Henrietta Lacks Enhancing Cancer Research Act of 2019 (Public Law No: 116-291)
- The Henrietta Lacks Enhancing Cancer Research Act of 2019 requires a Government Accountability Office (GAO) report on steps federal agencies are taking to reduce barriers for underrepresented populations to access federally funded cancer clinical trials.
- The legislation (signed into law on 1/05/2021) was introduced in the House as H.R. 1966 by the late Rep. Elijah E. Cummings (D-MD), along with Reps. John Sarbanes (D-MD) and Dutch Ruppersberger (D-MD) on 3/28/2019. Senators Chris Van Hollen (D-MD) and Ben Cardin (D-MD) introduced a companion bill, S. 946, in the Senate on the same date.
- Read the full bill text on Congress.gov.
- If you are interested in learning more about NCI-supported clinical trials networks and related efforts, more information is available here:
Implementation:
GAO issued its final report, “Cancer Clinical Trials: Federal Actions and Selected Non-Federal Practices to Facilitate Diversity of Patients,” on December 19, 2022. NCI subject matter experts from the Division of Cancer Treatment and Diagnosis, Division of Cancer Prevention, Center to Reduce Cancer Health Disparities, Division of Cancer Control and Population Sciences, Coordinating Center for Clinical Trials, Center for Cancer Research, and Office of Cancer Centers participated in the review.
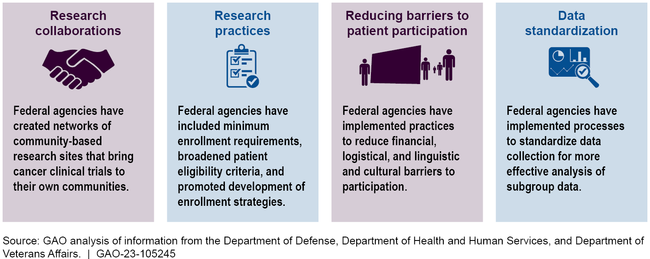
GAO found that both federal agencies and selected non-federal cancer centers took actions to facilitate participation of patients from diverse backgrounds in cancer clinical trials. Generally, these actions addressed a variety of barriers to participation that are often cited in the literature.
The Department of Health and Human Services (HHS), Department of Defense, and Department of Veterans Affairs took actions that have the goal of increasing the proportion of patients from diverse backgrounds enrolled in federally funded cancer clinical trials. These efforts are focused on developing research collaborations, modifying research practices, reducing barriers to patient participation, and collecting and sharing data.
Specifically, the report highlights NCI-supported clinical trials infrastructure that facilitates enrollment in clinical trials, including the National Clinical Trials Network and NCI Community Oncology Research Program, as well as more targeted efforts, such as the Partnerships to Advance Cancer Health Equity program and recent expansion of eligibility criteria.
Federal Actions to Facilitate Diversity in Cancer Clinical Trials
Further Consolidated Appropriations Act, 2020, P.L. 116-94, Sec. 603 Minimum age of sale of tobacco products
- Language raising the federal minimum age to purchase tobacco products from 18 to 21 was included in several pieces of legislation introduced in the 116th Congress, including the Tobacco to 21 Act (S.1258, H.R. 2411) and the Tobacco-Free Youth Act (S. 1541). Provisions from the Tobacco-Free Youth Act were included in P.L. 116-94 as Section 603, “Minimum age of sale of tobacco products.”
- The legislation amends Section 906(d) of the Federal Food, Drug, and Cosmetic Act by raising the federal minimum age for sale of tobacco products from 18 to 21 years of age.
- The law applies to sales of all tobacco products including cigarettes, smokeless tobacco, hookah tobacco, cigars, pipe tobacco, and electronic nicotine delivery systems.
- The President signed the bill on 12/20/19 and the law raising the minimum age to 21 became effective immediately.
- Read the full bill text on Congress.gov (Sec. 603)
FDA Reauthorization Act of 2017 (Public Law No: 115-52)
The FDA Reauthorization Act of 2017 (FDARA) amends the Federal Food, Drug, and Cosmetic Act to revise and extend the user-fee programs for prescription drugs, medical devices, generic drugs, and biosimilar biological products. H.R. 2430 was introduced by Rep. Greg Walden (R-OR) on 5/16/2017 and was signed into law on 8/18/2017, becoming Public Law No: 115-52.
