KRAS-related long noncoding RNAs in human cancers
Reza Ahmadian, a professor of Biochemistry & Molecular biology at the Heinrich-Heine University Düsseldorf, works on the molecular mechanisms of disease-related signaling pathways by (i) purifying and identifying native RAS/RHO protein complexes in cell-specific context and subcellular sites, (ii) elucidating the structure-function relationships of protein-protein and protein-ligand interactions, (iii) characterizing and in vitro reconstituting signaling networks in solution and on synthetic liposomes, and (iv) identifying and validating new interacting surfaces as potential drug target sites. Mahsa Saliani, a PhD student in Biochemistry at the Ferdowsi university of Mashhad in Iran, works together with Ahmadian Group on understanding KRAS-driven cancers by studying KRAS-related oncogenic lncRNAs and their potential role in KRAS signal transduction.
Intensive efforts to understand the mechanisms underlying the intracellular trafficking, regulation, and signaling pathways of KRAS have suggested several therapeutic strategies 1. Despite its well-recognized importance in cancer promotion, only a few efforts in the past four decades have resulted in approved clinical therapeutic strategies for KRAS-mutant cancers 2. Obviously, other alternative therapeutic approaches are needed to elucidate additional mechanisms responsible for the modulation of KRAS and to identify new therapeutic targets.
Long noncoding RNAs (lncRNAs), as key regulators of gene expression, are involved in the progression of many human cancers 3. Many lncRNAs have been recognized and several mechanisms of action have been suggested, including functioning as signal, decoy, scaffold, and guide molecules 4. The principle function of a signal lncRNA is to serve as a molecular signal to regulate transcription in response to various stimuli. Decoy lncRNAs limit the availability of regulatory elements by presenting ‘‘decoy” binding sites. These lncRNAs modulate transcription by binding to the regulatory factors such as transcription factors, catalytic proteins, subunits of larger chromatin remodeling complexes, as well as miRNAs, thereby decreasing their availability. Scaffold class of lncRNAs play an architectural role through providing a structural platform for assembly of multi-component complexes, including protein complexes. Guide lncRNAs interact with ribonucleoproteins (RNPs) and direct them to specific target genes, thereby they are essential for the proper localization of RNPs 4.
Emerging evidence suggests that various oncogenic lncRNAs are likely to function as competing endogenous RNAs (ceRNAs) with a decoy activity by sponging tumor suppressor microRNAs (miRNAs) 5, thereby modulating the translation of the mRNAs targeted by these miRNAs. Many tumor-suppressor miRNAs have inhibitory effects on KRAS-associated tumorigenesis by downregulating KRAS expression 6. Therefore oncogenic lncRNAs, as sponges of tumor suppressor miRNAs that target KRAS mRNA, promote cancer development via the upregulation of the KRAS oncogene 7.
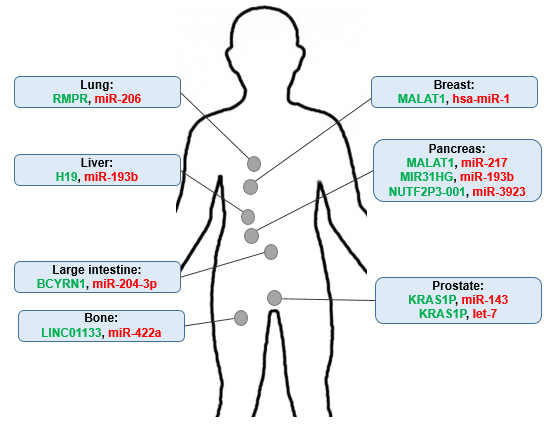
The ever-increasing number of KRAS-specific lncRNAs strongly indicates their potential contribution to and critical roles in the entire process of KRAS-driven carcinogenesis. This review compiles the current knowledge of KRAS-related oncogenic lncRNAs by considering their aberrant expression and their mechanism of action through sponging effects on KRAS-targeting miRNAs (Fig. 1). Identification of mechanisms involved in KRAS regulation by lncRNAs is expected to greatly enhance our understanding of the mechanisms of tumorigenesis associated with KRAS regulation.
MALAT1
MALAT1, which was first identified in lung cancer, plays an important role in the pathogenesis of various human diseases, such as cancer 8. MALAT1, as a molecular sponge of miR-217, an inhibitor of KRAS 9, promotes KRAS signaling in pancreatic ductal adenocarcinoma (PDAC) 10. Moreover, miR-1 has been shown to suppress breast cancer development by downregulating KRAS and MALAT1 transcription, which emphasizes the potential role of miR-1 as a tumor-suppressive miRNA and MALAT1 as an oncogenic lncRNA via the regulation of KRAS 11.
