About the RAS Initiative
Contact the NCI RAS Initiative
General Comments and Questions
Email Erin Alvey at SolveRAS@nih.gov.
Partnership Development
Email Vladimir Popov at vladimir.popov@nih.gov.
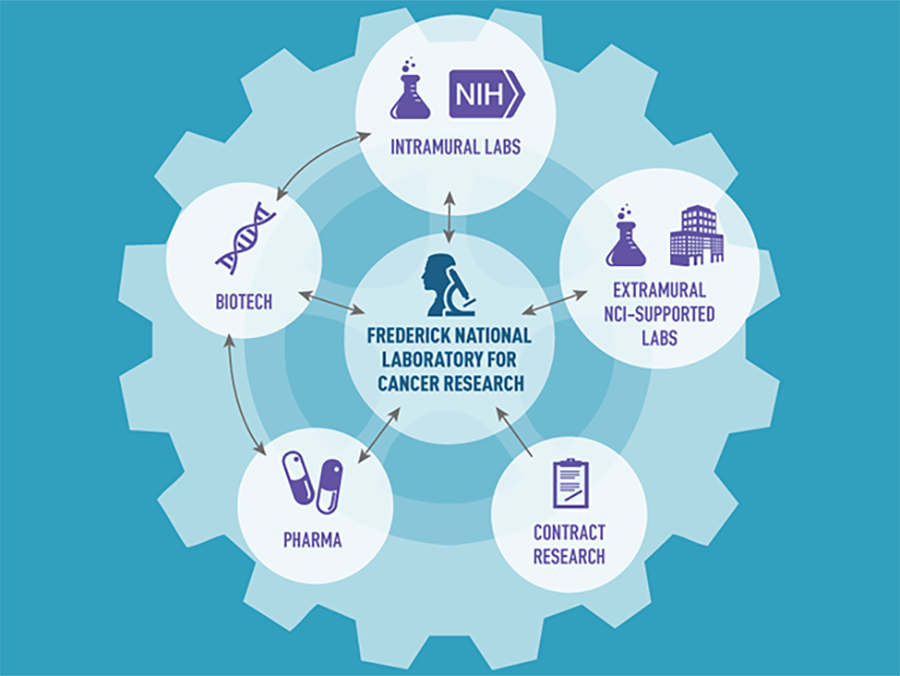
The overarching goal of the NCI RAS Initiative is to mobilize the cancer research community to develop ways to understand and target cancers driven by mutant RAS in an open model of collaboration among government, academic, and industry researchers. This approach is called a "hub and spoke" model. The Frederick National Laboratory for Cancer Research (FNLCR) acts as the hub that connects to the larger community of RAS researchers around the world combining efforts and creating new ways to approach the complex issue of RAS.
Addressing the Problem with RAS Genes
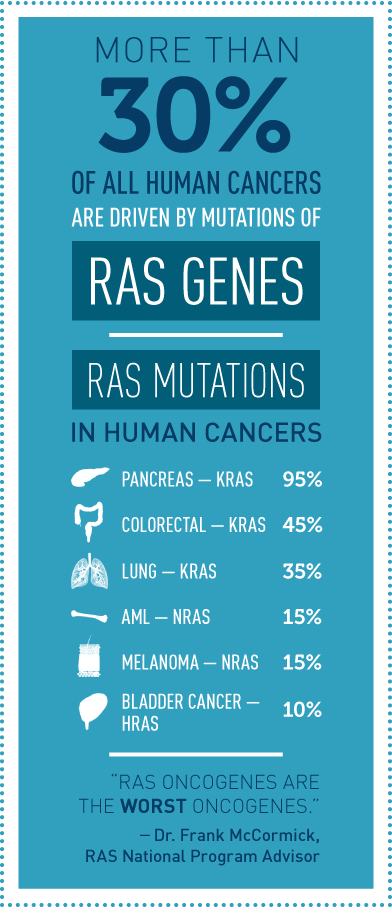
It has been known for more than three decades that about a third of all human cancers, including a high percentage of pancreatic, lung, and colorectal cancers, are driven by mutations in RAS genes. The main members of the RAS gene family— KRAS, HRAS, and NRAS—encode proteins that have a pivotal role in cell signaling. When RAS genes are mutated, cells grow uncontrollably and evade death signals. RAS mutations also make cells resistant to most available cancer therapies. NCI launched the RAS Initiative due to the magnitude of this challenge, as well as the potential clinical benefit.
RAS Proteins and Their Regulators in Human Disease provides a recent review of the RAS problem. For a community view of the genes that comprise the larger RAS pathway, see our blog post, RAS Pathway v2.0.
