Bridging the Gap: CTD² Network Research Findings Undergoing Testing in Clinical Trials
, by Subhashini Jagu, Ph.D.
The Cancer Target Discovery and Development (CTD2) initiative aims to not only understand the molecular mechanisms of cancer, but also develop therapies to overcome metastasis, tumor heterogeneity, and drug resistance. CTD2 Network Centers utilize advanced computational and systems biology methods, functional genomics and immunological approaches, and small molecule and genetic screens to functionally validate discoveries with preclinical and clinical relevance. Following the vision outlined in its name, the CTD2 Network has made many biological discoveries leading to mechanistic, hypothesis-driven, targeted immunotherapies, combinatorial therapies, and window of opportunity clinical trials.
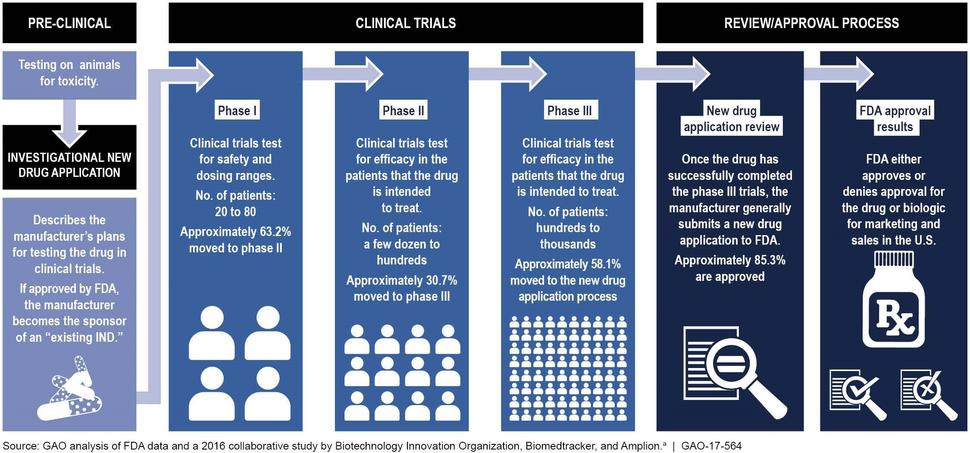
Clinical trials are done in people to answer specific research questions. These trials follow a protocol developed by the researcher or manufacturer and advance through different phases (I, II, and III) to test a treatment. There is a variation in the number of people involved in the different phases of clinical trials. A phase I trial is an early stage, small-scale study, and tests the safety of the treatment, phase II trial tests the effectiveness of the treatment, and a phase III trial is done on large scale to test the safety and effectiveness in different populations. The drug development process can be accelerated by going through only one or two phases of clinical trials when it is being tested for a life-threatening disease (Figure 1).
The following are some examples of how CTD2 Network research has informed clinical trials for various cancers:
Small molecule inhibitors
Acute Myeloid Leukemia (AML): AML is one of the most common forms of hematologic malignancies. The Beat AML program is a major collaborative effort led by CTD2 scientists at Oregon Health and Science University . The project integrates molecular, drug response, and clinical data for progress towards individualized therapies for AML1,2. These studies revealed potential novel treatment options that are being tested in clinical trials: a phase II clinical trial of a CSF1R inhibitor and a phase I clinical trial with the combination of ruxolitinib and venetoclax for patients with relapsed/refractory AML.
Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs): GEP-NETs are a rare class of tumors arising in the pancreas and gastrointestinal tract. The OncoTreat algorithm developed by CTD2 scientists at Columbia University was used to prioritize compounds for treatment. The algorithm predicted entinostat as the most effective treatment for about half (47%) of the metastatic patients. Entinostat also induced significant tumor volume reduction in NET xenograft models3. These data led to rapid investigational new drug (IND) approval by the FDA for a phase 2 clinical trial to treat metastatic GEP-NET.
