Sloan Kettering and Cornell University — MSKCC-Cornell Center for Translation of Cancer Nanomedicine
Center Overview
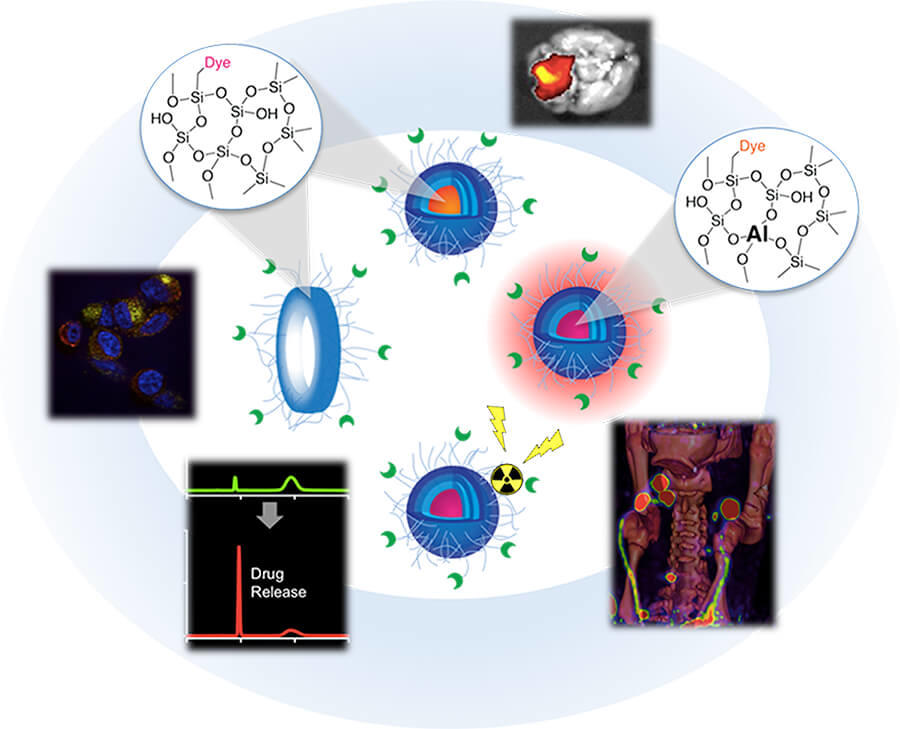
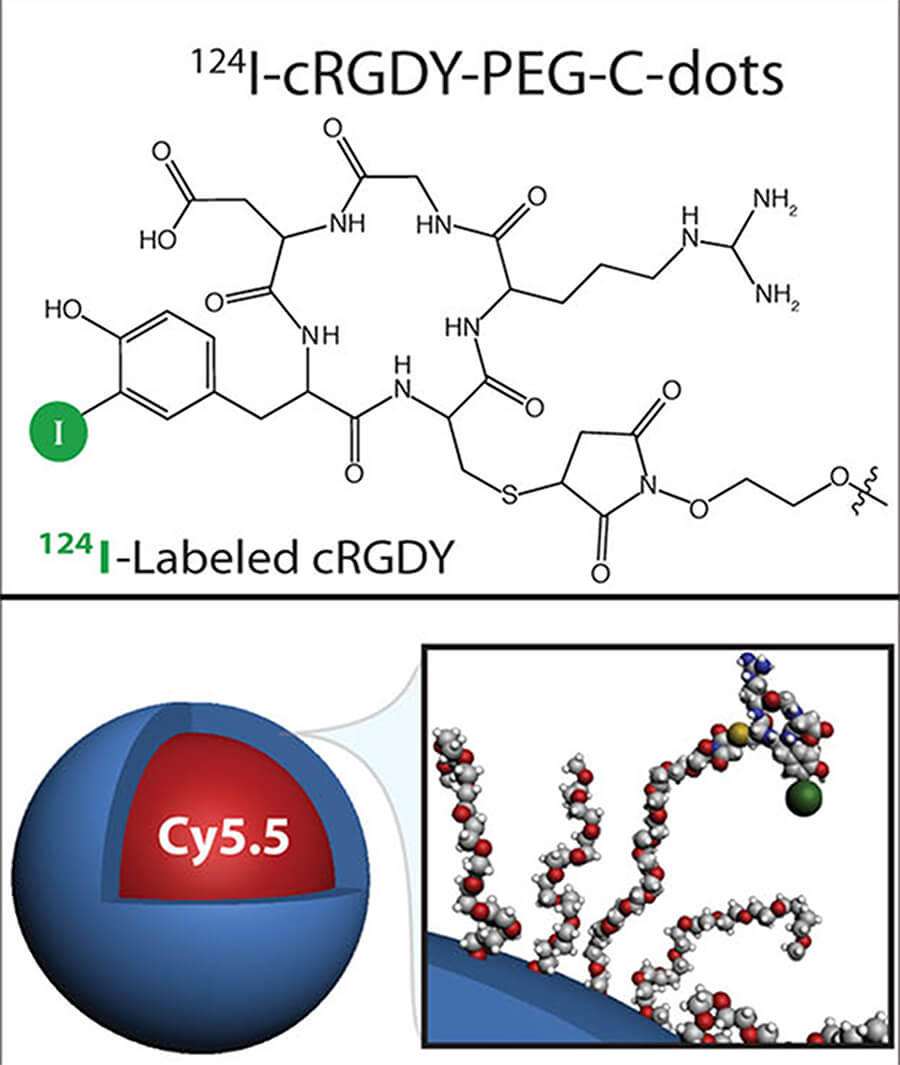
The scientific theme of the MC2TCN is based on advancing and translating a suite of ultrasmall (<10 nm) fluorescent silica organic hybrid nanoparticles, referred to as Cornell dots (or C dots), with tunable size, brightness, and geometry that may dramatically impact the way we diagnose and treat tumors on the basis of their favorable physicochemical, imaging, and biological properties. Earlier generation cancer-targeted C dots for both PET and optical imaging received FDA investigational new drug (IND) approvals for first-in-human clinical trials. Informed by these successes, MC2TCN will develop real-time intraoperative near infra-red (NIR) fluorescence detection tools to improve localization, staging, and treatment of melanoma, in addition to therapeutic delivery vehicles for enhancing targeted particle uptake, penetration, and efficacy in malignant brain tumors. The Center addresses common problems in oncology related to a lack of exquisitely bright, cancer-specific intraoperative visualization tools for targeted treatment of poorly prognostic nodal metastases, as well as the need for better targeted therapeutic delivery vehicles that may overcome the unfavorable biological properties, dose-limiting toxicity, and modest efficacy