Quantifying the Timing of Metastatic Progression from Patient Genomic Data: Spatial Computational Inference of MEtastatic Timing (SCIMET)
, by Christina Curtis, Ph.D.
Metastasis, the systemic spread of malignancy to an organ other than the primary site of cancer, is the chief cause of cancer related death. Consequently, understanding and controlling metastasis is one of the most pressing issues in cancer medicine. Despite its obvious clinical significance, many aspects of this lethal process remain elusive. In particular, when and how tumor cells become competent to disseminate and seed distant metastases is unknown. Indeed, to date, few functional determinants or ‘drivers’ of metastasis have been identified.
Our limited understanding of the metastatic process is due, in part, to the difficulty in obtaining paired primary cancers and distant metastases for genomic characterization. This task is practically challenging since relapses can occur many years apart such that patients are lost to clinical follow-up. Moreover, often only the first site of metastasis is biopsied. Further complicating matters, electronic medical records do not necessarily capture information on recurrence, making it challenging to retrospectively identify individuals with metastatic disease. For the aforementioned reasons, there remains considerable debate regarding the timing of metastasis although it has traditionally been assumed to that the ability to metastasize is acquired late during growth of the primary cancer, such that only a subset of cells have the capacity to disseminate and successfully seed metastases at foreign tissue sites1-3.
Tumor progression and metastasis have long been appreciated to be somatic (clonal) evolutionary processes4 driven by somatic mutations (genetic and epigenetic), natural selection and neutral genetic drift. While the elements of clonal evolution are well defined, the dynamics that govern tumor initiation, subsequent growth of the founding tumor clone and progression are poorly understood5. Fortunately, tumors covertly record their ancestry in the form of somatic genomic alterations that are stably inherited during cell divisions. Thus, mutational data can be used to reconstruct the lineage relationship amongst cells within a tumor using phylogenetic tools, thereby providing a window into this otherwise unobservable process. However, phylogenetic approaches cannot accurately time metastasis as they do not account for factors that can confound the interpretation of tumor evolutionary dynamics, including tumor spatial architecture, differences in growth and mutation rates and the extent of subclonal selection5,6.
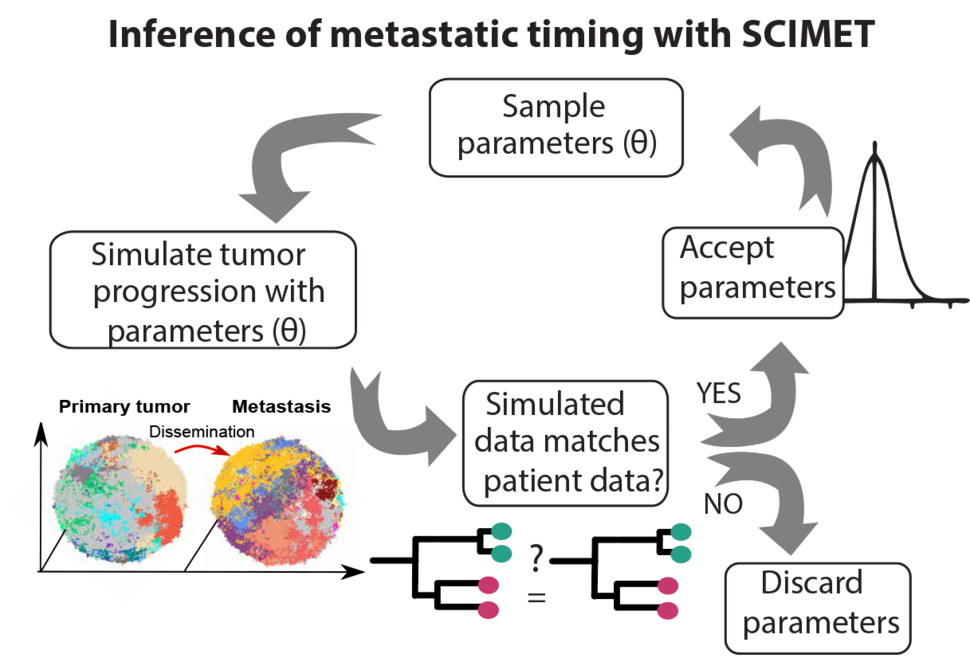
In order to estimate the timing of metastasis, the Curtis lab pioneered a new technique termed Spatial Computational Inference of MEtastatic Timing (SCIMET)7. This new method, developed by postdoctoral fellow Zheng Hu, leverages a spatial (3-D) computational model to simulate the spatial growth of realistically sized tumors (composed of ~109 cells or 10 cm3) and a robust statistical inference framework to measure evolutionary parameters, such as the timing of metastatic dissemination and the mutation rate from genomic data in a patient-specific manner. SCIMET enables the efficient simulation of the dynamics of metastasis in thousands of virtual patients under a range of parameters while explicitly accounting for tumor spatial architecture and the ‘mode’ of tumor evolution, both of which critically influence clonal dynamics and can otherwise confound the interpretation of metastatic timing.
