A Spirit of Scientific Creativity and Excitement: Celebrating the Career of Dr. Jim Hartley
Dom Esposito earned his PhD in Biochemistry and Biophysics at Johns Hopkins University, and completed postdoctoral work in the Laboratory of Molecular Biology at the National Institute of Diabetes and Digestive and Kidney Diseases. He worked closely with Dr. Jim Hartley to develop the Gateway recombinational cloning system—a discovery that marked the beginning of a decades-long, productive professional relationship. He currently heads the RAS Reagents Core, which provides high-quality DNA cloning, cell lines, and protein expression and purification services to the RAS community.
Many readers of RAS Lab will know Jim Hartley only as a frequent contributor to and moderator of this forum over the past 8 years. But with his recent retirement from the Frederick National Laboratory, his colleagues and I felt that it would be worthwhile to give readers a better look at the long scientific legacy that Jim leaves behind. Many readers will not even realize the impact that Jim’s scientific inventions have had and continue to have on their daily work in areas ranging from molecular biology to protein production. So, allow me the opportunity to shed some light on Jim’s legacy as one who was along for the ride for several decades of his lengthy career.
Jim did his undergraduate studies in Microbiology at Washington State University, and obtained his Ph.D. at Colorado State University. It was in his postdoctoral work with Dr. John Donnelson at the University of Iowa in the late 1970’s where Jim first began his impact on the science of thousands of researchers. In that laboratory, focused at the forefront of DNA sequencing technology in the early days of recombinant DNA, Jim developed and patented methods for producing plasmids containing multiple repeated copies of DNA segments1. Ultimately, this technology led Jim to invent the standardized DNA ladder2 which freed the field from restriction digest-based DNA ladders which had large gaps in DNA sizes. For those who remember the frustration of trying to size DNA fragments between the widely dispersed lambda-HindIII markers, or locating an esoteric restriction enzyme to attempt to accurately size your PCR fragments, the production of the 100 bp and 1 kB DNA ladders was a true revolution in molecular biology.
By the time Jim moved to Bethesda Research Laboratories (BRL) in 1981 as a Research Scientist, he had already turned his eye towards other equally creative improvements. At this time, several years before the invention of the polymerase chain reaction, detection of specific sequences of DNA was extremely challenging, generally requiring radioactive probe annealing to get high sensitivity. Jim’s solution, published in an elegant 1986 manuscript3 was the invention of the “probe-vector” process in which the power of E. coli transformation could be harnessed to identify specific DNA sequences. The success of this process led BRL to market E. coli competent cells, helping to usher in the first generation of vital molecular biology reagents for the recombinant DNA revolution.
Jim stayed at BRL for 20 years as the company merged to form Gibco-BRL, then Life Technologies, and finally Invitrogen. Over that period, Jim was an inventor on more than 40 patents covering an incredibly diverse set of technologies. Some of his work was seemingly mundane (developing stick-on tube labels that would remain intact when vials were frozen at -70°C) while others continued to have massive impacts across molecular biology. In 1990, Jim was responsible for the invention of the uracil DNA glycosylase (UDG) method of decontaminating PCR reactions4 to eliminate DNA carryover—a vital technology deployed by thousands of laboratories in the early days of PCR.
