Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment (PDQ®)–Patient Version
General Information About Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor
Key Points
- Central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT) is a cancer that forms in the tissues of the brain.
- Certain genetic changes may increase the risk of AT/RT.
- The symptoms of AT/RT are not the same in every person.
- CNS AT/RT is found with tests that examine the brain and spinal cord.
- Childhood AT/RT is diagnosed using a biopsy, and the tumor may be removed in the same surgery.
- Certain factors affect prognosis (chance of recovery) and treatment options.
Central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT) is a cancer that forms in the tissues of the brain.
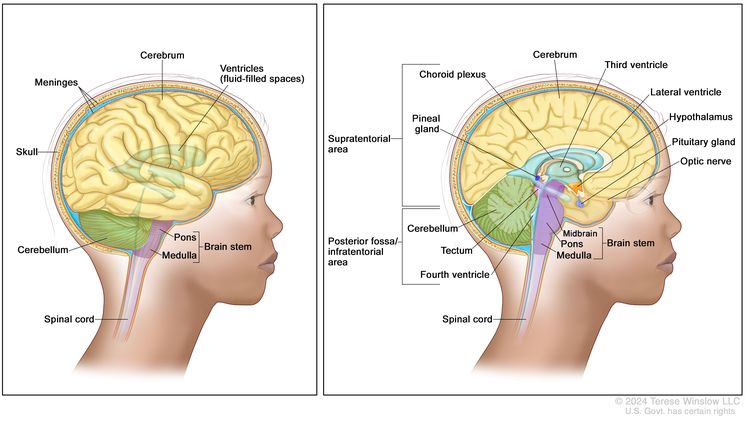
Central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT) is a very rare, fast-growing cancer that begins in the brain and spinal cord. It usually occurs in children aged 3 years and younger, although it can occur in older children and adults.
About half of these tumors form in the cerebellum or brain stem. The cerebellum is the part of the brain that controls movement, balance, and posture. The brain stem controls breathing, heart rate, and the nerves and muscles used in seeing, hearing, walking, talking, and eating. AT/RT can also begin in other parts of the brain and spinal cord.
Certain genetic changes may increase the risk of AT/RT.
A risk factor is anything that increases the chance of getting a disease. Not every child with one or more of these risk factors will develop AT/RT. And it will develop in some children who don't have a known risk factor.
AT/RT may be linked to changes in the tumor suppressor genes SMARCB1 or SMARCA4. Tumor suppressor genes make a protein that helps control how and when cells grow. Changes in the DNA of tumor suppressor genes like SMARCB1 or SMARCA4 may lead to cancer.
The changes in the SMARCB1 or SMARCA4 genes may be inherited (passed on from parents to offspring). When this gene change is inherited, tumors may form in two parts of the body at the same time (for example, in the brain and the kidney). For children with AT/RT, genetic counseling (a discussion with a trained professional about inherited diseases and a possible need for gene testing) may be recommended.
Talk with your child's doctor if you think your child may be at risk.
The symptoms of AT/RT are not the same in every person.
Symptoms depend on:
- the child's age
- where the tumor has formed
Because AT/RT is fast growing, symptoms may develop quickly and get worse over a period of days or weeks. It's important to check with your child's doctor if your child has:
- a morning headache or headache that goes away after vomiting
- nausea and vomiting
- unusual sleepiness or change in activity level
- loss of balance, lack of coordination, or trouble walking
- an increase in head size (in infants)
- pain, tingling, numbness, or paralysis in the face
These symptoms may be caused by problems other than AT/RT. The only way to know is to see your child's doctor.
CNS AT/RT is found with tests that examine the brain and spinal cord.
If your child has symptoms that suggest AT/RT, the doctor will need to find out if these are due to cancer or another problem. The doctor will ask when the symptoms started and how often your child has been having them. They will also ask about your child's personal and family health history and do a physical exam, including a neurological exam. Depending on these results, they may recommend other tests. If your child is diagnosed with AT/RT, the results of these tests will help you and your child's doctor plan treatment.
The tests used to diagnose AT/RT may include:
- Magnetic resonance imaging (MRI) uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the brain and spinal cord. This procedure is also called nuclear magnetic resonance imaging (NMRI).
- Lumbar puncture is a procedure used to collect cerebrospinal fluid (CSF) from the spinal column. This is done by placing a needle between two bones in the spine and into the lining around the spinal cord to remove a sample of the CSF. The sample of CSF is checked under a microscope for signs of tumor cells. The sample may also be checked for the amounts of protein and glucose. This procedure is also called an LP or spinal tap.
- SMARCB1 and SMARCA4 gene testing is a laboratory test in which a sample of blood or tissue is tested for certain changes in the SMARCB1 and SMARCA4 genes. Children with AT/RT may be eligible for gene testing through the Molecular Characterization Initiative.
