Dissecting Cellular Heterogeneity Using Single-Cell RNA Sequencing
, by Anuja Sathe, M.B.B.S., Ph.D. and Hanlee P. Ji, M.D.
Gene expression analysis using RNA sequencing has contributed immensely to our knowledge of cancer. However, bulk sequencing methods average signals by pooling information from a mass of cells. These methods are thus unable to fully resolve the complexity of intra-tumor heterogeneity in cancer.1
Single-cell RNA sequencing (scRNA-seq) enables the analysis of the transcriptome of each individual cell and allows a high-resolution characterization of tumors. Moreover, tumors are not isolated masses of cancer cells but are surrounded by a unique microenvironment composed of different cell types. scRNA-seq enables the analysis of this heterogenous tumor microenvironment (TME) that is increasingly important in improving treatment strategies such as immunotherapy. Unlike other single-cell analysis methods such as mass cytometry or CyTOF, scRNA-seq allows an unbiased assessment of cellular phenotypes. This enables the identification of not just heterogenous cell types but provides information on individual cell states.
In recent years, scRNA-seq has been used to construct single cell atlases of several tumor types.2 These studies have revealed novel targets in sub-populations of cancer cells as well as in the TME. We have successfully used scRNA-seq in the characterization of lymphoma TME using patient biopsies and in an organoid mouse model of gastric cancer.3,4 We are applying it to understand the TME of gastrointestinal cancers from fresh surgical specimens as well as in cell line models to delineate clonal heterogeneity.5
Several technologies have been developed for scRNA-seq.6 They differ in their method of cell isolation (e.g. plate or microfluidics-based), capture and length of transcript (full-length, 3’ or 5’ end) as well as the chemistry used for reverse transcription and amplification. Choosing a particular platform for an experiment depends on the question being investigated. For example, microfluidics-based techniques are more high-throughput than plate-based ones. Methods that capture the 3’ or 5’ transcript do not allow the detection of splicing events, isoforms, or quantifying allelic expression.
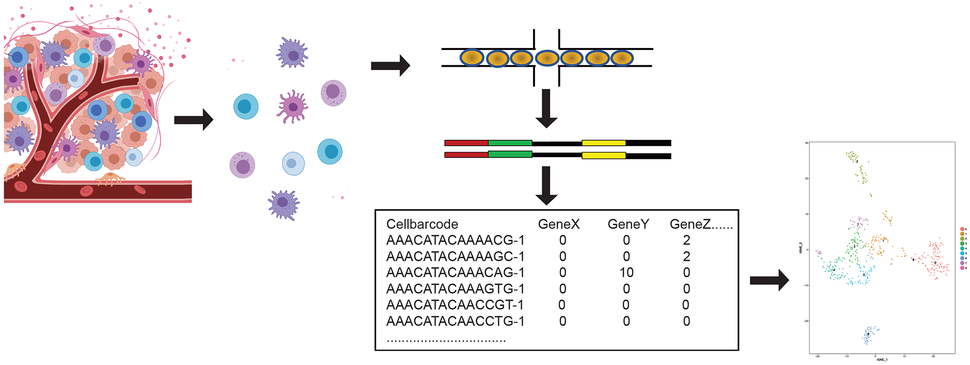
Following sequencing, a typical analytical workflow begins with data matrices containing entries of molecular counts corresponding to each gene and cell, which are represented in respective rows or columns. This high-dimensional data is generally analyzed using dimensionality reduction (e.g. with principal component analysis (PCA) or t-Distributed Stochastic Neighbor Embedding (t-SNE)) followed by clustering of cells with similar transcriptional profiles (Figure). Understanding differences across clusters is aided by differential expression testing.
A major challenge in scRNA-seq analysis results from the noise and variability of the assay owing to a high number of zero transcript counts. These dropouts can be biological, related to the stochasticity of mRNA expression, or technical, owing to the low amount of transcript material and limited efficiency of its capture. This can result in distorted false negative or false positive profiles. scRNA-seq requires careful attention to quality control and use of appropriate computational methods that are suited for such data distribution.7
Another limitation of scRNA-seq is that it does not retain any spatial information. Moreover, disaggregation methods are required to produce a single-cell suspension that could introduce artifacts in the gene expression program. Commercial assays and instrumentation can also be expensive.
A number of technical and computational approaches are enabling improvements in scRNA-seq that overcome many of these challenges. For example, conducting parallel experiments such as RNA in situ hybridization, imaging mass cytometry, or spatial transcriptomics can enable integration of spatial information. As sequencing costs reduce, the current cost for an scRNA-seq experiment with a microfluidics-based platform works out to be less than 1 USD/cell (https://satijalab.org/costpercell). This is additionally aided by modifications to the assay using antibodies to tag each cell that enable sample multiplexing.8
Several quality control and imputation methodologies are also being developed to improve data analytics.9,10 Novel assay developments such as single-cell DNA sequencing, single-cell Assay for Transposase Accessible Chromatin (ATAC) sequencing, single-cell T Cell Receptor (TCR) sequencing, and single-cell epitope sequencing can also be integrated with scRNA-seq to reveal a wealth of information at high granularity.
scRNA-seq is thus equipped to answer several important questions in cancer biology including target discovery and can enable a mechanistic understanding of target inhibition. It also has tremendous potential in translational applications such as longitudinal patient monitoring of treatment response.11
References
- Andor N, Graham TA, Jansen M, et al. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med. 2016 Jan;22(1):105-13. (PMID: 26618723)
- Valdes-Mora F, Handler K, Law AMK, et al. Single-Cell Transcriptomics in Cancer Immunobiology: The Future of Precision Oncology. Front Immunol. 2018 Nov 12;9:2582. (PMID: 30483257)
- Chen J, Lau BT, Andor N, et al. Single-cell transcriptome analysis identifies distinct cell types and niche signaling in a primary gastric organoid model. Sci Rep. 2019 Mar 14;9(1):4536. (PMID: 30872643)
- Andor N, Simonds EF, Czerwinski DK, et al. Single-cell RNA-Seq of follicular lymphoma reveals malignant B-cell types and coexpression of T-cell immune checkpoints. Blood. 2019 Mar 7;133(10):1119-1129. (PMID: 30591526)
- Andor N, Lau BT, Catalanotti C, et al. Joint single cell DNA-Seq and RNA-Seq of gastric cancer reveals subclonal signatures of genomic instability and gene expression. bioRxiv. 2018 Oct 17. doi: 10.1101/445932
- Haque A, Engel J, Teichmann SA, et al. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017 Aug 18;9(1):75. (PMID: 28821273)
- Chen G, Ning B, Shi T. Single-Cell RNA-Seq Technologies and Related Computational Data Analysis. Front Genet. 2019 Apr 5;10:317. (PMID: 31024627)
- Stoeckius M, Zheng S, Houck-Loomis B, et al. Cell Hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 2018 Dec 19;19(1):224. (PMID: 30567574)
- Huang M, Wang J, Torre E, et al. SAVER: gene expression recovery for single-cell RNA sequencing. Nat Methods. 2018 Jul;15(7):539-542. (PMID: 29941873)
- Ilicic T, Kim JK, Kolodziejczyk AA, et al. Classification of low quality cells from single-cell RNA-seq data. Genome Biol. 2016 Feb 17;17:29. (PMID: 26887813)
- Shalek AK, Benson M. Single-cell analyses to tailor treatments. Sci Transl Med. 2017 Sep 20;9(408). (PMID: 28931656)