Testicular Cancer Treatment (PDQ®)–Patient Version
General Information About Testicular Cancer
Key Points
- Testicular cancer is a disease in which malignant (cancer) cells form in the tissues of one or both testicles.
- Health history can affect the risk of testicular cancer.
- Signs and symptoms of testicular cancer include swelling or discomfort in the scrotum.
- Tests that examine the testicles and blood are used to diagnose testicular cancer.
- Certain factors affect prognosis (chance of recovery) and treatment options.
- Treatment for testicular cancer can cause infertility.
Testicular cancer is a disease in which malignant (cancer) cells form in the tissues of one or both testicles.
The testicles are 2 egg-shaped glands located inside the scrotum (a sac of loose skin that lies
directly below the penis). The testicles are held within the scrotum by the spermatic cord, which also contains the vas deferens and vessels and nerves of the testicles. 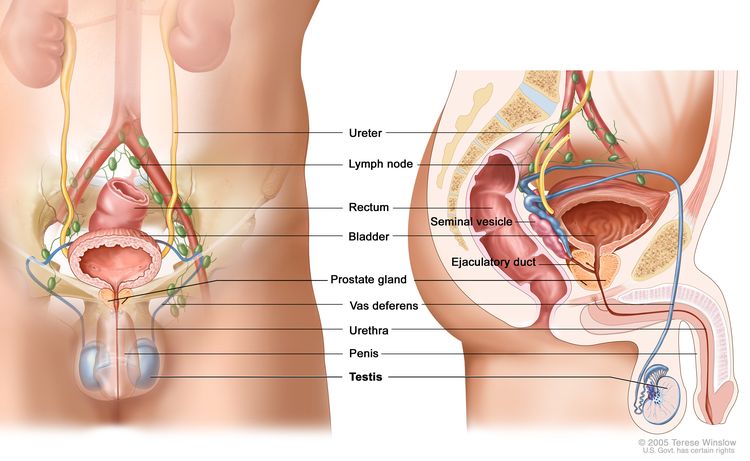
The testicles are the male sex glands and produce testosterone and sperm. Germ cells within the testicles produce immature sperm that travel through a network of tubules (tiny tubes) and larger tubes into the epididymis (a long coiled tube next to the testicles) where the sperm mature and are stored.
Almost all testicular cancers start in the germ cells. The two main types of testicular germ cell tumors are seminomas and nonseminomas. These 2 types grow and spread differently and are treated differently. Nonseminomas tend to grow and spread more quickly than seminomas. Seminomas are more sensitive to radiation. A testicular tumor that contains both seminoma and nonseminoma cells is treated as a nonseminoma.
Testicular cancer is the most common cancer in men 20 to 35 years old.
Health history can affect the risk of testicular cancer.
Anything that increases a person's chance of getting a disease is called a risk factor. Not every person with one or more of these risk factors will develop testicular cancer, and it will develop in people who don't have any known risk factors. Talk with your doctor if you think you may be at risk. Risk factors for testicular cancer include:
- Having had an undescended testicle.
- Having had abnormal development of the testicles.
- Having a personal history of testicular cancer.
- Having a family history of testicular cancer (especially in a father or brother).
- Being White.
Signs and symptoms of testicular cancer include swelling or discomfort in the scrotum.
These and other signs and symptoms may be caused by testicular cancer or by other conditions. Check with your doctor if you have any of the following:
Tests that examine the testicles and blood are used to diagnose testicular cancer.
In addition to asking about your personal and family health history and doing a physical exam, your doctor may perform the following tests and procedures:
- Physical exam of the testes: An exam in which a doctor checks for lumps, swelling, or pain in the testicles.
- Ultrasound exam of the testes: A procedure in which high-energy sound waves (ultrasound) are bounced off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram.
- Serum tumor marker test: A procedure in which a sample of blood is examined to measure the amounts of certain substances released into the blood by organs, tissues, or tumor cells in the body. Certain substances are linked to specific types of cancer when found in increased levels in the blood. These are called tumor markers. The following tumor markers are used to detect testicular cancer:Tumor marker levels are measured before inguinal orchiectomy and biopsy, to help diagnose testicular cancer.
- Alpha-fetoprotein (AFP).
- Beta-human chorionic gonadotropin (beta-hCG).