Mutated RAS Proteins: Long Considered Undruggable
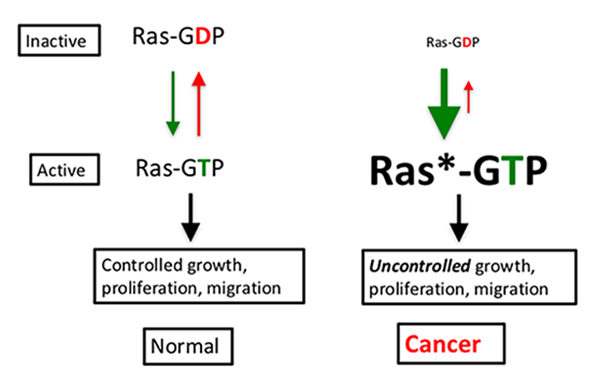
Mutant RAS proteins have been difficult to target, in part, because they are defective in an intrinsic enzyme activity, freezing them in the “on” (GTP-bound) state. (See diagram.). It is like a car with an accelerator that won’t release (green down arrow) and brakes that won’t engage (red up arrow).
Despite repeated attempts, researchers failed to develop a drug that could inhibit the activity of RAS proteins in cancers. They had hoped to find a small molecule that could preferentially bind the unique features of the RAS protein, but they couldn’t find a surface on the protein that would bind a drug. Many researchers in the drug discovery field became convinced that the RAS protein was undruggable. But advances in technology and improved understanding of RAS signaling and regulation have created opportunities to address this situation.
Binding RAS: Hope from Groundbreaking Research
After years with little success, researchers developed groundbreaking techniques that rapidly advanced the field of RAS research. In the 2010s, the Shokat Lab at the University of California San Francisco and the Fesik Lab at Vanderbilt University developed innovative drug screening and medicinal chemistry techniques that identified molecules that could bind mutated KRAS proteins. In May 2021, the Federal Drug Administration approved a treatment for non-small-cell lung cancer (NSCLC), which targets the KRAS G12C mutation, giving new hope to NSCLC patients.
Drugging the Once-Undruggable: Research Continues
The RAS research community has much to discover, and now it continues its work with renewed optimism. After developing an approved treatment for NSCLC, many researchers seek to apply what they’ve learned to other tumors. For example, mutated RAS proteins are present in 90% of pancreatic tumors, a cancer for which there are not yet any effective treatments. Clearly, the need for additional treatments targeting mutated RAS proteins in more types of cancers remains strong convincing.
An Open Model that Advances RAS Research
The RAS Initiative has adopted the “hub and spoke” model to mobilize the RAS research community. Based out of the Frederick National Laboratory for Cancer Research (FNLCR), the RAS Initiative acts as the hub that connects the larger community of RAS researchers located around the world. This open model allows government, academic, and industry researchers around the world to collaborate and more effectively advance the field of RAS research.
Key Accomplishments of the RAS Initiative
The RAS Initiative was launched in 2013 to continue to spur innovation in the field through drug discovery and detailed studies on the structure, biochemistry, and biophysics of RAS. The overarching model of the RAS Initiative is to nucleate more energy and focus on RAS biology and drug discovery through state-of-the-art science, and by providing resources, de-risking some of that effort by providing structures, assays, and reagents, and by coalescing the community. Accomplishments of the RAS Initiative include key structural, biochemical, biophysical, technological advances and, drug discovery.
Here are some major accomplishments:
- The RAS Initiative was the first research team to produce, purify, and generate a crystal structure of fully processed KRAS4b. This advancement enabled biophysical analyses of KRAS in synthetic membranes and showed how processing is critical in complexes interacting with chaperone proteins [citation link].
- The RAS Initiative solved additional crystal structures of oncogenic KRAS proteins in complex with GTPase-activating proteins (GAPs) [citation link].
- The RAS Initiative Research Team developed a range of cell-based, imaging, and biochemical screens to examine RAS activity, which have facilitated academic and industry collaborations [citation link].
- The RAS Initiative formed a, strategic, high-performance computing alliance with the U.S. Department of Energy (DOE) National Laboratories to bring new insight into RAS membrane dynamics [citation link].
- The RAS Initiative Research Team have active inhibitor programs that are progressing toward the clinic.

Deputy NCI Director Discusses the RAS Initiative
In 2016, Deputy NCI Director Dr. Lowy discussed the progress of the RAS Initiative and its goal to create a virtual network of scientists collaborating to solve the RAS problem.