The Molecular Characterization Initiative offers free molecular testing to children, adolescents, and young adults with certain types of newly diagnosed cancer. The program is offered through NCI's Childhood Cancer Data Initiative. To learn more, visit About the Molecular Characterization Initiative.
- Ultrasound exam uses high-energy sound waves (ultrasound) that bounce off internal tissues or organs, such as the kidney, and make echoes. The echoes form a picture of body tissues called a sonogram. This procedure is done to check for tumors that may also have formed in the kidney.
Childhood AT/RT is diagnosed using a biopsy, and the tumor may be removed in the same surgery.
If doctors think there might be a brain tumor, a biopsy may be done to remove a sample of tissue. For brain tumors, the biopsy can be done by removing part of the skull or making a small hole in the skull and using a needle or surgical device to remove a sample of tissue. Sometimes, when a needle is used, it is guided by a computer to remove the tissue sample. A pathologist views the tissue under a microscope to look for cancer cells. If cancer cells are found, the doctor may remove as much tumor as safely possible during the same surgery. The pathologist checks the cancer cells to find out the type of brain tumor. It is often difficult to completely remove AT/RT because of where the tumor is in the brain and because it may already have spread at the time of diagnosis. The piece of skull is usually put back in place after the procedure.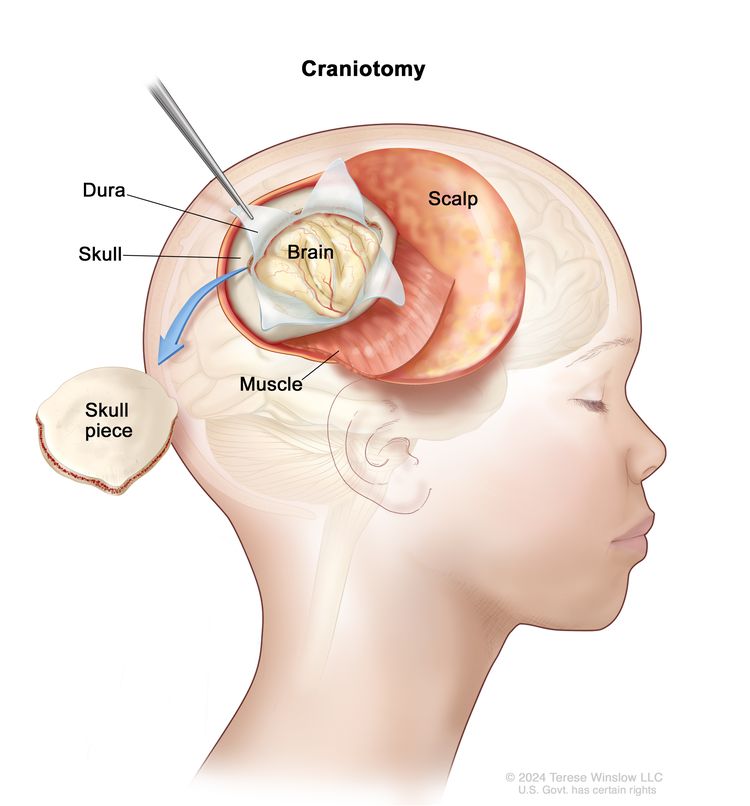
The following test may be done on the sample of tissue that is removed:
- Immunohistochemistry uses antibodies to check for certain antigens (markers) in a sample of a patient's tissue. The antibodies are usually linked to an enzyme or a fluorescent dye. After the antibodies bind to a specific antigen in the tissue sample, the enzyme or dye is activated, and the antigen can then be seen under a microscope. This type of test is used to help diagnose cancer and to help tell one type of cancer from another type of cancer.
Certain factors affect prognosis (chance of recovery) and treatment options.
If your child has been diagnosed with AT/RT, you likely have questions about how serious the cancer is and your child's chances of survival. The likely outcome or course of a disease is called prognosis.
The prognosis depends on:
- whether your child has certain inherited gene changes
- whether the tumor has certain gene changes
- your child's age
- the amount of tumor remaining after surgery
- whether the cancer has spread to other parts of the brain and spinal cord or to the kidney at the time of diagnosis
- whether the cancer has just been diagnosed or has recurred (come back)
No two people are alike, and responses to treatment can vary greatly. Your child's cancer care team is in the best position to talk with you about your child's prognosis.