HR-/HER2+ Breast Cancers: HER2-positive breast cancers express higher than normal levels of HER2 protein and make up approximately 25 % of breast cancers. CTD2 scientists at Columbia University have identified that a combination of HER2 and JAK/STAT3 inhibitors reduced the tumorigenicity in HER2+ breast cancer cell lines4. These preclinical findings served as a basis for a phase I/II multicenter clinical trial to investigate the maximum tolerated dose of ruxolitinib in combination with trastuzumab (both FDA-approved drugs) in patients with metastatic HER2+ breast cancer.
Head and Neck Squamous Cell Carcinoma (HNSCC): HNSCC develops in the mucous membranes of the mouth, nose, and throat. Aggressive treatment of HNSCC with surgery, radiation and cisplatin therapies, is disfiguring and induces high-grade toxicities which limit effectiveness of the drug. CTD2 scientists at Fred Hutchinson Cancer Research Center have identified WEE1 kinase (a regulator of the cell cycle) as a therapeutic target for the kinase inhibitor MK-17755,6. This data supported the initiation of a phase I clinical trial of MK-1775 with docetaxel and cisplatin before surgery in patients with Stage III-IVB HNSCC.
Inflammatory Breast Cancers (IBC): IBC is the most lethal form of breast cancer and its treatment options are limited. CTD2 researchers at the Columbia University developed new genetic tools, experimental and analytical strategies, and identified that activity of the gene HDAC6 is essential to maintain IBC cells7 viability and proliferation. This preclinical data provided a rationale for a phase I clinical trial studying ricolinostat (ACY1215), an HDAC6 inhibitor, in combination with standard nab-paclitaxel therapy to treat metastatic breast cancer.
Non-Small Cell Lung Cancer (NSCLC): Lung cancer is the leading cause of death in the United States, and NSCLC accounts for 85%-90% of lung cancers which display resistance to chemotherapy. CTD2 researchers at University of California San Francisco identified a synthetic lethal interaction between EGFR tyrosine kinase inhibitors and Aurora kinase inhibitors8. These findings led to the initiation of a phase I/Ib clinical trial testing the combination of EGFR inhibitor, osimertinib and Aurora kinase inhibitor alisertib.
In other work, CTD2 researchers at University of Texas Southwestern Medical Center identified XPO1, a nuclear export receptor, as an essential gene for survival in KRAS mutant NSCLC9. This preclinical study provided supporting evidence for the initiation of a phase 1/2 clinical trial of selinexor with docetaxel.
Multiple Myeloma (MM): MM is an incurable plasma cell neoplasm with a median survival of 3-4 years. CTD2 researchers at University of California San Francisco uncovered a network of chaperones and stress-response regulators involved in MM patient response and eventual resistance to carfilzomib, a proteasome inhibitor10. Their work suggested a combination of carfilzomib with lenalidomide, an angiogenesis inhibitor, and steroid dexamethasone to treat MM; currently being tested in a phase 1/2 clinical trial.
Rhabdoid Tumors (RT): RTs are a rare pediatric cancer that usually arise in the kidneys but can also occur in other soft tissues. Genes MDM2 and MDM4 were identified as actionable targets in malignant RTs in large-scale genetic and chemical perturbation studies11 performed by CTD2 researchers at Dana-Farber Cancer Institute. A phase 1 clinical study was initiated to examine the effect of the dual MDM2/MDMX inhibitor ALRN-6924 in treatment-resistant solid tumors.
Immunological agents
Solid Tumors: Solid tumors are an abnormal clump of cells and are named after the type of cells that form them. CTD2 scientists at University of California San Diego demonstrated that natural killer (NK) cells derived from human-induced pluripotent stem cells are effective against refractory cancers in a mouse xenograft model12. NK cells may provide an off-the-shelf resource for anti-cancer immunotherapy. NK cells as monotherapy and in combination with an immune checkpoint inhibitor (nivolumab, pembrolizumab or atezolizumab) are being assessed in a phase I/II clinical trial.
Squamous Cell Carcinomas (SCC): SCC is characterized by abnormal, accelerated growth of squamous cells. CTD2 scientists at University of California San Francisco showed that anti-PD-1 therapy initiates a tumor rejection program and induces a TGFβ immune-suppressive program in SCCs with high mutational load13. This study formed the basis for a phase I/Ib clinical trial of NIS793 in combination with PDR001 in patients with advanced malignancies.
