Cervical Cancer Screening
Screening means checking for a disease before there are symptoms. Cervical cancer screening is an important part of routine health care for people who have a cervix.
What is cervical cancer screening?
The goal of screening for cervical cancer is to find precancerous cervical cell changes, when treatment can prevent cervical cancer from developing. Sometimes, cancer is found during cervical screening. Cervical cancer found at an early stage is usually easier to treat. By the time symptoms appear, cervical cancer may have begun to spread, making treatment more difficult.
There are three main ways to screen for cervical cancer:
- The human papillomavirus (HPV) test checks cells for infection with high-risk HPV types that can cause cervical cancer.
- The Pap test (also called a Pap smear or cervical cytology) collects cervical cells so they can be checked for changes caused by HPV that may—if left untreated—turn into cervical cancer. It can find precancerous cells and cervical cancer cells. A Pap test also sometimes finds conditions that are not cancer, such as infection or inflammation.
- The HPV/Pap cotest uses an HPV test and Pap test together to check for both high-risk HPV and cervical cell changes.
When to get screened for cervical cancer
Cervical screening recommendations are developed by several organizations, including the United States Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS). How often you should be screened for cervical cancer and which tests you should get will depend on your age and health history. Because HPV vaccination does not prevent infection with all high-risk HPV types, vaccinated people who have a cervix should follow cervical cancer screening recommendations.
Age 21-29 years
If you are in this age group, USPSTF recommends getting your first Pap test at age 21, followed by Pap testing every 3 years. Even if you are sexually active, you do not need a Pap test before age 21.
Age 30-65 years
If you are in this age group, USPSTF recommends getting screened for cervical cancer using one of the following methods:
- HPV test every 5 years
- HPV/Pap cotest every 5 years
- Pap test every 3 years
Updated cervical cancer screening guidelines from ACS recommend starting screening at age 25 with an HPV test and having HPV testing every 5 years through age 65. However, testing with an HPV/Pap cotest every 5 years or with a Pap test every 3 years is still acceptable. To read about the reasons for updates to the guidelines, visit ACS's Updated Cervical Cancer Screening Guidelines Explained.
Older than 65 years
If you are in this age group, talk with your health care provider to learn if screening is still needed. If you have been screened regularly and had normal test results, your health care provider will probably advise you that you no longer need screening. However, if your recent test results were abnormal or you have not been screened regularly, you may need to continue screening beyond age 65.
Exceptions to the cervical cancer screening guidelines
Your health care provider may recommend more frequent screening if:
- you are HIV positive
- you have a weakened immune system
- you were exposed before birth to a medicine called diethylstilbestrol (DES), which was prescribed to some pregnant women through the mid 1970s
- you had a recent abnormal cervical screening test or biopsy result
- you have had cervical cancer
If you've had an operation to remove both the uterus and cervix (called a total hysterectomy) for reasons not related to cancer or abnormal cervical cells you do not need to be screened for cervical cancer. However, if your hysterectomy was related to cervical cancer or precancer, talk with your health care provider to learn what follow-up care you need. If you've had an operation to remove the uterus but not the cervix (sometimes called a partial or supracervical hysterectomy) you should continue routine cervical cancer screening.
Where to get screened for cervical cancer
Doctors' offices, clinics, and community health centers offer HPV and Pap tests. Many people receive these tests from their ob/gyn (obstetrics/gynecology) or primary care provider.
If you don't have a primary care provider or doctor you see regularly, you can find a clinic near you that offers cervical cancer screening by contacting:
- your state or local health department
- the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) or call 1-800-232-4636; NBCCEDP provides low-income, uninsured, and underserved people access to timely cervical cancer screening and diagnostic services
- a Planned Parenthood clinic, or call 1-800-230-7526
- NCI's Cancer Information Service, or call 1-800-422-6237
Cervical screening test results usually come back from the lab in about 1–3 weeks. If you don't hear from your health care provider, call and ask for your test results. Make sure you understand any follow-up visits or tests you may need.