Section 504 of FDARA, The Research to Accelerate Cures and Equity (RACE) for Children Act
The RACE for Children Act was introduced as stand-alone legislation in both the House and Senate, and key provisions of the bill were included in FDARA as Section 504, Development of drugs and biological products for pediatric cancers. These provisions amend current study requirements under the Pediatric Research Equity Act (PREA) so that requirements for pediatric studies are based on relevant molecular targets rather than the current requirements, based on cancer site of origin. Additionally, the provisions amend PREA by ending the exemption of PREA obligations for cancer drugs with orphan designations if the molecular target of their drug is relevant to a pediatric cancer.
Implementation:
The Act includes two provisions specifically relevant to NCI, and NCI collaborates with FDA’s Oncology Center of Excellence Pediatric Oncology Program to implement these provisions:
- The Act directed the HHS Secretary to consult with both FDA and NCI to develop a list of relevant molecular targets. The Act describes this as "a list of molecular targets considered, on the basis of data the Secretary determines to be adequate, to be substantially relevant to the growth and progression of a pediatric cancer, and that may trigger the requirements under this section."
- The Act also directed the HHS Secretary to consult with FDA and NCI and in convening a public meeting within one year after the Act is signed into law to solicit feedback from physicians, researchers, patients, and other stakeholders regarding various aspects of implementation, including development of the list of relevant molecular targets.
Applicable FDA guidance, relevant meeting information, as well as the pediatric Relevant Molecular Target List developed through this collaboration are available here on the FDA website.
21st Century Cures Act, P.L. 114-255 (H.R. 6/H.R. 34)
On 12/13/2016, President Obama signed the 21st Century Cures Act (“Cures”) into law. The nearly 1,000-page bill passed the House 392-26 and the Senate by a vote of 94-5. Key provisions for NIH aim to coordinate policies relating to early career investigators, improve loan repayment programs, and streamline procedural requirements for attendance at scientific meetings. Cures reauthorizes the NIH for FY2018-FY2020 at the following levels:
- $34,851,000,000 for FY 2018
- $35,585,871,000 for FY 2019
- $36,472,442,775 for FY 2020
In addition, Cures creates a $4.8 billion "NIH Innovation Account." The funds in the Innovation Account support these specific projects:
- Precision Medicine Initiative -- $1.45 billion over the next 10 years
- Beau Biden Cancer Moonshot -- $1.8 billion over the next seven years
- BRAIN Initiative -- $1.511 million over the next 10 years
- Regenerative Medicines -- $30 million over the next four years
Beau Biden Cancer Moonshot: One of the key features of the NIH Innovation Account is the Beau Biden Cancer Moonshot. This provision of the law was renamed via a joint amendment introduced by Senate Majority Leader Mitch McConnell (R-KY) and Senate Minority Leader Harry Reid (D-NV), in honor of Vice President Joe Biden's son Beau, who passed away from cancer in 2015. The $1.8 billion for the Cancer Moonshot was authorized to be appropriated as follows:
- $300 million for FY 2017
- $300 million for FY 2018
- $400 million for FY 2019
- $195 million for FY 2020
- $195 million for FY 2021
- $194 million for FY 2022
- $216 million for FY 2023
Per the Cures statute, the purpose of the Cancer Moonshot funding is: To support cancer research, such as the development of cancer vaccines, the development of more sensitive diagnostic tests for cancer, immunotherapy and the development of combination therapies, and research that has the potential to transform the scientific field, that has inherently higher risk, and that seeks to address major challenges related to cancer.
- Read the full bill text on Congress.gov.
- Additional information about NCI research efforts supported through the Cancer Moonshot is available at: https://www.cancer.gov/research/progress/moonshot-cancer-initiative
- Additional information about all NIH Innovation Fund efforts is available at: https://www.nih.gov/research-training/medical-research-initiatives/cures