lonardi S, Elez E, et al.: Nivolumab (NIVO) plus ipilimumab (IPI) vs chemotherapy (chemo) as first-line (1L) treatment for microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): Expanded efficacy analysis from CheckMate 8HW. [Abstract] J Clin Oncol 42 (Suppl 16): A-3503, 2024.
- Overman MJ, McDermott R, Leach JL, et al.: Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 18 (9): 1182-1191, 2017. [PUBMED Abstract]
- Valone FH, Friedman MA, Wittlinger PS, et al.: Treatment of patients with advanced colorectal carcinomas with fluorouracil alone, high-dose leucovorin plus fluorouracil, or sequential methotrexate, fluorouracil, and leucovorin: a randomized trial of the Northern California Oncology Group. J Clin Oncol 7 (10): 1427-36, 1989. [PUBMED Abstract]
- Erlichman C, Fine S, Wong A, et al.: A randomized trial of fluorouracil and folinic acid in patients with metastatic colorectal carcinoma. J Clin Oncol 6 (3): 469-75, 1988. [PUBMED Abstract]
- Doroshow JH, Multhauf P, Leong L, et al.: Prospective randomized comparison of fluorouracil versus fluorouracil and high-dose continuous infusion leucovorin calcium for the treatment of advanced measurable colorectal cancer in patients previously unexposed to chemotherapy. J Clin Oncol 8 (3): 491-501, 1990. [PUBMED Abstract]
- Poon MA, O'Connell MJ, Wieand HS, et al.: Biochemical modulation of fluorouracil with leucovorin: confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J Clin Oncol 9 (11): 1967-72, 1991. [PUBMED Abstract]
- Wadler S, Lembersky B, Atkins M, et al.: Phase II trial of fluorouracil and recombinant interferon alfa-2a in patients with advanced colorectal carcinoma: an Eastern Cooperative Oncology Group study. J Clin Oncol 9 (10): 1806-10, 1991. [PUBMED Abstract]
- Grem JL, Jordan E, Robson ME, et al.: Phase II study of fluorouracil, leucovorin, and interferon alfa-2a in metastatic colorectal carcinoma. J Clin Oncol 11 (9): 1737-45, 1993. [PUBMED Abstract]
- Wong CS, Cummings BJ, Brierley JD, et al.: Treatment of locally recurrent rectal carcinoma--results and prognostic factors. Int J Radiat Oncol Biol Phys 40 (2): 427-35, 1998. [PUBMED Abstract]
- Crane CH, Janjan NA, Abbruzzese JL, et al.: Effective pelvic symptom control using initial chemoradiation without colostomy in metastatic rectal cancer. Int J Radiat Oncol Biol Phys 49 (1): 107-16, 2001. [PUBMED Abstract]
- Baron TH: Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 344 (22): 1681-7, 2001. [PUBMED Abstract]
- Power DG, Healey-Bird BR, Kemeny NE: Regional chemotherapy for liver-limited metastatic colorectal cancer. Clin Colorectal Cancer 7 (4): 247-59, 2008. [PUBMED Abstract]
- Khatri VP, Chee KG, Petrelli NJ: Modern multimodality approach to hepatic colorectal metastases: solutions and controversies. Surg Oncol 16 (1): 71-83, 2007. [PUBMED Abstract]
- Pawlik TM, Choti MA: Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 11 (8): 1057-77, 2007. [PUBMED Abstract]
- Adson MA, van Heerden JA, Adson MH, et al.: Resection of hepatic metastases from colorectal cancer. Arch Surg 119 (6): 647-51, 1984. [PUBMED Abstract]
- Gayowski TJ, Iwatsuki S, Madariaga JR, et al.: Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery 116 (4): 703-10; discussion 710-1, 1994. [PUBMED Abstract]
- Hughes KS, Simon R, Songhorabodi S, et al.: Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery 100 (2): 278-84, 1986. [PUBMED Abstract]
- Schlag P, Hohenberger P, Herfarth C: Resection of liver metastases in colorectal cancer--competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol 16 (4): 360-5, 1990. [PUBMED Abstract]
- Rosen CB, Nagorney DM, Taswell HF, et al.: Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 216 (4): 493-504; discussion 504-5, 1992. [PUBMED Abstract]
- Fong Y, Fortner J, Sun RL, et al.: Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230 (3): 309-18; discussion 318-21, 1999. [PUBMED Abstract]
- Scheele J, Stangl R, Altendorf-Hofmann A: Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 77 (11): 1241-6, 1990. [PUBMED Abstract]
- Scheele J, Stangl R, Altendorf-Hofmann A, et al.: Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 110 (1): 13-29, 1991. [PUBMED Abstract]
- Wagman LD, Kemeny MM, Leong L, et al.: A prospective, randomized evaluation of the treatment of colorectal cancer metastatic to the liver. J Clin Oncol 8 (11): 1885-93, 1990. [PUBMED Abstract]
- Coppa GF, Eng K, Ranson JH, et al.: Hepatic resection for metastatic colon and rectal cancer. An evaluation of preoperative and postoperative factors. Ann Surg 202 (2): 203-8, 1985. [PUBMED Abstract]
- Taylor M, Forster J, Langer B, et al.: A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg 173 (6): 467-71, 1997. [PUBMED Abstract]
- Jaeck D, Bachellier P, Guiguet M, et al.: Long-term survival following resection of colorectal hepatic metastases. Association Française de Chirurgie. Br J Surg 84 (7): 977-80, 1997. [PUBMED Abstract]
