Treatment and Therapy
Traditional cancer treatments are currently limited to surgery, radiation, and chemotherapy, all of which carry the risk for damaging normal tissues or incomplete eradication of the cancer. Nanotechnology offers the means to target therapies directly and selectively to cancerous cells and neoplasms, guide surgical resection of tumors, and enhance the therapeutic efficacy of radiation-based treatments. Nanotechnology also presents a unique set of tools to overcome drug resistance and enable the use of novel immunotherapies (Chunbai He, et al., Nature Communications, 2016; Darrell J. Irvine and Eric L. Dane, Nature Reviews Immunology, 2020), as one exemplified in the right image. Collectively, these advancements can add up to a decreased risk to the patient and an increased probability of effective treatment.
Research on nanotechnology cancer therapy goes beyond drug delivery, extending to the creation of new therapeutics available only through the use of nanomaterial properties themselves. First, some physical properties of nanoparticles, such as energy absorption and re-radiation, can be used to disrupt diseased tissue, as seen in laser ablation and hyperthermia applications. Second, nanoparticles are small enough to accumulate at the tumor sites, whereas they are large enough to encapsulate many therapeutic compounds, like radionuclides and active pharmaceutical ingredients. Third, their ample surface area can be functionalized with ligands including DNA or RNA strands, peptides, aptamers, or antibodies, which can actively direct their destination in vivo. Furthermore, nanostructured architectures are innovatively utilized to design artificial antigen presenting cells and create in vivo depots of immunostimulatory factors for sustained anti-tumor activity. Altogether, these applications empower efficient drug delivery, multi-modality treatment, and theranostics.
Delivering Chemotherapy
Nanotechnology’s role in cancer therapeutics has been to improve the pharmacokinetics and reduce the systemic toxicities of chemotherapies by selectively targeting and delivering anticancer drugs to tumor tissues. Nanosized carriers can increase the overall therapeutic index of delivered drugs through nanoformulations, where chemotherapeutic is either encapsulated or conjugated to the surface of nanoparticles. The selective delivery of nanotherapeutic platforms primarily depends on the passive targeting of tumors through the enhanced permeability and retention (EPR) effect, although other mechanisms were also proposed. The EPR phenomenon relies on tumor microenvironment defects like lymphatic drainage and increased tumor vasculature permeability, which facilitate the accumulation of nanoparticles (<200 nm) in the tumor. Additionally, the timing or site of drug release can be controlled by the use of external or internal trigger mechanisms such as ultrasound, pH, heat, or material composition itself.
Several researchers are working towards developing nanomaterial-based delivery platforms to enhance chemotherapy’s effectiveness while reducing its toxicity. For example, they are developing a strategy for photodynamic therapy, specifically tailored for bone marrow application (Alexander Zheleznyak, Monica Shokeen, and Samuel Achilefu, WIREs Nanomedicine and Nanobiotechnology, 2018), an area usually inaccessible to external radiation sources. Others are investigating the fundamental interactions between nanomaterials and biological systems to advance cancer diagnostics and therapeutics. This involves a focus on nanoparticle-based delivery systems that can penetrate physiological barriers to achieve targeted access to specific tumors, utilizing methods like mechanical particle deformation or using a synergistic approach for the delivery of paclitaxel and gemcitabine chemotherapeutics in mesoporous silica nano-constructs (Huan Meng, et al., ACS Nano, 2015).
Delivering or Augmenting Radiotherapy
Roughly half of all cancer patients receive radiation therapy as part of their treatment regimen. This therapy uses high-energy radiation to shrink tumors and kill cancer cells by inducing cellular apoptosis. Radiation therapy achieves this by either directly damaging DNA or generating charged particles (atoms with an odd or unpaired number of electrons) within the cells that can in turn damage the DNA. Most types of radiation used for cancer treatment are X-rays, gamma rays, and charged particles. Given their inherent toxicity to all cells, not solely cancer cells, these radiation treatments are administered at dosages balancing the effectiveness and safety, aiming to be potent while preventing excessive harm to surrounding tissue or organs near the tumor mass. This compromise considers factors like tumor type, tumor location, and stage.
Nanotechnology-specific research has been focusing on radiotherapy, elevating capabilities of this treatment modality through the unique properties of nanoscale materials. Specifically, most of the nanotechnology platforms designed for radiotherapy treatments rely on the interaction between X-rays and nanoparticles, driven by the inherent atomic level properties of the materials. The nanoparticles with high atomic number can enhance the Compton and photoelectric effects of conventional radiation therapy, thus increasing efficacy while reducing the existing radiotherapy dosage and its subsequent toxicity to the surrounding tissue. Other platforms make use of nanoparticles that release drug upon X-ray radiation, enabling localized drug delivery at tumor site and sensitizing cancer cells to radiotherapy.
Another type of therapy that benefits from external electromagnetic radiation is photodynamic therapy (PDT). It is an effective anticancer procedure for superficial tumors that relies on tumor localization of a photosensitizer followed by its light activation to generate cytotoxic reactive oxygen species (ROS). Upon X-rays irradiation, some nanoparticles that contain lanthanide or hafnium can emit visible light photons locally at the tumor site to generate ROS for tumor destruction. This approach serves as an alternative therapy for cancer cells that have become radiotherapy resistant. Moreover, certain nanomaterials can function as dual agents, facilitating both PDT and enhanced radiation therapy. In addition, some platforms utilize Cherenkov radiation for localized photon emission, acting as a trigger for local PDT in deep-tissue targets.