- Inguinal orchiectomy: A procedure to remove the entire testicle through an incision in the groin. A tissue sample from the testicle is then viewed under a microscope to check for cancer cells. (The surgeon does not cut through the scrotum into the testicle to remove a sample of tissue for biopsy, because if cancer is present, this procedure could cause it to spread into the scrotum and lymph nodes. It's important to choose a surgeon who has experience with this kind of surgery.) If cancer is found, the cell type (seminoma or nonseminoma) is determined in order to help plan treatment.
Certain factors affect prognosis (chance of recovery) and treatment options.
The prognosis and treatment options depend on the following:
- Stage of the cancer (whether it is in or near the testicle or has spread to other places in the body, and blood levels of AFP, beta-hCG, and LDH).
- Type of cancer.
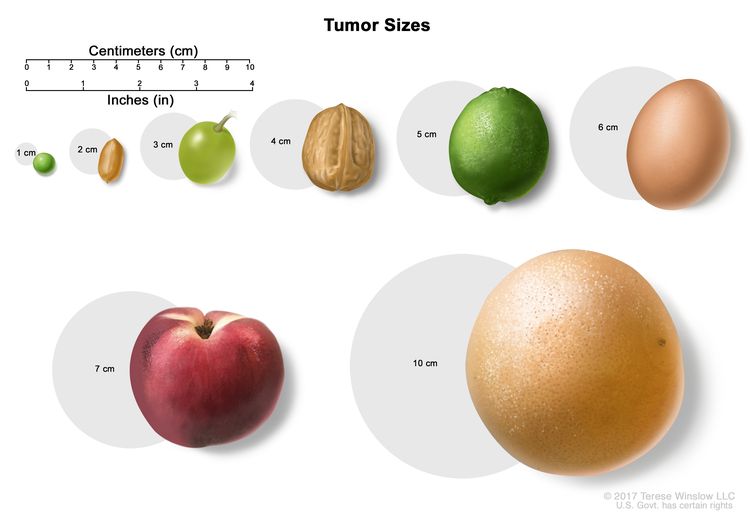
- Size of the tumor.
- Number and size of retroperitoneal lymph nodes.
Testicular cancer can usually be cured in patients who receive adjuvant chemotherapy or radiation therapy after their primary treatment.
Treatment for testicular cancer can cause infertility.
Certain treatments for testicular cancer can cause infertility that may be permanent. Patients who may wish to have children should consider sperm banking before having treatment. Sperm banking is the process of freezing sperm and storing it for later use.
Stages of Testicular Cancer
Key Points
- After testicular cancer has been diagnosed, tests are done to find out if cancer cells have spread within the testicles or to other parts of the body.
- There are three ways that cancer spreads in the body.
- Cancer may spread from where it began to other parts of the body.
- The following stages are used for testicular cancer:
- Stage 0
- Stage I
- Stage II
- Stage III
- Testicular cancer can recur (come back) after it has been treated.
After testicular cancer has been diagnosed, tests are done to find out if cancer cells have spread within the testicles or to other parts of the body.
The process used to find out if cancer has spread within the testicles or to other parts of the body is called staging. The information gathered from the staging process determines the stage of the disease. It is important to know the stage in order to plan treatment.
The following tests and procedures may be used in the staging process:
- Chest x-ray: An x-ray of the organs and bones inside the chest. An x-ray is a type of energy beam that can go through the body and onto film, making a picture of areas inside the body.
- CT scan (CAT scan): A procedure that makes a series of detailed pictures of areas inside the body, such as the abdomen, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
- MRI (magnetic resonance imaging): A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body, such as the abdomen. This procedure is also called nuclear magnetic resonance imaging (NMRI).
- Abdominal lymph node dissection: A surgical procedure in which lymph nodes in the abdomen are removed and a sample of tissue is checked under a microscope for signs of cancer. This procedure is also called lymphadenectomy. For patients with nonseminoma, removing the lymph nodes may help stop the spread of disease. Cancer cells in the lymph nodes of seminoma patients can be treated with radiation therapy.
- Serum tumor marker test: A procedure in which a sample of blood is examined to measure the amounts of certain substances released into the blood by organs, tissues, or tumor cells in the body. Certain substances are linked to specific types of cancer when found in increased levels in the blood. These are called tumor markers. The following 3 tumor markers are used in staging testicular cancer:Tumor marker levels are measured again, after inguinal orchiectomy and biopsy, in order to determine the stage of the cancer. This helps to show if all of the cancer has been removed or if more treatment is needed. Tumor marker levels are also measured during follow-up as a way of checking if the cancer has come back.