MIR31HG
MIR31HG is markedly upregulated in cancer tissues, with potential roles in cancer initiation, progression, and metastasis. It was reported that, MIR31HG may act as a ceRNA for miR-193b binding to regulate the miRNA targets such as KRAS 12. Taken together, results demonstrated that MIR31HG functions as an oncogenic lncRNA which could promote tumor progression, and miR-193b targets not only protein-coding genes but also the lncRNAs including MIR31HG
KRAS1P
KRAS1P is considered as a pseudogene of KRAS. Its expression is amplified in most cancers with mutated KRAS, which indicates a positive correlation between these genes. Two studies have reported the possible role of KRAS1P as a ceRNA with binding sites for some KRAS-targeting miRNAs, such as miR-143 and the let-7 miRNA family 13,14. Thus, KRAS1P can potentially act as an oncogenic lncRNA to inhibit degradation of the KRAS transcript 15.
BCYRN1
The high expression of BCYRN1 in various tumor cell lines suggests the role of BCYRN1 as an oncogenic lncRNA 16. Strikingly, as a ceRNA, BCYRN1 affects the development of colorectal cancer via regulation of the miR-204- 3p/KRAS axis 17. Therefore, negative regulation of KRAS by miR-204-3p suggests BCYRN1 as another confirmed KRAS-related lncRNA.
NUTF2P3-001
Overexpression of NUTF2P3-001 in pancreatic cancer and chronic pancreatitis tissues is positively correlated with cancer cell characteristics 18. It was reported that NUTF2P3-001, as an oncogenic lncRNA, competes with the 3′UTR of KRAS mRNA for binding to miR-3923. In addition, downregulation of NUTF2P3-001 inhibits the viability, proliferation, and invasion of pancreatic cancer cells and contributes to a decrease in KRAS expression 18. Hence, these data provide an alternative lncRNA-mediated regulatory mechanism for the tumor oncogene KRAS.
RMRP
RMRP lncRNA is widely expressed in different human and mouse tissues 19. Suppression of miR-206 by RMRP positively modulates Cyclin D2 expression and cell cycle progression, which provides us with a better understanding of the mechanism underlying RMRP carcinogenesis 20. It was indicated that miR-206 acts as a tumor suppressor miRNA in oral squamous cell carcinoma by directly targeting KRAS 21. Inhibition of miR-206 by RMRP was demonstrated to result in overexpression of KRAS, FMNL2, and SOX9 in lung adenocarcinoma 22, confirming RMRP as one of the KRAS-related lncRNAs.
H19
H19, with both oncogenic and tumor suppressor activities, acts as a double-edged sword via mechanisms such as miRNA sponging 23. The let-7 family miRNAs that control human RAS oncogene expression are often downregulated in human cancers 24. This can also be mediated by miR-193b, another KRAS-regulating miRNA 25. Overexpression of H19 has been shown to attenuate miR-193b-mediated inhibition of multiple driver oncogenes, including EGFR, KRAS, PTEN, IGF1R, and MAPK1, suggesting that lncRNA H19 serves as a KRAS regulator through miR-193b sponging 26.
LINC01133
LINC01133, with a length of 1154 nucleotides, is located on chromosome 1q23.2 and was first reported to be involved in CRC and NSCLC 27. Other results showed that LINC01133 aggravates the proliferation, migration, and invasion of osteosarcoma by sponging miR-422a, which targets KRAS, exerting antitumor effects 28.
SLCO4A1-AS1
The role of SLCO4A1-AS1 in the tumorigenesis of CRC has been demonstrated in several studies, confirming its upregulation in CRC tissues and its relation with poor prognosis and tumor metastasis 29. SLCO4A1-AS1 knockdown in HCT116 and SW480 cells led to the downregulation of EGFR, KRAS, BRAF, and MAP3K1 expression 30. Therefore, SLCO4A1-AS1 can be considered as a KRAS-related lncRNA. However, the corresponding miRNA has not yet been identified.
CONCLUSION AND PERSPECTIVE
Approximately 25% of all human cancers have oncogenic mutations in the RAS family of oncogenes, most frequently the KRAS gene, resulting in the aberrant activation of RAS proteins and leading to malignant transformation. One approach is to interfere with oncoprotein production by targeting tissue-specific KRAS-related lncRNAs, thus restoring translational suppression of KRAS mRNA by miRNAs. The increased success rate of nucleic acid therapeutics provides an outstanding opportunity to pursue lncRNAs as therapeutic candidate targets in KRAS-dependent malignant transformation.