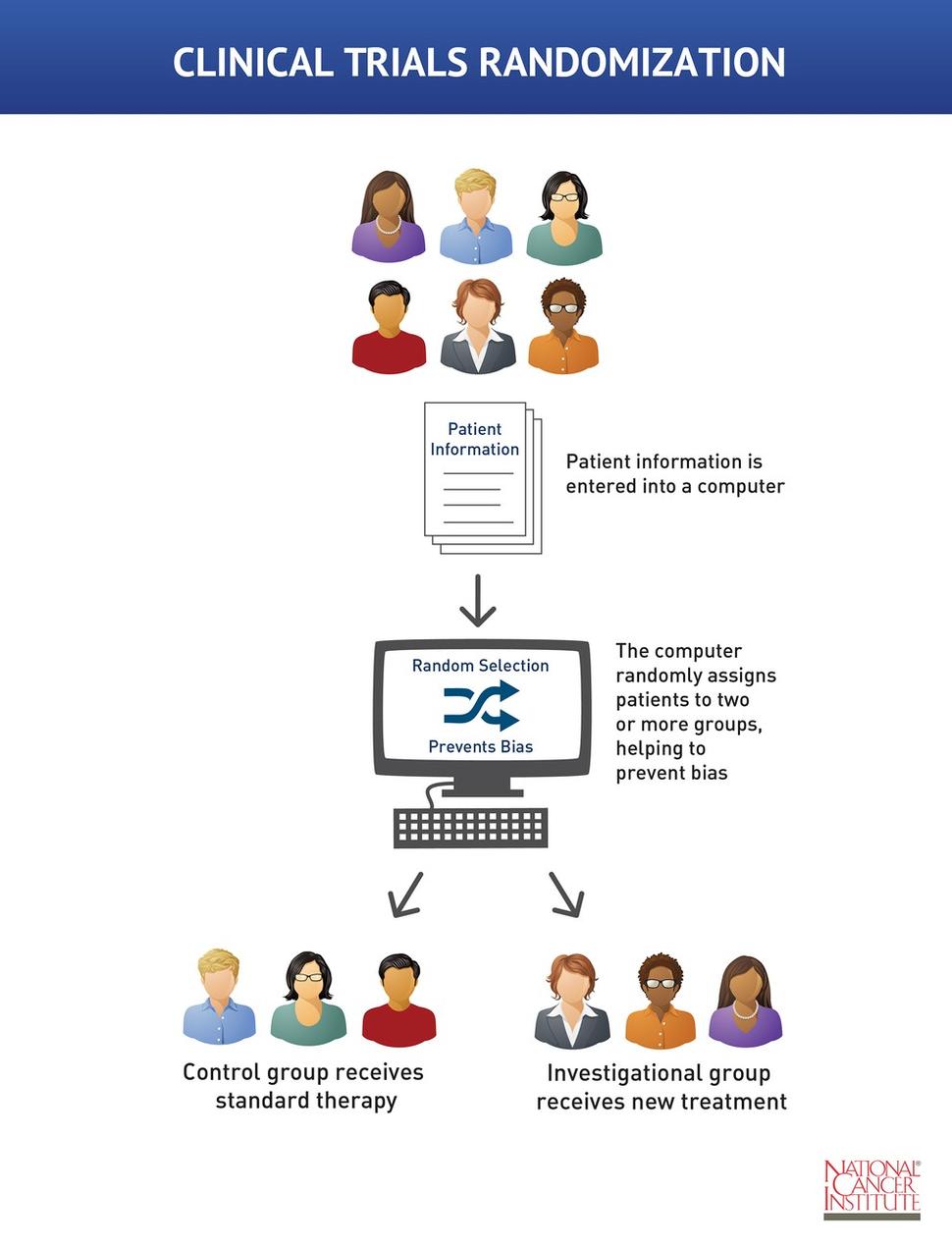
Clinical Trial Randomization
Clinical trial randomization is the process of assigning patients by chance to groups that receive different treatments. In the simplest trial design, the investigational group receives the new treatment and the control group receives standard therapy. At several points during and at the end of the clinical trial, researchers compare the groups to see which treatment is more effective or has fewer side effects.
Randomization helps prevent bias. Bias occurs when a trial's results are affected by human choices or other factors not related to the treatment being tested.