associated with native small molecule inhibitors (SMIs). The Center will (1) create new <10 nm probes with finely-tuned properties for reliably and accurately detecting cancer and/or normal tissue targets in surgical settings; (2) engineer <10 nm delivery vehicles incorporating SMIs or radiotherapies that can individually, or in combination with immunotherapies, selectively and efficaciously treat cancer, and (3) identify and qualify potential staging, prognostic and/or predictive biomarkers of response that can be validated by future clinical trial designs.
The two PIs leading the Center will be Drs. Michelle Bradbury (MSKCC) and Ulrich Wiesner (Cornell), who have been inter-institutional collaborators on the development and translation of C dots for over nine years. Dr. Michelle Bradbury, the Director of Intraoperative Imaging, Department of Radiology (MSKCC), holds a Joint appointment at Sloan Kettering Institute, and is an Associate Professor of Radiology at Weill Cornell Medical College. Her expertise is largely translational, and based in the areas of nanomedicine and molecular imaging. Prof. Wiesner is the Spencer T. Olin Professor of Engineering in the Materials Science and Engineering Department of Cornell University, with expertise in polymer and silica sol-gel derived nanomaterials.
Projects
Project 1: Highly-Integrated Ultrasmall and Bright Fluorescent Silica Particle Architectures
Project Investigator: Ulrich Wiesner, Ph.D.