In particular, the Curtis lab previously described a Big Bang model of colorectal tumor growth wherein after transformation, tumor growth is propagated by numerous equally fit subclones compatible with effectively neutral evolution or nearly neutral evolution8. This new model stands in contrast to the de facto sequential clonal evolution model of continuous positive selection during tumor progression and highlights the early origin of intra-tumor heterogeneity with several clinical implications. Building on the finding that distinct modes of evolution can be operative in different tumors and at different stages of disease progression, the Curtis lab developed a method to classify the two dominant ‘modes’ of tumor evolution, namely effective neutrality and subclonal selection from tumor sequencing data5.
Since the mode and tempo of progression differ between tumors evolving neutrally and those subject to stringent subclonal selection, it is critical to account for this when characterizing the dynamics of metastasis.
By simulating tumor progression and the underlying genomic profiles for thousands for virtual tumors, SCIMET can delineate the contribution of different factors to the metastatic process and define the parameter sets that best explain the observed patient genomic data. This is of critical importance since ground truth measurements in the human system are not feasible. SCIMET can be used to determine optimal study designs and tissue sampling schemes and generates testable predictions regarding the metastatic process that can be evaluated experimentally.
Application of SCIMET to primary cancers and paired metastases has already begun to reveal mechanistic insights into this otherwise unobservable process, including the temporal dynamics and routes of metastasis. SCIMET will be an important resource for the cancer genomics community in light of the increasing focus on profiling metastatic disease and its broad generalizability for studying diverse solid tumors. Indeed, the systematic characterization of tumor evolutionary dynamics and the determinants of metastasis in clinically annotated patient cohorts has the potential to identify new therapeutic targets, as well as biomarkers that may ultimately be used to inform patient stratification and predict disease progression.
References
- Jones S, et al. Comparative lesion sequencing provides insights into tumor evolution. Proceedings of the National Academy of Sciences of the USA. 2008 Mar 18;105(11):4283-8. (PMID: 18337506)
- Yachida S, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010 Oct 28;467(7319):1114-7. (PMID: 20981102)
- Yates LR, Campbell PJ. Evolution of the cancer genome. Nature Reviews Genetics. 2012 Nov;13(11):795-806. (PMID: 23044827)
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23-8.
- Sun R, et al. Between-region genetic divergence reflects the mode and tempo of tumor evolution. Nature Genetics. 2017 Jul;49(7):1015-24. (PMID: 28581503)
- Hu Z, Sun R, Curtis C. A population genetics perspective on the determinants of intra-tumor heterogeneity. Biochimica et Biophysica Acta 2017 Apr;1867(2):109-26. (PMID: 28274726)
- Hu Z, Ding J, Ma Z, et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nature Genetics. 2019 Jun 17. doi: 10.1038/s41588-019-0423-x. (PMID: 31209394)
- Sottoriva A, et al. A Big Bang model of human colorectal tumor growth. Nature Genetics. 2015 Mar;47(3):209-16. (PMID: 25665006)