- Alpha-fetoprotein (AFP)
- Beta-human chorionic gonadotropin (beta-hCG).
- Lactate dehydrogenase (LDH).
There are three ways that cancer spreads in the body.
Cancer can spread through tissue, the lymph system, and the blood:
- Tissue. The cancer spreads from where it began by growing into nearby areas.
- Lymph system. The cancer spreads from where it began by getting into the lymph system. The cancer travels through the lymph vessels to other parts of the body.
- Blood. The cancer spreads from where it began by getting into the blood. The cancer travels through the blood vessels to other parts of the body.
Cancer may spread from where it began to other parts of the body.
When cancer spreads to another part of the body, it is called metastasis. Cancer cells break away from where they began (the primary tumor) and travel through the lymph system or blood.
- Lymph system. The cancer gets into the lymph system, travels through the lymph vessels, and forms a tumor (metastatic tumor) in another part of the body.
- Blood. The cancer gets into the blood, travels through the blood vessels, and forms a tumor (metastatic tumor) in another part of the body.
The metastatic tumor is the same type of cancer as the primary tumor. For example, if testicular cancer spreads to the lung, the cancer cells in the lung are actually testicular cancer cells. The disease is metastatic testicular cancer, not lung cancer.
The following stages are used for testicular cancer:
Stage 0
In stage 0, abnormal cells are found in the tiny tubules where the sperm cells begin to develop. These abnormal cells may become cancer and spread into nearby normal tissue. All tumor marker levels are normal. Stage 0 is also called germ cell neoplasia in situ.
Stage I
In stage I, cancer has formed. Stage I is divided into stages IA, IB, and IS.
- In stage IA, cancer is found in the testicle, including the rete testis, but has not spread to the blood vessels or lymph vessels in the testicle.
All tumor marker levels are normal.
- In stage IB, cancer:
- is found in the testicle, including the rete testis, and has spread to the blood vessels or lymph vessels in the testicle; or
- has spread into the hilar soft tissue (tissue made of fibers and fat with blood vessels and lymph vessels), the epididymis, or the outer membranes around the testicle; or
- has spread to the spermatic cord; or
- has spread to the scrotum.
All tumor marker levels are normal.
- In stage IS, cancer is found anywhere in the testicle and may have spread into the spermatic cord or scrotum.
Tumor marker levels range from slightly above normal to high.
Stage II
Stage II is divided into stages IIA, IIB, and IIC.
- In stage IIA, cancer is found anywhere in the testicle and may have spread into the spermatic cord or scrotum. Cancer has spread to 1 to 5 nearby lymph nodes and the lymph nodes are 2 centimeters or smaller.
All tumor marker levels are normal or slightly above normal.
- In stage IIB, cancer is found anywhere in the testicle and may have spread into the spermatic cord or scrotum. Cancer has spread to:
- 1 nearby lymph node and the lymph node is larger than 2 centimeters but not larger than 5 centimeters; or
- more than 5 nearby lymph nodes and the lymph nodes are not larger than 5 centimeters; or
- a nearby lymph node and the cancer has spread outside the lymph node.
All tumor marker levels are normal or slightly above normal.
- In stage IIC, cancer is found anywhere in the testicle and may have spread into the spermatic cord or scrotum. Cancer has spread to a nearby lymph node and the lymph node is larger than 5 centimeters.
All tumor marker levels are normal or slightly above normal.
Stage III
Stage III is divided into stages IIIA, IIIB, and IIIC.
- In stage IIIA, cancer is found anywhere in the testicle and may have spread into the spermatic cord or scrotum. Cancer may have spread to one or more nearby lymph nodes. Cancer has spread to distant lymph nodes or to the lungs.
All tumor marker levels are normal or slightly above normal.
- In stage IIIB, cancer is found anywhere in the testicle and may have spread into the spermatic cord or scrotum. Cancer has spread:
- to one or more nearby lymph nodes and has not spread to other parts of the body; or
- to one or more nearby lymph nodes. Cancer has spread to distant lymph nodes or to the lungs.
The level of one or more tumor markers is moderately above normal.
- In stage IIIC, cancer is found anywhere in the testicle and may have spread into the spermatic cord or scrotum. Cancer has spread:
- to one or more nearby lymph nodes and has not spread to other parts of the body; or
- to one or more nearby lymph nodes. Cancer has spread to distant lymph nodes or to the lungs.