The primary objective of Project 1, a basic research effort led by Ulrich Wiesner, is the synthesis and exploration of novel classes of < 10 nm C dots synthesized in water for use as cancer-targeted diagnostic and therapeutic probes. Major goals include using high-resolution analytical tools to characterize variations in particle size, composition, geometry, surface chemistry, and material heterogeneity, and correlate these variations with pharmacokinetics, renal clearance, and tumor-to-background ratios in melanoma models. Expected outcomes are particle structure-biological property correlations enabling economic particle synthesis in water and clinical translation.
Project 2: Real-time Intraoperative Imaging of Cancer Biomarkers and Peripheral Nerves
Project Investigators: Michelle Bradbury, M.D., Ph.D., and Snehal Patel, M.D.
The primary objective of Project 2, a translational research effort led by Michelle Bradbury and co-lead Snehal Patel, Associate Professor of Surgery at MSKCC, is optimization of new generation C dots for earlier and more reliable detection of multiple targets on melanoma-bearing lymph nodes and / or adjacent nerves, using an FDA-approved handheld intraoperative fluorescence camera. Major goals include development and screening of NIR dye-containing melanoma-targeting particles; selection of lead candidates to assess tumor-specific accumulation and pharmacokinetic profiles in conventional / GEMM animal models; and development of spectrally-distinct NIR dye-containing products for simultaneous differentiation of distinct pathological markers on diseased nodal tissue and normal tissue types. Expected outcomes include novel image-guided multiplexing tools to visualize both disease and normal critical structures for better surgical management and reduced surgical risks.
Project 3: Targeted Ultrasmall Silica Nanoparticles for Alpha- and Beta-Emitting Radiotherapy and Delivery of Small Molecule Inhibitors
Project Investigators: Thomas Quinn, Ph.D., Michael McDevitt, Ph.D., Cameron Brennan, M.D., and Charles Rudin, M.D., Ph.D.
The primary objectives of translational research Project 3 are to (1) target radiolabeled C dots to melanoma to deliver highly localized radiation doses and (2) deliver and release SMIs from nanoparticle-drug conjugates (NDCs) to malignant brain tumors. Melanoma studies will be led by Thomas Quinn, Professor of Biochemistry, University of Missouri, with co-lead Michael McDevitt, Radiologist, MSKCC. Major goals for melanoma models include the generation of optimized targeted C dots exhibiting favorable biological properties in melanoma models, as well as the development of safe and efficacious alpha- and beta-emitting C dots for clinical translation. Expected outcomes include the advancement of targeted C dot radiotherapeutics as part of combinatorial treatment paradigms with immunotherapies to improve melanoma management. Major goals of the malignant brain tumor studies, led by Cameron Brennan, M.D., Associate Member, Human Oncology and Pathogenesis Program, Sloan Kettering Institute, and Associate Professor of Neurosurgery, Weill Cornell Medical Center with co-lead Charles Rudin, M.D., Ph.D., Chief, Thoracic Oncology, MSKCC, will include the creation of optimized NDCs that exhibit favorable biological properties and overcome dose-limiting toxicity for use in combinatorial brain tumor treatment strategies. Expected outcomes include the development of potent NDCs with improved PK profiles, tumor accumulation, and enhanced therapeutic index.
Cores
Nanoparticle Synthesis and Characterization Core
Core Investigator: Teresa Porri, Ph.D.
This core, at Cornell and led by Teresa Porri, will support center activities for particle synthesis and characterization, as well as gel permeation chromatography and high performance liquid chromatography equipment for detailed particle characterization.
Imaging and Radiochemistry Core
Core Investigator: Pat Zanzonico, Ph.D.
This core, at MSKCC and led by Pat Zanzonico, will support center activities for imaging and radiochemistry and will provide expertise and state-of-the-art instrumentation in positron emission tomography, fluorescence imaging, and cyclotron production of radionuclides.
Developmental Program
Center Program Director: Michelle Bradbury, M.D., Ph.D.
The goals of MC2TCN will be further enhanced by a developmental program supporting pilot projects, with primary focus on probes below 10 nm.