How OCG’s Programs Support the Innovative Science that Will Improve Patient Care
, by OCG Staff
The mission of the National Cancer Institute’s (NCI) Office of Cancer Genomics (OCG) is to enhance the understanding of the molecular mechanisms of cancer; advance and accelerate technology development; and efficiently translate high-throughput data to improve cancer research, prevention, early detection, diagnosis, and treatment. OCG provides resources that bolster research aimed at reducing the burden of cancer; thus, aligning its mission with this year’s theme from the American Association for Cancer Research’s (AACR) annual meeting: “Driving Innovative Cancer Science to Patient Care”. Program managers for the current OCG initiatives share their perspectives on the important themes highlighted throughout the conference, along with how their respective programs contribute to those and further generate excitement and inspiration for the future.
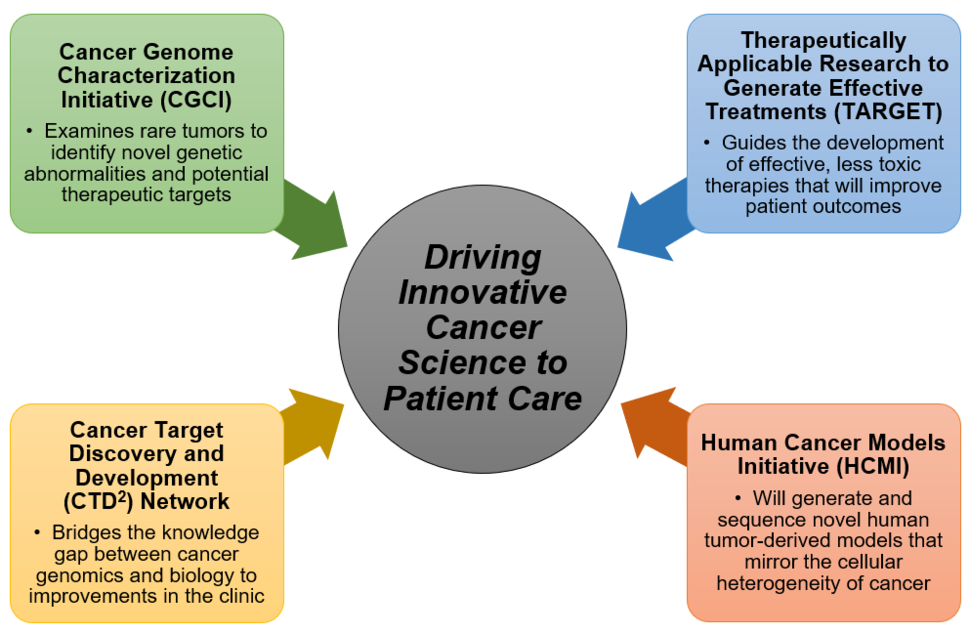
Cancer Genome Characterization Initiative (CGCI)
Nicholas Griner, Ph.D.
The Cancer Genome Characterization Initiative (CGCI) projects, such as the Burkitt Lymphoma Genome Sequencing Project (BLGSP) and the HIV+ Tumor Molecular Characterization Project (HTMCP), represent rare cancer studies where available research can be sparse. Large annual meetings, like this year’s AACR conference, allow investigators from around the world to congregate and share scientific knowledge. This is very beneficial for many involved in the study of common cancers, but it is especially useful for rare cancer type research. Large conferences like AACR allow rare cancer studies to be shared with a larger audience of the scientific community, which can spur new ideas that may be absent at tailored meetings. In addition, since tissue accrual can be difficult for rare cancer studies, large conferences allow greater networking opportunities leading to unexpected sources for tissues.
As scientific advancement continues to grow at an unprecedented rate, so do technologies for more efficient and precise analysis. The 2018 AACR annual meeting had a great number of technologies in the spotlight, including those from vendors and research labs demonstrating the efficacy of such technology. This is very beneficial for rare cancer studies as the quality of tissue accrual, from such areas as third world countries, can present a great challenge to the quality and quantity of tissue accrued. Attending seminars and posters unrelated to rare cancers still provides a glimpse as to how these technologies can be applied resulting in more accurate data sets for rare cancer studies.
Cancer Target Discovery and Development (CTD²) Network
Subhashini Jagu, Ph.D.
The current rate of accumulation of genomic data has far outpaced the rate of interpretation of this data. The next phase of translational cancer research is functional genomics, using varied methods of experimentation to determine the function of observed genomic alterations in cancerous cells. Cancer is a constellation of diseases with common characteristics. It is critical to develop a better understanding of the cause of initiation, progression, and metastasis by bringing together multidisciplinary research teams. Hence, the Cancer Target Discovery and Development (CTD²) Network, a functional genomics initiative, was established to bridge the knowledge gap between cancer genomics and biology for the development of human therapeutics. The CTD² Network aims to understand the tumor heterogeneity and drug resistance for the development of efficient strategies to identify optimal combinations of small-molecules or immunotherapy with small molecules. The Network Centers utilize a distinct array of advanced computational and systems biology methods, functional genomics and immunological approaches, small molecule and genetic screens to translate existing genomic data into biomarkers and therapeutic targets in cancer. They share data and resources (e.g. analytical tools, reagents, etc.) across the Network and with the research community.
