Genomic Discoveries in Pediatric Mixed Phenotype Acute Leukemia Inform Therapeutic Approaches
, by Thomas B. Alexander, MD, MPH and Charles G. Mullighan, MBBS (Hons), MSc, MD
The principal subtypes of acute leukemia are acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). These are driven by distinct mutational profiles and arise from different developing blood cells. The treatment for these diseases involves contrasting combinations and schedules of drugs. Exploring the biology of these leukemia subtypes has improved disease classification and clinical management. However, these advances have not carried over to patients diagnosed with mixed phenotype acute leukemia (MPAL), a subtype of acute leukemia of ambiguous lineage (ALAL) and a disease characterized by features of both ALL and AML.
MPAL carries a poor prognosis and accounts for about 3 percent of the estimated 4,000 cases of pediatric acute leukemia diagnosed annually in the U.S. The biology driving this complicated leukemia subtype, and the optimal treatment, are largely undefined, leaving physicians and patients with limited information to help guide therapy. The recent study in Nature by Alexander et al1 explored the biology of pediatric ALAL, mostly MPAL, by genomic interrogation, analysis of multiple leukemia cell subpopulations within each patient sample, and in vivo xenograft models. The investigation provided conceptual insights into the cell of origin of each subtype of MPAL and the genetic alterations that drive them, as well as the basis for the unusual, aberrant immunophenotype. These insights provide a framework for future preclinical research, as well as clinical trials and therapeutic decisions for patients with MPAL. The research was a combined effort of many clinicians and researchers. Thirteen institutions or consortiums provided samples to create a large cohort of 115 cases.
MPAL includes multiple subtypes, defined by immunophenotype, that have distinct genetic features driving leukemia biology. The most common subtypes are B/Myeloid (B/M) and T/Myeloid (T/M). B/M MPAL has immunophenotypic features of both B-cell ALL (B-ALL) and AML, and similarity to B-ALL in both the DNA mutation profile of transcription factor genes ETV6, PAX5, IKZF1 and patterns of gene expression. Rearrangements of the transcription factor gene ZNF384, which have also been observed in B-ALL, were present in nearly half of the cases analyzed by RNA-sequencing and this fusion has been well-characterized in B-ALL2,3. These biological features support ALL directed therapy as the appropriate initial therapeutic choice for B/M MPAL, consistent with recent clinical studies 4,5 .T/M MPAL shares features with a subtype of ALL called early T-cell precursor (ETP) ALL. Both T/M MPAL and ETP-ALL have frequent alterations in FLT3 (tyrosine kinase receptor gene), WT1 (tumor suppressor gene), and developmental pathway genes RUNX1 and ETV6. The gene expression profiles of both T/M MPAL and ETP-ALL are in between T-ALL and AML. Previously, ETP-ALL was shown to have a poor prognosis with some investigators advocating for incorporation of AML-directed therapy 6,7. However, recent trials have demonstrated improved prognosis of ETP-ALL when patients are treated on response-adapted ALL protocols 8,9.
The similarities between T/M MPAL and ETP-ALL suggest that ALL therapy may be appropriate for many T/M MPAL patients.
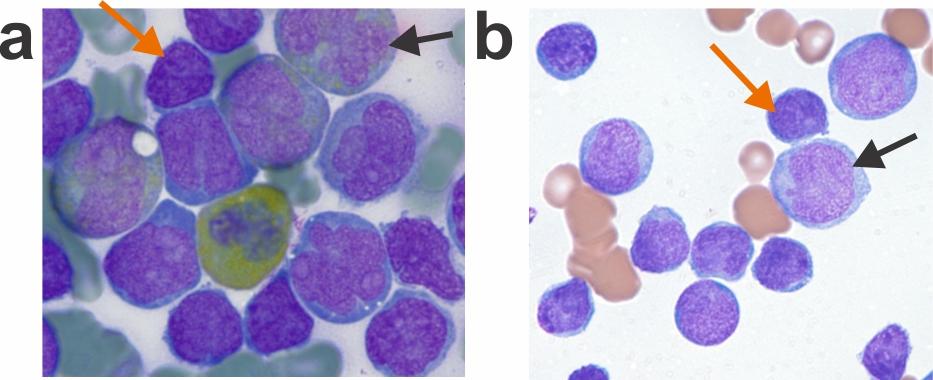
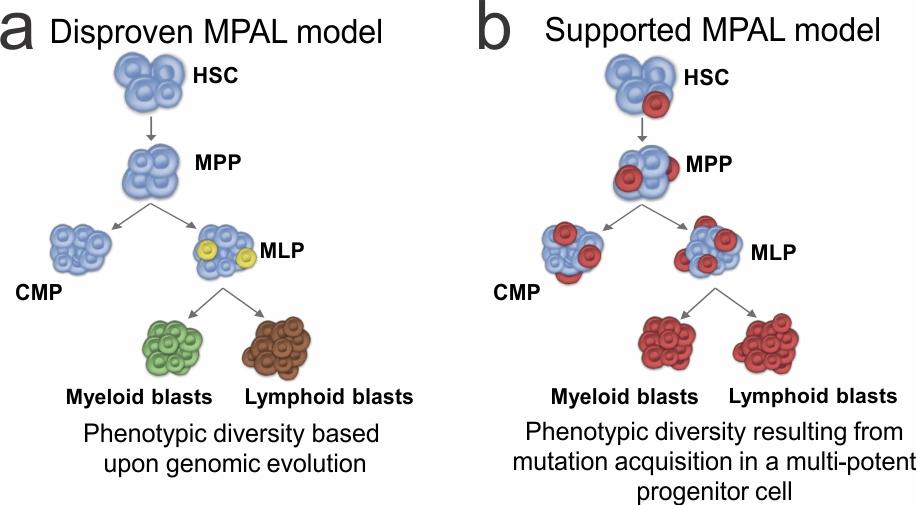