Nano-enabled Immunotherapy
Immunotherapy is a promising modality in cancer treatment, encompassing various approaches such as checkpoint inhibitors, lymphocyte-promoting cytokines, engineered T cells, and cancer vaccines. However, a key challenge in the widespread implementation of cancer immunotherapy is the precise regulation of the immune system, as these treatments can lead to serious adverse effects including autoimmunity and nonspecific inflammation. Understanding of tumor-host immune system interactions is pivotal for improving efficacy and controlling these adverse effects. New technologies for molecular and functional analysis of single cells are being used to interrogate tumor and immune cells, shedding light on molecular indicators and functional immune responses to therapy. To this end, nano-enabled devices and materials are strategically employed to efficiently sort, image, and comprehensively characterize immune cells.
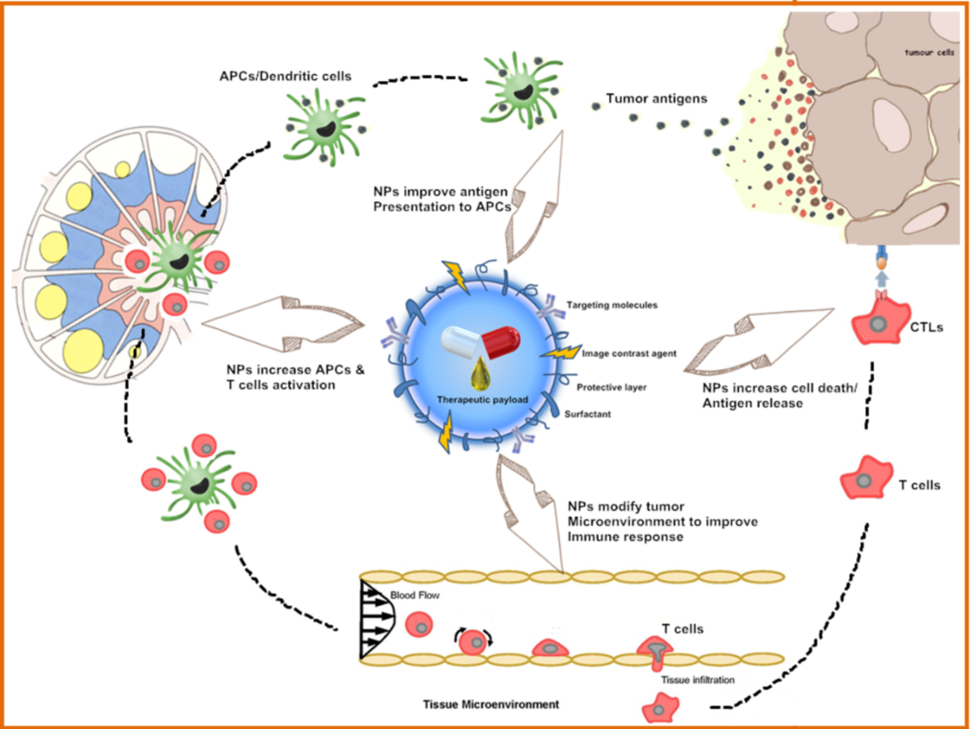
Nanotechnologies are also being investigated to deliver immunotherapy while reducing toxic side effects, as illustrated in the figure above. This includes use of nanoparticles for delivering immunostimulatory or immunomodulatory molecules in combination with chemo- or radiotherapy, or as adjuvants to other immunotherapies. Nanoparticles are also used to capture antigens shed from tumors following radiotherapy. Furthermore, nanoparticle-based vaccines (as exemplified in the image below) are being designed for raising T cell responses through antigen-adjuvant co-delivery, multi-antigen activation of dendritic cells, and continuous antigen release, as exemplified by the image below. Other applications of nanotechnology here include in situ vaccination with artificial antigen presenting cells or immune depots placed near tumors. These strategies will advance and be refined as our understanding of cancer immunotherapy deepens.
Delivering Gene Therapy
The value of nanomaterial-based delivery has become apparent for new types of therapeutics, particularly those using nucleic acids. Nuclei acids are highly unstable in systemic circulation and sensitive to degradation. These include DNA and RNA-based gene therapeutics, such as small interfering RNAs (siRNAs), messenger RNAs (mRNAs), and microRNAs (miRNAs), which have been reported to have significantly extended half-lives when delivered either encapsulated or conjugated to the surface of nanoparticles. These therapeutics are frequently used to target ‘undruggable’ cancer proteins. Additionally, the increased stability of genetic therapies delivered via nanocarriers, often combined with controlled release, has been shown to prolong their effects.
Researchers are exploring nanotechnology-based delivery of nucleic acids as effective treatment strategies for a variety of cancers. These efforts center on designing and characterizing spherical nucleic acids (SNAs) for the delivery of RNA therapeutics to treat brain cancer (Priya Kumthekar, et al., Science Translational Medicine, 2021). Another strategy is tackling vemurafenib resistant melanoma by direct suppressing drug resistance through siRNA delivery using polymetformin nanoparticles (Yi zhao, et al., Nature Communications, 2016). Similarly, other works focus on systematically characterizing the in vitro and in vivo RNA nanoparticle (Peixuan Guo, Nature Nanotechnology, 2010) behavior for optimized delivery of siRNA to tumor cells, as well as cancer immunotherapy.