RAS Initiative Imaging Research
The RAS Initiative Imaging Research Team develops and applies new optical microscopy techniques to understand how RAS molecules move, interact, and signal in living cells. Signaling by oncogenic RAS is a major driver of cancer.
Published evidence suggests that RAS signaling may be mediated by and dependent on higher order clustering of RAS molecules. We use insights gained from our imaging to develop assays to screen for disruption and mislocalization of RAS proteins and complexes.
Progress
We use a variety of microscopy techniques to study RAS dynamically in live cells. We also make synthetic membranes in the lab and use purified RAS proteins to reduce the complexity of the system. Using these imaging approaches, we have conducted projects where we successfully:
- Developed image-based assays for localization of KRAS and effectors.
- Developed bioluminescence-based assays for monitoring interactions of RAS molecules with effectors in living cells and screening for inhibitors, screening more than 100,000 molecules.
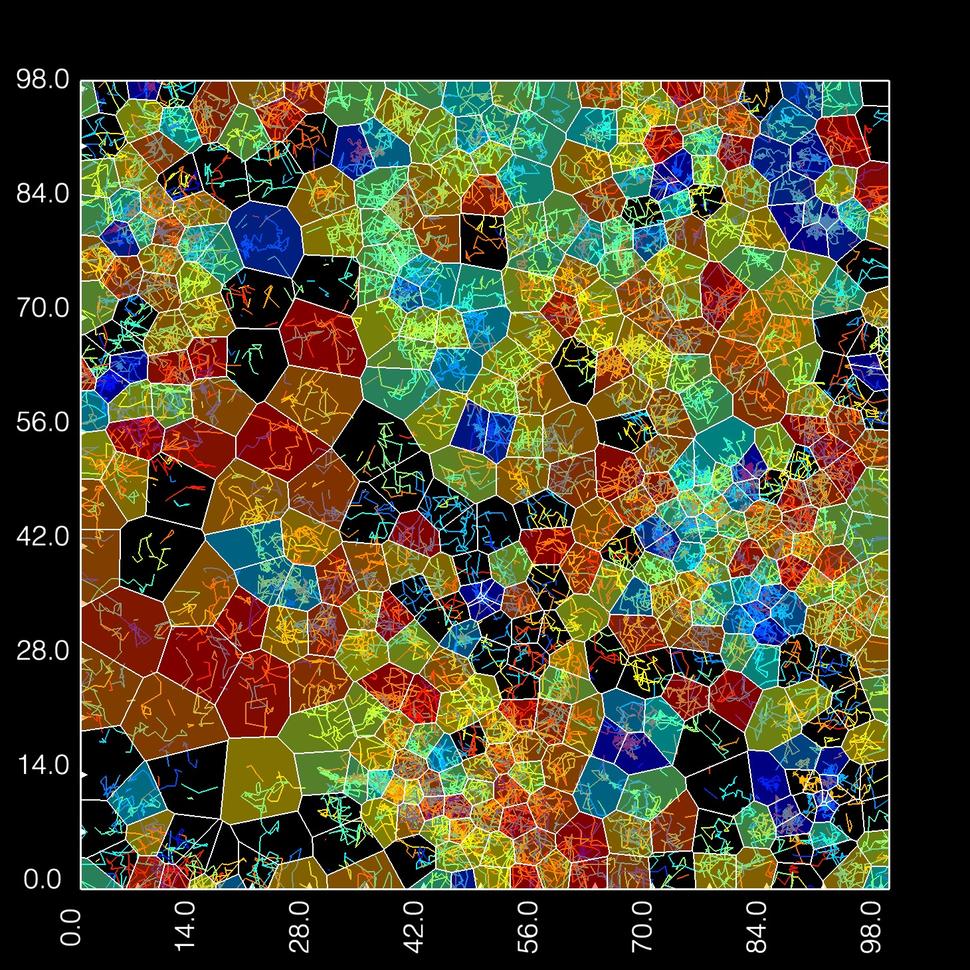
- Modeled the mobility and clustering of single Halo-tagged KRAS molecules in the membranes of living cells using single-molecule microscopy.
- Used supported lipid bilayers and purified proteins to study RAS dynamics.
Projects
In our collaborative work with Lawrence Livermore, we continue to use microscopy to understand how RAS activates its primary effector protein, RAF, in cancer signaling:
- Measure and characterize the dynamics of KRAS molecules in living cells using single molecule microscopy.
- Monitor interactions of KRAS with itself and other proteins in the membranes of living cells.
- Measure diffusion and conformational dynamics of RAS and effectors on supported lipid bilayers
Tools
Optical microscopy and atomic force microscopy both offer outstanding temporal and spatial resolution. We can use these technologies to measure single molecule events that give us valuable information about the local environment of RAS, its conformational changes, and how it interacts and moves with lipids and other proteins.
- Single-molecule tracking
- Fluorescence correlation and lifetime correlation spectroscopy (FCS and FLCS)
- Step photobleaching
- Photo-activated localization microscopy and stochastic optical reconstruction microscopy (PALM/Storm) imaging
- Fluorescence lifetime imaging microscopy (FLIM)
- Labeling proteins with fluorescent dyes
- BRET (bioluminescence resonance energy transfer)
- Confocal microscopy and live cell imaging
- Atomic force microscopy (AFM)
The RAS Initiative Imaging Research Team
Team lead and contact
Dr. Tommy Turbyville
Thomas.Turbyville@nih.gov
301-846-6892
Collaborators
Our team has collaborated with the following:
- Lawrence Livermore National Laboratory
- The Broad Institute
- Eli Lilly and Company
- University of California San Francisco