Stages of Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor
Key Points
- There is no standard staging system for central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
There is no standard staging system for central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
The process used to find out if cancer has spread to other parts of the body is called staging. There is no standard staging system for central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
For treatment, this tumor is grouped as newly diagnosed or recurrent. Treatment depends on:
- your child's age
- how much cancer remains after surgery to remove the tumor
- whether the cancer has spread to other parts of the CNS
- the results of tests and procedures done to diagnose the cancer
Treatment Option Overview
Key Points
- There are different types of treatment for children with central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
- Children with AT/RT should have their treatment planned by a team of health care providers who are experts in treating cancer in children.
- Childhood brain tumors may cause symptoms that begin before the cancer is diagnosed and continue for months or years.
- The following types of treatment may be used:
- Surgery
- Chemotherapy
- Radiation therapy
- Stem cell transplant
- Clinical trials
- Treatment for childhood CNS AT/RT may cause side effects.
- Follow-up care may be needed.
- Resources and support are available to help you cope with your child's cancer.
There are different types of treatment for children with central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
There are different types of treatment for children with AT/RT. You and your child's care team will work together to decide treatment. Many factors will be considered, such as where the cancer is located and your child's age and overall health.
Your child's treatment plan will include information about the tumor, the goals of treatment, treatment options, and the possible side effects. It will be helpful to talk with your child's care team before treatment begins about what to expect. For help every step of the way, see our booklet, Children with Cancer: A Guide for Parents.
Children with AT/RT should have their treatment planned by a team of health care providers who are experts in treating cancer in children.
A pediatric oncologist, a doctor who specializes in treating children with cancer, oversees treatment of AT/RT. The pediatric oncologist works with other health care providers who are experts in treating children with CNS cancer and also specialize in other areas of medicine. Other specialists may include:
Childhood brain tumors may cause symptoms that begin before the cancer is diagnosed and continue for months or years.
Symptoms caused by the tumor may begin before diagnosis. These signs or symptoms may continue for months or years. It is important to talk with your child's doctors about symptoms caused by the tumor that may continue after treatment.
The following types of treatment may be used:
Surgery
Surgery is used to treat CNS AT/RT. Learn more about how this tumor is diagnosed.
After the doctor removes all the cancer that can be seen at the time of the surgery, most children will receive chemotherapy and possibly radiation therapy to try to kill any cancer cells that are left. Treatment given after surgery to lower the risk that the cancer will come back is called adjuvant therapy.
Chemotherapy
Chemotherapy uses drugs to stop the growth of cancer cells. Chemotherapy either kills the cells or stops them from dividing. Chemotherapy may be given with other types of treatments.
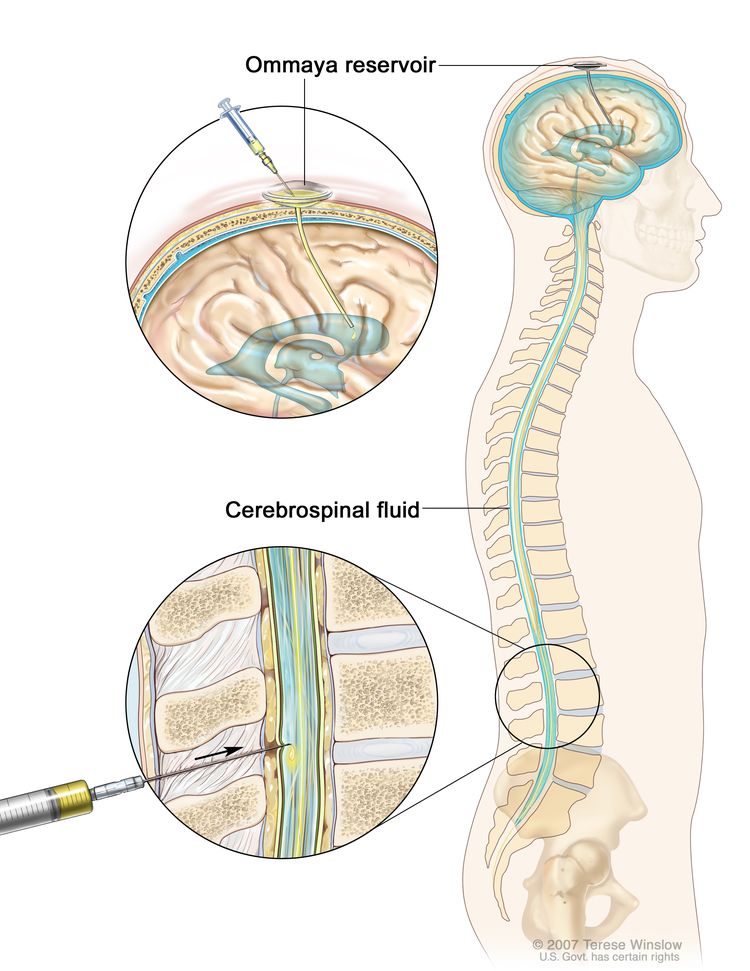
Chemotherapy for AT/RT is injected into a vein. When given this way, the drugs enter the bloodstream and can reach tumor cells throughout the body. High doses of some chemotherapy drugs given into a vein can cross the blood-brain barrier and reach the tumor. Chemotherapy for AT/RT is also placed directly into the cerebrospinal fluid (intrathecal chemotherapy). Combination chemotherapy uses more than one anticancer drug.