- Fernández-Trigo V, Shamsa F, Sugarbaker PH: Repeat liver resections from colorectal metastasis. Repeat Hepatic Metastases Registry. Surgery 117 (3): 296-304, 1995. [PUBMED Abstract]
- Weeks JC, Nelson H, Gelber S, et al.: Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA 287 (3): 321-8, 2002. [PUBMED Abstract]
- Higgins GA, Amadeo JH, McElhinney J, et al.: Efficacy of prolonged intermittent therapy with combined 5-fluorouracil and methyl-CCNU following resection for carcinoma of the large bowel. A Veterans Administration Surgical Oncology Group report. Cancer 53 (1): 1-8, 1984. [PUBMED Abstract]
- Buyse M, Zeleniuch-Jacquotte A, Chalmers TC: Adjuvant therapy of colorectal cancer. Why we still don't know. JAMA 259 (24): 3571-8, 1988. [PUBMED Abstract]
- Laurie JA, Moertel CG, Fleming TR, et al.: Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol 7 (10): 1447-56, 1989. [PUBMED Abstract]
- Moertel CG, Fleming TR, Macdonald JS, et al.: Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322 (6): 352-8, 1990. [PUBMED Abstract]
- Leonard GD, Brenner B, Kemeny NE: Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol 23 (9): 2038-48, 2005. [PUBMED Abstract]
- Hernandez-Alejandro R, Ruffolo LI, Sasaki K, et al.: Recipient and Donor Outcomes After Living-Donor Liver Transplant for Unresectable Colorectal Liver Metastases. JAMA Surg 157 (6): 524-530, 2022. [PUBMED Abstract]
- Adam R, Piedvache C, Chiche L, et al.: Chemotherapy and liver transplantation versus chemotherapy alone in patients with definitively unresectable colorectal liver metastases: A prospective multicentric randomized trial (TRANSMET). [Abstract] J Clin Oncol 46 (Suppl 16): A-3500, 2024.
- Rossi S, Buscarini E, Garbagnati F, et al.: Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol 170 (4): 1015-22, 1998. [PUBMED Abstract]
- Solbiati L, Livraghi T, Goldberg SN, et al.: Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 221 (1): 159-66, 2001. [PUBMED Abstract]
- Lencioni R, Goletti O, Armillotta N, et al.: Radio-frequency thermal ablation of liver metastases with a cooled-tip electrode needle: results of a pilot clinical trial. Eur Radiol 8 (7): 1205-11, 1998. [PUBMED Abstract]
- Curley SA, Izzo F, Delrio P, et al.: Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 230 (1): 1-8, 1999. [PUBMED Abstract]
- Oshowo A, Gillams A, Harrison E, et al.: Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 90 (10): 1240-3, 2003. [PUBMED Abstract]
- Livraghi T, Solbiati L, Meloni F, et al.: Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the "test-of-time approach". Cancer 97 (12): 3027-35, 2003. [PUBMED Abstract]
- Pawlik TM, Izzo F, Cohen DS, et al.: Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 10 (9): 1059-69, 2003. [PUBMED Abstract]
- Kemeny N, Huang Y, Cohen AM, et al.: Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 341 (27): 2039-48, 1999. [PUBMED Abstract]
- Kemeny MM, Adak S, Gray B, et al.: Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol 20 (6): 1499-505, 2002. [PUBMED Abstract]
- Kemeny N, Daly J, Reichman B, et al.: Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med 107 (4): 459-65, 1987. [PUBMED Abstract]
- Chang AE, Schneider PD, Sugarbaker PH, et al.: A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg 206 (6): 685-93, 1987. [PUBMED Abstract]
- Rougier P, Laplanche A, Huguier M, et al.: Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol 10 (7): 1112-8, 1992. [PUBMED Abstract]
- Kemeny N, Cohen A, Seiter K, et al.: Randomized trial of hepatic arterial floxuridine, mitomycin, and carmustine versus floxuridine alone in previously treated patients with liver metastases from colorectal cancer. J Clin Oncol 11 (2): 330-5, 1993. [PUBMED Abstract]
- Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. Meta-Analysis Group in Cancer. J Natl Cancer Inst 88 (5): 252-8, 1996. [PUBMED Abstract]
- McGinn CJ, Lawrence TS: Clinical Results of the Combination of Radiation and Fluoropyrimidines in the Treatment of Intrahepatic Cancer. Semin Radiat Oncol 7 (4): 313-323, 1997. [PUBMED Abstract]
Latest Updates to This Summary (02/12/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Rectal Cancer
Updated statistics with estimated worldwide new cases and deaths for 2022 (cited Bray et al. as reference 1).
Updated statistics with estimated new cases and deaths for the United States in 2025 (cited American Cancer Society as reference 2).
Treatment of Stage IV and Recurrent Rectal Cancer
Added Fruquintinib as a new subsection.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of rectal cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Rectal Cancer Treatment are:
- Amit Chowdhry, MD, PhD (University of Rochester Medical Center)
- Leon Pappas, MD, PhD (Massachusetts General Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Rectal Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/colorectal/hp/rectal-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389402]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.