Benefits of Nanotechnology for Cancer
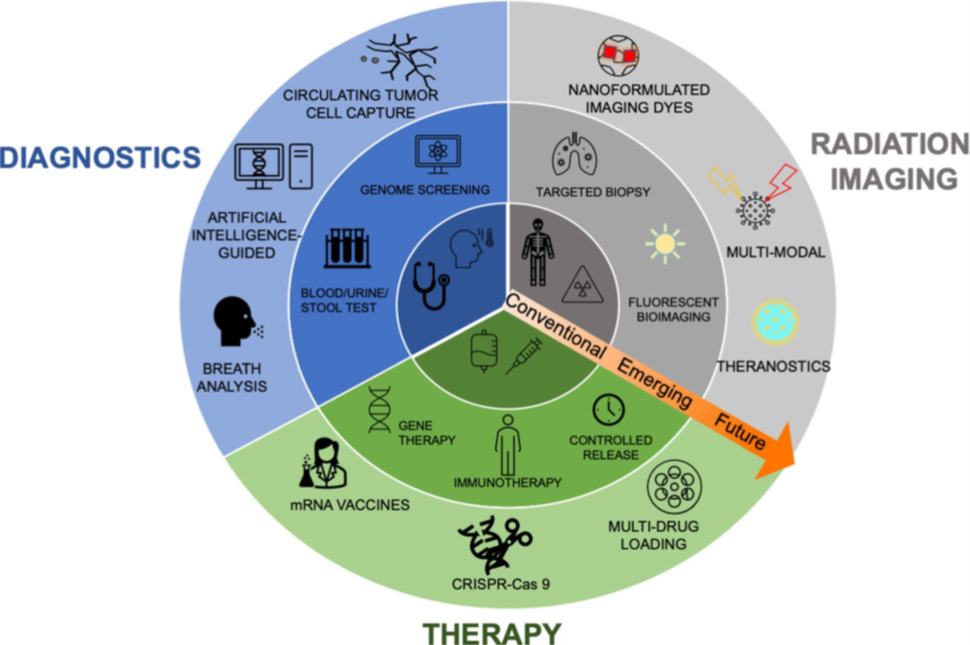
Modern medicine has significantly improved outcomes in cancer management, however the disease still causes over 600,000 deaths in the United States every year. While cancer treatments have advanced significantly, the need to increase their specificity and reduce systemic toxicity remains a challenge. As illustrated in the diagram below, nanotechnology presents opportunity to (1) enhance earlier diagnosis through in vitro assays, (2) enhance imaging capabilities for diagnosis and treatment monitoring, and (3) improve therapeutic outcomes by refining targeting precision, augmenting localized drug efficacy, and minimizing systemic toxicity.
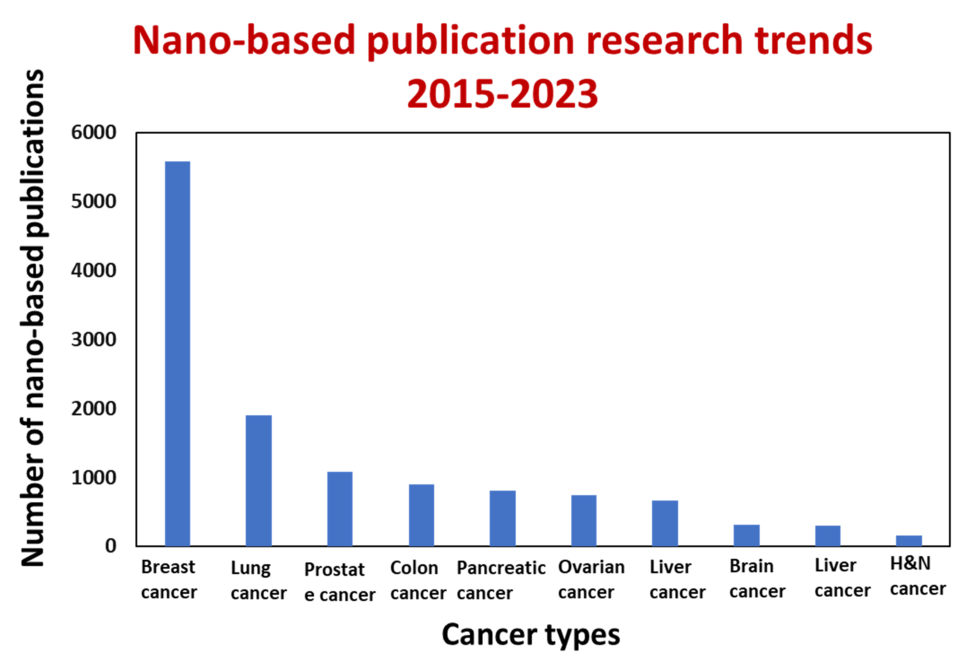
Nanoparticles, being one hundred to ten thousand times smaller than human cells, possess the unique ability to interact with biomolecules on both the surface and inside cells. This broad access across various organs holds the potential to change how we think about cancer detection and treatment, leading to a multitude of research publications (as depicted in the figure below, Carolina Salvador Morales and Piotr Grodzinski, WIREs Nanomedicine and Nanobiotechnology, 2023). The application of nanoscale materials in cancer research relies on their design flexibility and tunability. For example, gold nanoparticles can be readily decorated with different ligands on their surfaces to facilitate an understanding of how they interact with live cancer cells (as shown in the top right image). By enabling rapid and sensitive detection of cancer-related molecules, nanotechnology empowers scientists to detect molecular changes, even in a small percentage of cells. Moreover, nanotechnology has the potential to generate entirely novel and effective therapeutic agents. In addition, nanomaterials exhibit the ability to passively accumulate at the tumor sites, actively target cancer cells, and traverse physiological barriers such as the blood-brain barrier or the stomach epithelium.