Chemotherapy drugs used alone or in combination to treat AT/RT in children include:
Other chemotherapy drugs not listed here may also be used.
Learn more about how chemotherapy works, how it is given, and common side effects at Chemotherapy to Treat Cancer.
Radiation therapy
Radiation therapy uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer.
Because radiation therapy can affect growth and brain development in young children, especially children who are 3 years old or younger, the dose of radiation therapy may be lower than in older children.
Learn more about External Beam Radiation Therapy for Cancer and Radiation Therapy Side Effects.
Stem cell transplant
High doses of chemotherapy are given to kill cancer cells. This treatment destroys healthy cells, including blood-forming cells. Stem cell transplant is a treatment to replace the blood-forming cells. Stem cells (immature blood cells) are removed from the blood or bone marrow of the patient and are frozen and stored. After the patient completes chemotherapy, the stored stem cells are thawed and given back to the patient through an infusion. These reinfused stem cells grow into (and restore) the body's blood cells.
Clinical trials
For some children, joining a clinical trial may be an option. There are different types of clinical trials for childhood cancer. For example, a treatment trial tests new treatments or new ways of using current treatments. Supportive care and palliative care trials look at ways to improve quality of life, especially for those who have side effects from cancer and its treatment.
You can find clinical trials for people with atypical teratoid/rhabdoid tumor at Treatment Clinical Trials for Atypical Teratoid/Rhabdoid Tumor or use the clinical trial search to find NCI-supported cancer clinical trials accepting participants. The search allows you to filter trials based on the type of cancer, your child's age, and where the trials are being done. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Learn more about clinical trials, including how to find and join one, at Clinical Trials Information for Patients and Caregivers.
Treatment for childhood CNS AT/RT may cause side effects.
Cancer treatments can cause side effects. Which side effects your child might have depends on the type of treatment they receive, the dose, and how their body reacts. Talk with your child's treatment team about which side effects to look for and ways to manage them.
To learn more about side effects that begin during treatment for cancer, visit Side Effects.
Problems from cancer treatment that begin 6 months or later after treatment and continue for months or years are called late effects. Late effects of cancer treatment may include:
- physical problems
- changes in mood, feelings, thinking, learning, or memory
- second cancers (new types of cancer)
Some late effects may be treated or controlled. It is important to talk with your child's doctors about the effects cancer treatment can have on your child. Learn more about Late Effects of Treatment for Childhood Cancer.
Follow-up care may be needed.
As your child goes through treatment, they will have follow-up tests or check-ups. Some of the tests that were done to diagnose the cancer may be repeated. Some tests will be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your child's condition has changed or if the cancer has recurred (come back).
Resources and support are available to help you cope with your child's cancer.
When your child has cancer, every member of the family needs support. Taking care of yourself during this difficult time is important. Reach out to your child's treatment team and to people in your family and community for support. To learn more, see Support for Families: Childhood Cancer and the booklet Children with Cancer: A Guide for Parents.
Treatment of Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor
For information about the treatments listed below, see the Treatment Option Overview section.
There is no standard treatment for children with newly diagnosed central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT). Because AT/RT is fast-growing, a combination of treatments is usually given.
After surgery to remove the tumor, treatment for AT/RT may include combinations of:
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Recurrent Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor
For information about the treatments listed below, see the Treatment Option Overview section.
There is no standard treatment for children with recurrent central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
Treatment for recurrent childhood AT/RT may include:
- radiation therapy
- palliative treatment to improve quality of life
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
To Learn More about Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor and Other Childhood Brain Tumors
For more information about childhood central nervous system atypical teratoid/rhabdoid tumor and other childhood brain tumors, visit:
For more childhood cancer information and other general cancer resources, visit:
- About Cancer
- Childhood Cancers
- CureSearch for Children's Cancer
- Late Effects of Treatment for Childhood Cancer
- Adolescents and Young Adults with Cancer
- Children with Cancer: A Guide for Parents
- Cancer in Children and Adolescents
- Cancer Staging
- Coping with Cancer
- Questions to Ask Your Doctor about Cancer
- For Survivors, Caregivers, and Advocates
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of childhood central nervous system atypical teratoid and rhabdoid tumor. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Pediatric Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/brain/patient/child-cns-atrt-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389341]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.