The level of one or more tumor markers is high.
or
Cancer is found anywhere in the testicle and may have spread into the spermatic cord or scrotum. Cancer has not spread to distant lymph nodes or the lung, but has spread to other parts of the body, such as the liver or bone.
Tumor marker levels may range from normal to high.
Testicular cancer can recur (come back) after it has been treated.
The cancer may come back many years after the initial cancer, in the other testicle or in other parts of the body.
Treatment Option Overview
Key Points
- There are different types of treatment for patients with testicular cancer.
- Testicular tumors are divided into 3 groups, based on how well the tumors are expected to respond to treatment.
- Good Prognosis
- Intermediate Prognosis
- Poor Prognosis
- The following types of treatment are used:
- Surgery
- Radiation therapy
- Chemotherapy
- Surveillance
- High-dose chemotherapy with stem cell transplant
- New types of treatment are being tested in clinical trials.
- Treatment for testicular cancer may cause side effects.
- Patients may want to think about taking part in a clinical trial.
- Patients can enter clinical trials before, during, or after starting their cancer treatment.
- Follow-up tests may be needed.
There are different types of treatment for patients with testicular cancer.
Different types of treatments are available for patients with testicular cancer. Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Testicular tumors are divided into 3 groups, based on how well the tumors are expected to respond to treatment.
Good Prognosis
For nonseminoma, all of the following must be true:
- The tumor is found only in the testicle or in the retroperitoneum (area outside or behind the abdominal wall); and
- The tumor has not spread to organs other than the lungs; and
- The levels of all the tumor markers are slightly above normal.
For seminoma, all of the following must be true:
- The tumor has not spread to organs other than the lungs; and
- The level of alpha-fetoprotein (AFP) is normal. Beta-human chorionic gonadotropin (beta-hCG) and lactate dehydrogenase (LDH) may be at any level.
Intermediate Prognosis
For nonseminoma, all of the following must be true:
- The tumor is found in one testicle only or in the retroperitoneum (area outside or behind the abdominal wall); and
- The tumor has not spread to organs other than the lungs; and
- The level of any one of the tumor markers is more than slightly above normal.
For seminoma, all of the following must be true:
- The tumor has spread to organs other than the lungs; and
- The level of AFP is normal. Beta-hCG and LDH may be at any level.
Poor Prognosis
For nonseminoma, at least one of the following must be true:
- The tumor is in the center of the chest between the lungs; or
- The tumor has spread to organs other than the lungs; or
- The level of any one of the tumor markers is high.
There is no poor prognosis grouping for seminoma testicular tumors.
The following types of treatment are used:
Surgery
Surgery to remove the testicle (inguinal orchiectomy) and some of the lymph nodes may be done at diagnosis and staging. (See the General Information and Stages sections of this summary.) Tumors that have spread to other places in the body may be partly or entirely removed by surgery.
After the doctor removes all the cancer that can be seen at the time of the surgery, some patients may be given chemotherapy or radiation therapy after surgery to kill any cancer cells that are left. Treatment given after the surgery, to lower the risk that the cancer will come back, is called adjuvant therapy.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping the cells from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy).
See Drugs Approved for Testicular Cancer for more information.
Surveillance
Surveillance is closely following a patient's condition without giving any treatment unless there are changes in test results. It is used to find early signs that the cancer has recurred (come back). In surveillance, patients are given certain exams and tests on a regular schedule.
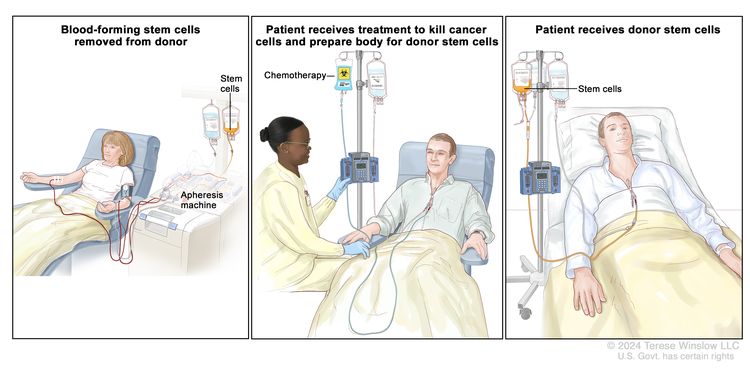
High-dose chemotherapy with stem cell transplant
High doses of chemotherapy are given to kill cancer cells. Healthy cells, including blood-forming cells, are also destroyed by the cancer treatment. Stem cell transplant is a treatment to replace the blood-forming cells. Stem cells (immature blood cells) are removed from the blood or bone marrow of the patient or a donor and are frozen and stored. After the patient completes chemotherapy, the stored stem cells are thawed and given back to the patient through an infusion. These reinfused stem cells grow into (and restore) the body's blood cells.
