RAS Initiative Drug Screening and Preclinical Research
The RAS Initiative Drug Screening Research Team strives to discover and develop compounds that directly target oncogenic RAS proteins.
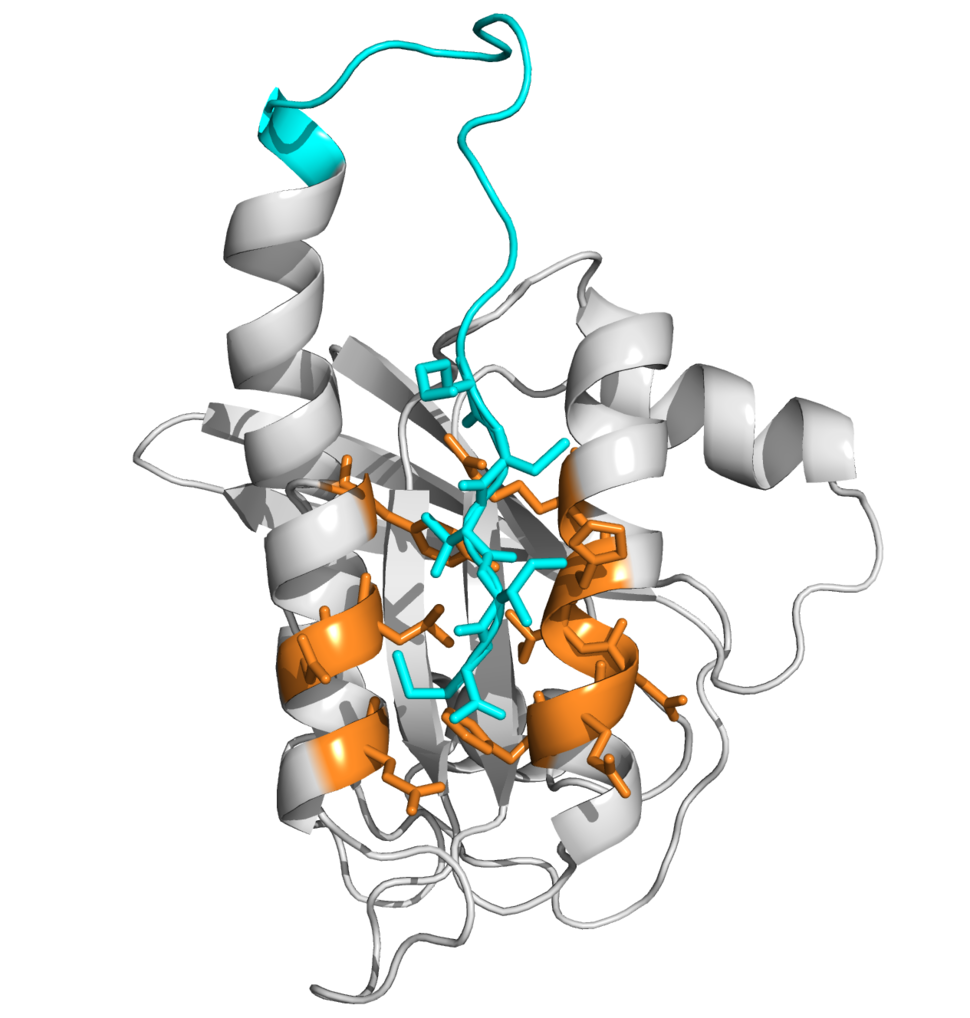
Our team uses the disulfide tethering technology first described by Wells and colleagues in 2000 and exemplified by Shokat, et al. in 2013. This site-directed method of fragment-based drug-discovery allows screening of low-affinity disulfide containing fragments against cysteine residues in a target protein. Fragment binding is not purely driven by cysteine reactivity; it is thermodynamically driven and strongly depends on protein-fragment interaction. Bound fragments are detected by mass spectrometry and provide a lead into the drug discovery process.
Progress
Initially we focused on developing covalent inhibitors targeting the C-terminal cysteine (C185) in the hypervariable region of KRAS4b, which is the site of post-translational modification that is crucial for KRAS membrane attachment and its oncogenic activity. Later, we focused on targeting histidine-95 that is found in KRAS but not HRAS or NRAS and localizes within the Switch2/Helix3 pocket identified by Shokat and colleagues as a docking site for their KRAS G12C covalent inhibitors.
Projects
With the highest RAS mutation frequencies seen with the top three causes of cancer deaths (lung, colorectal, and pancreatic cancer), the development of anti-RAS therapies is a major priority and a major challenge for cancer research. RAS proteins did not appear to present suitable pockets to which drugs could bind. Disulfide tethering could identify novel pockets induced in RAS upon small molecule binding.
- Development of mutant allele-specific RAS inhibitors
- Disulfide tethering to discover novel/cryptic pockets in RAS
- Targeting RAS family oncogenic small GTPases
Tools
We use a suite of tools, including biophysical, biochemical, and cell-based screens to test compounds for activity in recombinant proteins and in cells, including RAS-dependent mouse embryonic fibroblasts (MEFs), and other engineered/cancer or cancer cell lines.
- Tethering screens utilizing in-house 2,200 disulfide fragment library
- MALDI-TOF mass spectrometry-based screens to identify covalent engagement
- Biochemical and cell-based screens for compounds progression
- NMR
- Cell biology for mechanism of action studies
The RAS Initiative Drug Screening and Preclinical Research Team
Team lead and contact
Dr. Anna E Maciag
anna.maciag@nih.gov
301-846-6347
Collaborators
We are involved in a collaboration with biotech company TheRas and LLNL, co-developing inhibitors of mutant RAS. Collaborations with USCF and JHU bring expertise in cancer biology and single cell proteomics.
- TheRas Therapeutics, Inc.
- University of California San Francisco
- Lawrence Livermore National Laboratory
- Johns Hopkins University