The CTD² Network demonstrated how OCG supported “Driving Innovative Cancer Science to Patient Care” at AACR’s 2018 annual meeting. The meeting hosted several talks and posters by CTD² Network investigators, focusing on combining state-of-the-art high-throughput informatic and experimental approaches to functionally validate discoveries from genomic studies and high-throughput and high content small molecule and genetic screens and advance them toward precision oncology. For instance, in the “Translational Applications of Systems Biology” session, CTD² Network investigators discussed how systems biology approaches could be used to target adoptive responses; identify cancer dependencies, driver mutations, and tumor checkpoints; overcome treatment resistance; and rationalize combinatorial therapies.
Human Cancer Models Initiative (HCMI)
Caitlyn Barrett, Ph.D.
The field of patient-derived models has exploded in terms of the tissues from which models can be derived and the potential for model use. Culturing techniques, such as organoids and conditionally reprogrammed cells, offer a unique opportunity for the scientific community to study individual human tumors in vitro that closely represent the heterogeneity of the primary tumor from which they were derived. The NCI and founding institutions of the Human Cancer Models Initiative (HCMI) at the Wellcome Sanger Institute, Cancer Research UK, and the foundation Hubrecht Organoid Technology have developed large scale programs that are contributing to the development of patient-derived cancer models. Sequencing data from the model, normal tissue, and parent tumor; patient clinical data; and the models will be available to researchers worldwide as a community resource. It was thrilling to note that at this year’s AACR annual meeting, patient-derived cancer models were incorporated extensively into the science presented.
Models developed by the HCMI have great potential, and AACR was an excellent opportunity to see how scientists are beginning to use patient-derived models in their research. For example, to test targeted therapies, laboratories are developing new culturing methods, such as microfluidic devices for 3D culturing of cancer cells, which enable better penetrance of chemotherapeutics for screening. To assess mechanisms of drug resistance, models are being developed from resistant disease and tested for new drug susceptibilities. Importantly, genomic and proteomic assessment of patient-derived models are offering new insight into mechanisms of tumor evolution, progression, and drug response. The HCMI will be producing and distributing up to 1,000 models into a scientific field that will benefit from new cancer models, and the next couple of years will clarify the potential of patient-derived models. The HCMI is a work in progress; for up-to-date information visit the HCMI Program page.
Therapeutically Applicable Research to Generate Effective Treatments (TARGET)
Jaime M. Guidry Auvil, Ph.D.
Childhood cancer is emerging as its own critical field in the research community, separate from adult disease counterparts by more than merely age. While many patients can be cured of pediatric malignancy, cancer remains the leading cause of death in children over one year old in the United States. Childhood cancers devastate families, both those who lose children to the disease and those who support their children as they endure a lifetime of hardship resulting from toxic harsh treatments. Once thought to be similar diseases, it is now widely recognized that cancers arising earlier in life are molecularly and behaviorally distinct from those that develop in the aged. Consequently, new research directed toward advancing precision oncology is being pursued to improve clinical outcomes and subsequent quality of life for these young patients, particularly in genomics, model systems, and immunotherapies.
This year’s annual AACR meeting focused largely on pediatric areas of investigation and the “big data” volumes that funded programs are generating in their studies. Of interest for all research efforts is the critical need for broad, open, and ethical data sharing policies and procedures to advance discovery. Much of the pediatric data sessions, in line with the rest of AACR generally, focused strongly on data sharing perspectives and processes. An international panel of experts convened by the AACR Pediatric Cancer Working Group discussed this key topic in depth, including what data types are stored and where, barriers to access, need for standardization of data and metadata formats and terminology, consistent consent language and processes, and all on a global scale. It is clear that scientific advances have been compromised by intended and inadvertent data silos, and the pediatric community is coming together to find solutions for childhood cancers. OCG initiatives, including its large-scale pediatric molecular characterization program known as Therapeutically Applicable Research to Generate Effective Treatments (TARGET), have always promoted broad and equitable data sharing practices to support the NCI mission.
Bringing it All into Focus
Pamela Birriel, Ph.D.
NCI had a large presence at this year’s AACR annual meeting in covering the latest basic, translational, clinical, and prevention-focused research in the field. OCG program managers presented during an NCI-sponsored session focusing on how the initiatives support “driving innovative cancer science to patient care”. OCG, as part of NCI, is innovating across the field of cancer genomics and translational science: from basic cancer genomics research to improving data accessibility via bioinformatics infrastructure development; identifying therapeutic targets, perturbagens, and biomarkers; exploring cancer heterogeneities and developing strategies to overcome treatment resistance; as well as generating new cancer models to validate new therapeutic targets and diagnostic/prognostic markers. OCG unifies these activities by integrating research in different fields of cancer genomics––structural, functional, and computational––to advance precision oncology and improve clinical outcomes.