Research advocates play a vital role in shaping NCI’s work. They challenge us, and ensure that we never lose sight of what we are here to do – which is to improve patient outcomes by advancing cancer research. We can’t do this without the unique perspectives research advocates bring.
The advocacy community provides NCI with critical perspective and insights that are essential to advancing cancer research. Prioritizing and integrating the collective patient perspective across NCI-supported research activities helps to ensure scientific and medical advances are more timely, relevant, and effective for people living with and affected by cancer.
NCI’s Office of Advocacy Relations (OAR) works with all research advocates and organizations to ensure the community’s unique perspectives and ideas are integrated into NCI activities.
Advocate Roles at NCI
Both individual research advocates and national organizations play unique and critical roles that can broadly be categorized across four key functions.
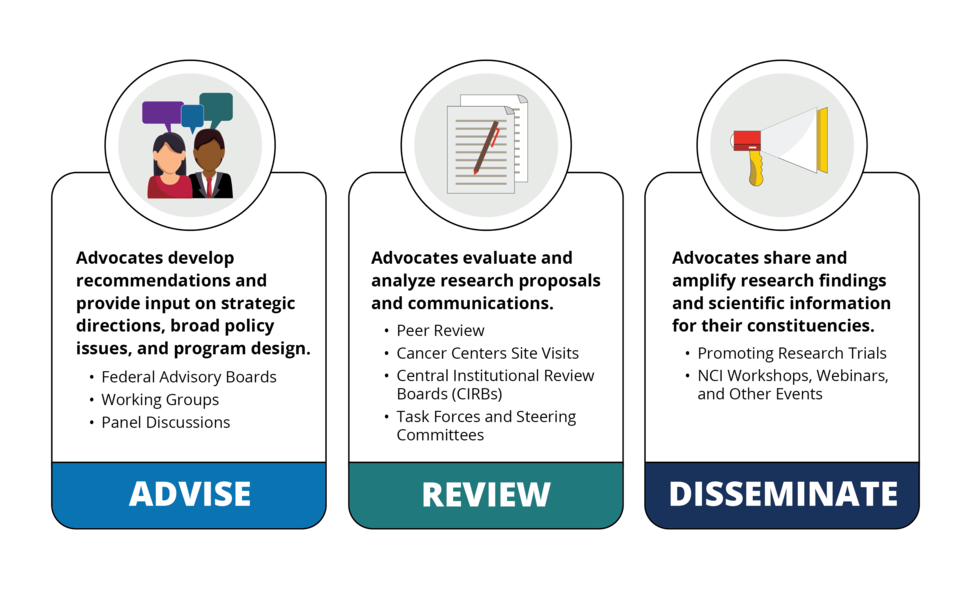
Advise
Advocates develop recommendations and provide input on strategic directions, broad policy issues, and program design. Their contributions may often help identify barriers to implementation. Activities may include:
- Participating on formal advisory boards, like the NCI Council of Research Advocates
- Participating in working groups
- Speaking on panel discussions at scientific meetings or conferences
- Supporting clinical trial development and accrual
Review
Advocates evaluate and analyze research proposals and communications. Activities may include:
- Participating in peer review panels
- Participating on task forces or steering committees
- Editing or translating scientific language to improve readability
Disseminate
Advocates share and amplify research findings and scientific information for their constituencies. Activities may include:
- Promoting new research findings or developments, events, or materials
- Promoting research trials
- Distributing NCI announcements like the Professional Judgment Budget Proposal
Case Studies
Advocates are engaged across the NCI in a variety of activities. Below are just a few examples of how advocates make an impact at NCI.
NCI Technology Research Advocacy Partnership
The NCI Technology Research Advocacy Partnership (NTRAP) incorporates the collective patient perspective into technology development research programs including the Innovative Molecular Analysis Technologies (IMAT) and Informatics Technology for Cancer Research (ITCR). Both the IMAT and ITCR grant programs support early-stage technology development to impact cancer research. Advocates on NTRAP:
- Assist in translation and dissemination efforts for novel technologies by developing messaging to communicate about the programs and technologies and identifying opportunities for dissemination.
- Provide input regarding patient needs, interests, concerns, and enthusiasms to guide program direction and decision-making on potential new technology projects.
- Interact with technology developers to help explain the potential for new tools and resources to end user and patient communities.
Example of Program Impact: Advocates drafted a ‘Sensitivity Expectations” document for IMAT and ITCR Investigators to guide presentations and communications. The guidelines emphasize the need to respect people living with cancer and encourages the use of appropriate language to credit the patient’s contributions to research.
Learn more about the NCI Technology Research Advocacy Partnership.
NCI Scientific Steering Committees and Task Forces
NCI Scientific Steering Committees and Task Forces evaluate and prioritize clinical trial concepts to be conducted through the National Clinical Trials Network (NCTN), NCI Clinical Oncology Program (NCORP), or Experimental Therapeutics Clinical Trials Network (ETCTN). Each steering committee and task force has two patient advocate members. Advocate members also serve on the Patient Advocate Steering Committee (PASC), which works to ensure that advocates are effectively and consistently integrated with the development and evaluation of clinical trials within those groups. Advocates on steering committees and task forces:
- Ensure that the concept evaluations include consideration for the patient community at large.
- Review trial inclusion and exclusion criteria for maximal patient participation.
- Review and update strategic priorities for a given disease or research area and review accrual to ongoing trials.
- Develop and share best practices for patient advocate interactions in scientific steering committees through PASC.
Example of Program Impact: Advocates participating on steering committees and task forces provide the patient perspective and ask critical questions that can guide the direction of conversation and may impact the final trial design. In past reviews they have emphasized topics such as reasonable timeline for results, burden on trial participants (side effects, number of visits), and evaluating the importance of research based on making a difference for patients.
Learn more about the NCI Scientific Steering Committees and Task Forces.
Cancer Grand Challenges Advocacy Panel
The Cancer Grand Challenges (CGC) program, co-funded by NCI, maintains an Advocacy Panel of international advocates who review applications for funding and help develop how CGC includes advocacy within the initiative. In addition to the Advocacy Panel, every funded team includes patient advocates who develop and execute involvement and engagement initiatives on behalf of the team and work closely with investigators.
Example of Program Impact: Advocacy Panel feedback is considered in the review of all applications and final funding decisions. A representative of the Advocacy Panel participates in team interviews. The Advocacy Panel supports funded teams in their engagement of team advocates and planning for advocacy activities.
Learn more about Cancer Grand Challenges.
Early-age Onset Colorectal Cancer Think Tank
NCI worked with Fight CRC in 2023 to co-sponsor and plan a meeting on the increasing issue of early-age onset colorectal cancer. The meeting brought together specialists in oncology, epidemiology, genetics, industry, and academia to advance research on early-age onset colorectal cancer.
Example of Program Impact: Fight CRC intends to host a follow up meeting to continue discussing how to advance this area of research.
Learn more about the 2023 meeting.
PedAL Trial
In June 2022, LLS launched the PedAL Master Clinical Trial, the first integrated, global, acute leukemia master clinical trial to test new, safer therapies on children, who will be matched to treatments based on their unique tumor biology. Through partnership with the National Cancer Institute (NCI) and Children’s Oncology Group the screening and treatment trials were made available in the U.S.
Example of Program Impact: Screening trials are ongoing with one treatment trial currently open and a second expected to open in 2024.
Learn more about the PedAL trial.
To find out how you or your group can get involved, email NCIadvocacy@nih.gov to connect with OAR today!