What to expect during a cervical cancer screening test
Cervical cancer screening tests are usually done during a pelvic exam, which takes only a few minutes. During the exam, you lie on your back on an exam table, bend your knees, and put your feet into supports at the end of the table. The health care provider uses a speculum to gently open your vagina to see the cervix. A soft, narrow brush or tiny spatula is used to collect a small sample of cells from your cervix. You may have the option to self-collect the cervical sample during your appointment. If you're interested in this option, talk with your health care provider to learn more.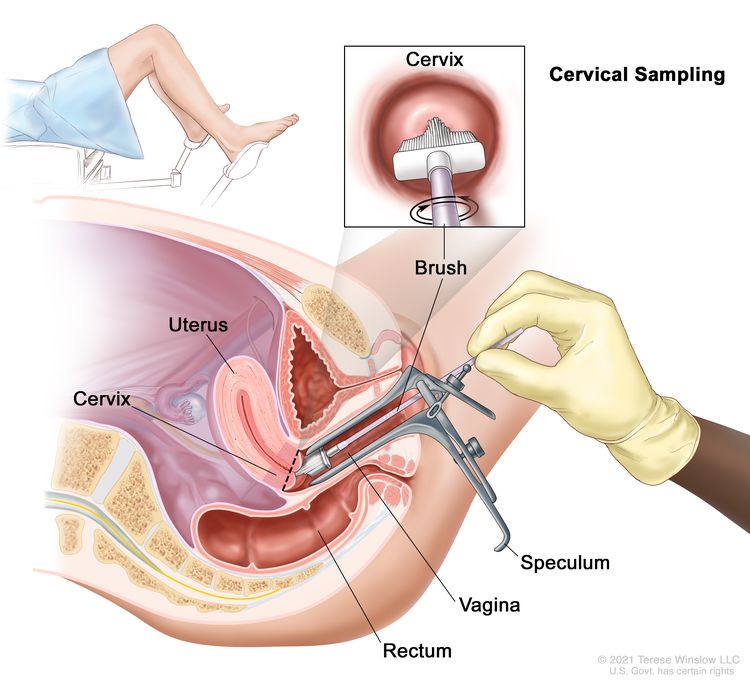
The sample is then sent to a lab, where the cells can be checked to see if they are infected with the types of HPV that cause cancer (an HPV test). The same sample can be checked for abnormal cells (a Pap test). When both an HPV test and a Pap test are done on the same sample, this is called an HPV/Pap cotest.
A pelvic exam may include more than taking samples for an HPV and/or Pap test. Your health care provider may also check the size, shape, and position of the uterus and ovaries and feel for any lumps or cysts. The rectum may also be checked for lumps or abnormal areas. You may talk with your health care provider about being tested for sexually transmitted infections.
Most health care providers will tell you what to expect at each step of the exam, so you will be at ease.
Researchers have found that cervical cancer screening may be less effective for people with obesity, possibly because of challenges in visualizing the cervix and obtaining a cell sample. Approaches to improve cervical visualization, including the use of larger speculum, may be helpful.
Does cervical cancer screening have any risks?
Cervical cancer screening saves lives. Very few people screened for cervical cancer at routine intervals develop cervical cancer. Screening can detect cervical changes early, lowering a person's chance of dying from cervical cancer. Despite these benefits, cervical screening is not perfect, and there are several possible harms to be aware of. Before having any screening test, you may want to discuss the test with your doctor.
Potential risks of harm from cervical cancer screening include:
- Unnecessary follow-up tests and treatment: Finding a condition through screening that would not have caused problems may lead to unnecessary follow-up tests and possibly treatment. The current recommended screening intervals and tests reduce the chance of finding and treating cervical cell abnormalities that would have gone away on their own.
- False-positive test results: Screening test results may sometimes appear abnormal even though no precancer or cancer is present. When a Pap test shows a false-positive result (one that shows there is precancer or cancer when there isn't), it can cause anxiety and is usually followed by more tests and procedures (such as colposcopy, cryotherapy, or loop electrosurgical excision procedure), which also have harms.
- False-negative test results: Screening test results may appear normal even though cervical precancer or cancer is present. A person who receives a false-negative test result (one that shows there is no cancer when there is) may delay seeking medical care even if there are symptoms.
For a downloadable booklet about cervical cancer screening, visit Understanding Cervical Changes: A Health Guide.