See Drugs Approved for Testicular Cancer for more information.
New types of treatment are being tested in clinical trials.
Information about clinical trials is available from the NCI website.
Treatment for testicular cancer may cause side effects.
For information about side effects caused by treatment for cancer, visit our Side Effects page.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
As you go through treatment, you will have follow-up tests or check-ups. Some tests that were done to diagnose or stage the cancer may be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back).
Men who have had testicular cancer have an increased risk of developing cancer in the other testicle. A patient is advised to regularly check the other testicle and report any unusual symptoms to a doctor right away.
Long-term clinical exams are very important. The patient will probably have check-ups frequently during the first year after surgery and less often after that.
Treatment of Stage 0 (Testicular Intraepithelial Neoplasia)
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage 0 may include the following:
- Radiation therapy.
- Surveillance.
- Surgery to remove the testicle.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Stage I Testicular Cancer
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage I testicular cancer depends on whether the cancer is a seminoma or a nonseminoma.
Treatment of seminoma may include the following:
- Surgery to remove the testicle, followed by surveillance.
- For patients who want active treatment rather than surveillance, treatment may include:
- Surgery to remove the testicle, followed by chemotherapy.
Treatment of nonseminoma may include the following:
- Surgery to remove the testicle, with long-term follow-up.
- Surgery to remove the testicle and lymph nodes in the abdomen, with long-term follow-up.
- Surgery followed by chemotherapy for patients at high risk of recurrence, with long-term follow-up.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Stage II Testicular Cancer
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage II testicular cancer depends on whether the cancer is a seminoma or a nonseminoma.
Treatment of seminoma may include the following:
- When the tumor is 5 centimeters or smaller:
- Surgery to remove the testicle, followed by radiation therapy to lymph nodes in the abdomen and pelvis.
- Combination chemotherapy.
- Surgery to remove the testicle and lymph nodes in the abdomen.
- When the tumor is larger than 5 centimeters:
- Surgery to remove the testicle, followed by combination chemotherapy or radiation therapy to lymph nodes in the abdomen and pelvis, with long-term follow-up.
Treatment of nonseminoma may include the following:
- Surgery to remove the testicle and lymph nodes, with long-term follow-up.
- Surgery to remove the testicle and lymph nodes, followed by combination chemotherapy and long-term follow-up.
- Surgery to remove the testicle, followed by combination chemotherapy and a second surgery if cancer remains, with long-term follow-up.
- Combination chemotherapy before surgery to remove the testicle, for cancer that has spread and is thought to be life-threatening.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Stage III Testicular Cancer
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage III testicular cancer depends on whether the cancer is a seminoma or a nonseminoma.
Treatment of seminoma may include the following:
- Surgery to remove the testicle, followed by combination chemotherapy. If there are tumors remaining after chemotherapy, treatment may be one of the following:
- Surveillance with no treatment unless tumors grow.
- Surveillance for tumors smaller than 3 centimeters and surgery to remove tumors larger than 3 centimeters.
- A PET scan two months after chemotherapy and surgery to remove tumors that show up with cancer on the scan.
- A clinical trial of chemotherapy.
Treatment of nonseminoma may include the following:
- Surgery to remove the testicle, followed by combination chemotherapy.
- Combination chemotherapy followed by surgery to remove the testicle and all remaining tumors. Additional chemotherapy may be given if the tumor tissue removed contains cancer cells that are growing or if follow-up tests show that cancer is progressing.
- Combination chemotherapy before surgery to remove the testicle, for cancer that has spread and is thought to be life-threatening.
- A clinical trial of chemotherapy.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Recurrent Testicular Cancer
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of recurrent testicular cancer may include the following:
- Combination chemotherapy.
- High-dose chemotherapy and stem cell transplant.
- Surgery to remove cancer that has either:
- come back more than 2 years after complete remission; or
- come back in only one place and does not respond to chemotherapy.
- A clinical trial of a new therapy.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
To Learn More About Testicular Cancer
For more information from the National Cancer Institute about testicular cancer, see the following:
For general cancer information and other resources from the National Cancer Institute, visit:
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of testicular cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Testicular Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/testicular/patient/testicular-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389286]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.