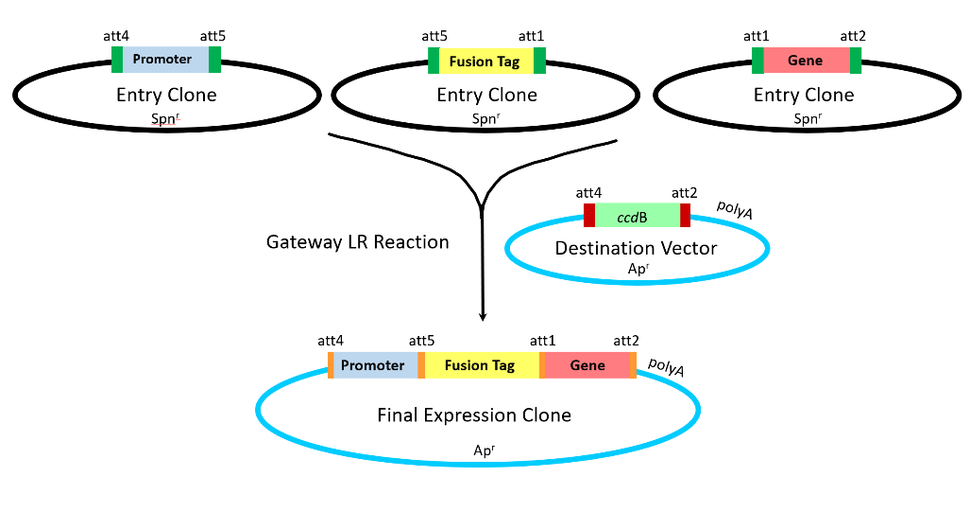
I met Jim in 1999 when I was finishing my postdoctoral work and applied for a position in the Protein Engineering and Analysis group at Life Technologies. The job advertisement was for an expert in bacteriophage site-specific recombination—an expertise which probably 5 people in the whole United States had at the time. I was baffled as to how anything related to this could be of interest to a company like Life Technologies, and approached my interview with Jim (who I was told was the person responsible for this new product) with great curiosity, but an equal level of skepticism that a “non-expert” in this field could have come up with anything that those of us working on it for years hadn’t already thought of. Within 60 seconds of sitting down in Jim’s office, he explained with a brief sketch a technology so simple and yet so creative that it was an utter embarrassment that none of the brilliant minds in this field (or I) had thought of it. This was the Gateway recombinational cloning system—a method for using site-specific recombination to move genes around without restriction enzymes or a need for continual DNA sequencing5. Not only essential for the boom of molecular biology in the early 2000’s, this technology was vital for the success of large-scale structural biology initiatives including the US based Protein Structure Initiative (PSI) and the European Structural Genomics Consortium (SGC). Hundreds of thousands of Gateway clones are available at commercial repositories, and every human protein-coding gene has been cloned into this system. The simplicity and ease of use of this system still remain today, especially after further tweaks by Jim and colleagues to allow recombination of multiple genes simultaneously in the same set of vectors.
Shortly after the commercial deployment of Gateway and the merger of Invitrogen with Life Technologies, the R&D site of the company in Maryland was shut down and Jim moved to the Frederick National Lab, then run by SAIC-Frederick, Inc. Jim was kind enough to invite me and a few other colleagues along to help him start up a new facility called the Protein Expression Laboratory (PEL) which would serve to assist NCI and NIH intramural investigators by generating high quality DNA and protein reagents, and to develop new technologies that improve protein production. In the two decades that followed, our group was responsible for the production of hundreds of proteins and thousands of DNA molecules used across the NIH in high profile research. Jim grew the group from a small handful of us in the early days to more than 20 people, adding personnel experienced in virus production, serology, large scale bacterial fermentation, and more. Jim continued his out-of-the-box thinking at FNLCR, helping to develop technologies for ORFeome-scale proteomics work, novel solubility strategies, and microscale purification processes. Jim’s leadership and vision put the PEL in the right place for the advent of the NCI’s RAS Initiative where the machine that Jim built was ready to generate high-quality DNA, cell line, and protein reagents to support not only the RAS Initiative’s internal efforts in structural biology and drug discovery, but also the efforts of the extramural RAS community. Without Jim’s leadership and foresight, the RAS Initiative likely would not have reached the level of success it has achieved.
On a personal note, Jim has been a phenomenal mentor to me over the last two decades. While I have never been able to match his level of creativity, I have learned a great deal about how to be a good scientist, leader, and mentor from him. His colleagues from his past life at Gibco-BRL agree as well. Dr. Gary Temple, who worked with Jim for many years, said ”what I most recall was Jim's creativity in conceiving solutions to obstacles faced by our research customers, invariably finding unexpected and clever approaches to solve these problems.” Gary went on to say that Jim’s creativity was greatly appreciated by the company’s many outside consultants—some of the most regarded scientists of the time—whose first question upon arrival for a meeting would always be "what is Jim Hartley up to lately?" Dr. Joel Jessee, former head of R&D at Life Technologies put it bluntly when he said “Jim is remarkable. He stands out as one of the most unique people I have met. I admire him greatly.”
Throughout my career, I have worked with many great scientists who did groundbreaking work. But the level of innovative thinking and creativity that Jim constantly showed did more than just lead to great science. It impacted those of us around him as well. As Gary Temple put it best, “the spirit of creativity and excitement he fostered among others in our research group, I believe, positively stimulated us all.” I send my thanks to Jim for all that he’s done for me and for science as a whole, and wish him a very well-deserved retirement!