MPAL cases often present with multiple distinct populations; some cells express predominantly myeloid features, some lymphoid only, some cells with features of both, and some cells with no lineage-defining features. In the current study, mutational analysis of purified progenitor subpopulations demonstrated the presence of hallmark mutations in progenitor cells. Conversely, distinct immunophenotypic populations of leukemia cells within individual cases typically harbored the same mutations. In addition, when immunophenotypically distinct subpopulations from several MPAL cases were inoculated into immunocompromised mice, the engrafted leukemias typically recapitulated the full phenotypic spectrum of each primary patient sample. These findings support the notion that causal mutations arise in early hematopoietic cells that have the potential for either lymphoid or myeloid differentiation, rather than mutational evolution determining the final phenotype.
These findings have multiple conceptual and clinical implications. Nearly half of the cases of T/M MPAL contained FLT3 alterations, and half of the B/M cases contained ZNF384 rearrangements which result in high levels of FLT3 expression even in the absence of FLT3 mutations. Hence, possibilities for translational approaches come to the forefront, e.g. inhibition of FLT3. Another translational challenge is to identify the optimal methods for monitoring dynamics of responses to therapy in MPAL, which is complicated by the unusual phenotype. Copy number analysis revealed rearrangements of immunoglobulin (Ig) and T-cell receptor (TCR) in MPAL cases. The potential for utilizing sequence-based methods to track MPAL responses to therapy using Ig/TCR rearrangements as a clonal marker remains to be explored. Finally, converging evidence supports ALL therapy as an appropriate choice in a majority of cases of MPAL. This evidence has contributed to the important decision of the Children’s Oncology Group to include patients with MPAL on an arm of the upcoming frontline ALL clinical trial (AALL1732, activation anticipated 2019).
The work was led by authors Charles G. Mullighan and Hiroto Inaba. The study’s co-first authors were Thomas B. Alexander, Zhaohui Gu, and Ilaria Iacobucci, with key contributions from the Children’s Oncology Group and the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) initiative of the National Cancer Institute.
References
- Alexander TB, Gu Z, Iacobucci I, et al. The genetic basis and cell of origin of mixed phenotype acute leukaemia. Nature. 2018 Oct;562(7727):373-379. (PMID: 30209392)
- Gu Z, Churchman M, Roberts K, et al. Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nature Communications. 2016;7:13331. (PMID: 27824051) .
- Yasuda T, Tsuzuki S, Kawazu M, et al. Recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults. Nature Genetics. 2016 May;48(5):569-574. (PMID: 27019113)
- Hrusak O, de Haas V, Stancikova J, et al. International cooperative study identifies treatment strategy in childhood ambiguous lineage leukemia. Blood. 2018;132(3):264-276. (PMID: 29720486)
- Maruffi M, Sposto R, Oberley MJ, Kysh L, & Orgel E. Therapy for children and adults with mixed phenotype acute leukemia: a systematic review and meta-analysis. Leukemia. 2018;32(7):1515-1528. (PMID: 29550836)
- Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. The Lancet. Oncology. 2009 Feb;10:147-156. (PMID: 19147408)
- Wood B, Winter S, Dunsmore K, et al. Patients with Early T-Cell Precursor (ETP) Acute Lymphoblastic Leukemia (ALL) Have High Levels of Minimal Residual Disease (MRD) at the End of induction—A Children's Oncology Group (COG) Study. Blood. 2009;114:9-9.
- Wood BL, Winter SS, Dunsmore KP, et al. T-Lymphoblastic Leukemia (T-ALL) Shows Excellent Outcome, Lack of Significance of the Early Thymic Precursor (ETP) Immunophenotype, and Validation of the Prognostic Value of End-Induction Minimal Residual Disease (MRD) in Children’s Oncology Group (COG) Study AALL0434. Blood. 2014;124:1-1.
- Conter V, Valsecchi MG, Buldini B, et al. Early T-cell precursor acute lymphoblastic leukaemia in children treated in AIEOP centres with AIEOP-BFM protocols: a retrospective analysis. The Lancet Haematology. 2016;3:e80-86. (PMID: 26853647)