Data and findings from the CTD2 Network are made public through the CTD2 Data Portal and Dashboard, leading to many trials by others in the research community. For example, work by CTD2 scientists on targeting adaptive responses to PARPi with inhibitors of the AKT (NCT02208375), PI3K (NCT03586661), MEK (NCT03162627), immune checkpoints (NCT03801369), and a novel sequential therapy with a WEE1 kinase inhibitor (NCT04197713) to decrease toxicity has led to numerous clinical trials with the potential to improve patient outcomes.
Table 1: List of clinical trials initiated based on the CTD2 Network research findings
References
-
Tyner JW, Tognon CE, Bottomly D, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562(7728):526-531. doi:10.1038/s41586-018-0623-z (PMID: 30333627)
-
Zhang H, Savage S, Schultz AR, et al. Clinical resistance to crenolanib in acute myeloid leukemia due to diverse molecular mechanisms. Nat Commun. 2019;10(1):244. Published 2019 Jan 16. doi:10.1038/s41467-018-08263-x (PMID: 30651561)
-
Alvarez MJ, Subramaniam PS, Tang LH, et al. A precision oncology approach to the pharmacological targeting of mechanistic dependencies in neuroendocrine tumors. Nat Genet. 2018;50(7):979-989. doi:10.1038/s41588-018-0138-4 (PMID: 29915428)
-
Rodriguez-Barrueco R, Yu J, Saucedo-Cuevas LP, et al. Inhibition of the autocrine IL-6-JAK2-STAT3-calprotectin axis as targeted therapy for HR-/HER2+ breast cancers. Genes Dev. 2015;29(15):1631-1648. doi:10.1101/gad.262642.115 (PMID: 26227964)
-
Moser R, Xu C, Kao M, et al. Functional kinomics identifies candidate therapeutic targets in head and neck cancer. Clin Cancer Res. 2014;20(16):4274-4288. doi:10.1158/1078-0432.CCR-13-2858 (PMID: 25125259)
-
Méndez E, Rodriguez CP, Kao MC, et al. A Phase I Clinical Trial of AZD1775 in Combination with Neoadjuvant Weekly Docetaxel and Cisplatin before Definitive Therapy in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2018;24(12):2740-2748. doi:10.1158/1078-0432.CCR-17-3796 (PMID: 29535125)
-
Putcha P, Yu J, Rodriguez-Barrueco R, et al. HDAC6 activity is a non-oncogene addiction hub for inflammatory breast cancers [published correction appears in Breast Cancer Res. 2017 Apr 19;19(1):49]. Breast Cancer Res. 2015;17(1):149. Published 2015 Dec 8. doi:10.1186/s13058-015-0658-0 (PMID: 26643555)
-
Shah KN, Bhatt R, Rotow J, et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat Med. 2019;25(1):111-118. doi:10.1038/s41591-018-0264-7 (PMID: 30478424)
-
Kim J, McMillan E, Kim HS, et al. XPO1-dependent nuclear export is a druggable vulnerability in KRAS-mutant lung cancer. Nature. 2016;538(7623):114-117. doi:10.1038/nature19771(PMID: 27680702)
-
Acosta-Alvear D, Cho MY, Wild T, et al. Paradoxical resistance of multiple myeloma to proteasome inhibitors by decreased levels of 19S proteasomal subunits. Elife. 2015;4:e08153. Published 2015 Sep 1. doi:10.7554/eLife.08153 (PMID: 26327694)
-
Howard TP, Arnoff TE, Song MR, et al. MDM2 and MDM4 Are Therapeutic Vulnerabilities in Malignant Rhabdoid Tumors. Cancer Res. 2019;79(9):2404-2414. doi:10.1158/0008-5472.CAN-18-3066 (PMID: 30755442)
-
Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell. 2018;23(2):181-192.e5. doi:10.1016/j.stem.2018.06.002 (PMID: 30082067)
-
Dodagatta-Marri E, Meyer DS, Reeves MQ, et al. α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J Immunother Cancer. 2019;7(1):62. Published 2019 Mar 4. doi:10.1186/s40425-018-0493-9 (PMID: 30832732)