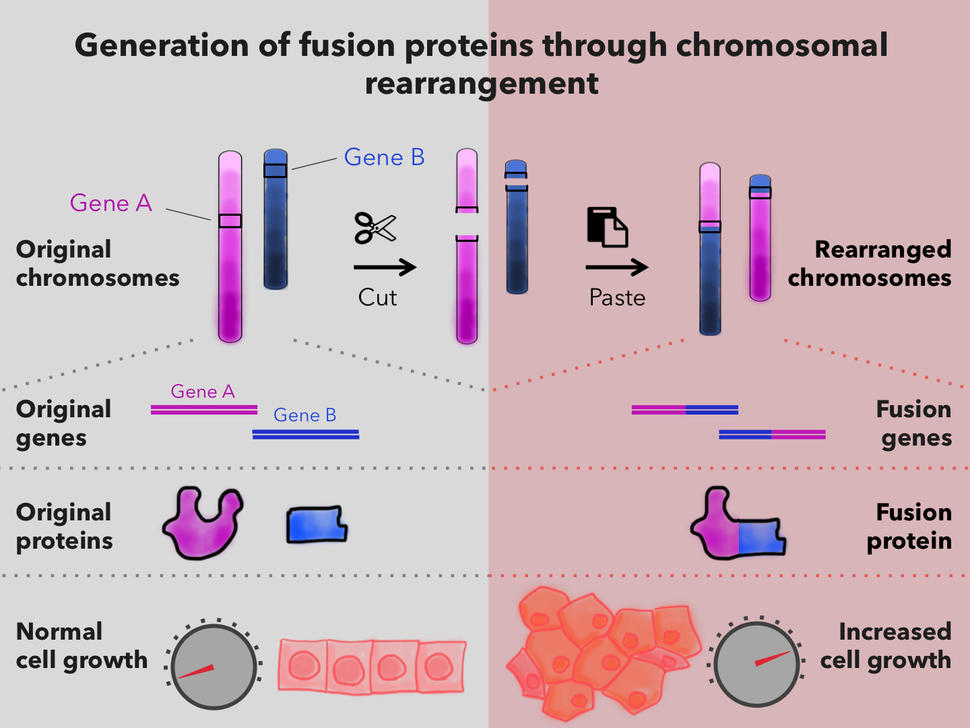
Potential Impact of Fusion Oncoprotein Research
Progress in our understanding of fusion oncoprotein-driven cancer biology through investigator-initiated research and the Cancer Moonshot-funded Fusion Oncoproteins in Childhood Cancers (FusOnC2) Consortium, along with recent technological developments, provides an opportunity for collaborative efforts towards the development of new treatment approaches for childhood cancers.
Advances in chemical biology have expanded the universe of “druggable” protein targets in pediatric cancers and new technological approaches provide strategies for the direct targeting of fusion oncoproteins and the proteins with which they interact within complexes. Progress in medicinal chemistry (such as PROTACs and molecular glues) provides a knowledge base for the design of new therapies for fusion-driven childhood cancers.
While directly targeting fusion oncoproteins would be ideal because of their exclusivity in tumors, other targets (e.g., interacting proteins, synthetic lethal partners, mediators of fusion oncoprotein function) may be more amenable to these strategies.
The TFCC aims to build on the progress of fusion oncoprotein research and translate preclinical discoveries into the development of effective treatments for fusion oncoprotein-driven childhood cancers.
TFCC News
Program Directors in DCB and DCTD discuss challenges and future opportunities for the development of therapies targeting fusion oncoproteins in childhood cancers (including sharing the progress of the FusOnC2 Program, describing the Novel Chemical Approaches for Targeting Fusion Oncoproteins Webinar Series, and sharing the goals of the TFCC) in a JNCI Perspective.
Contacts for TFCC
For additional information about TFCC, please contact Dr. Keren Witkin in DCB or Dr. Joseph Agyin in DCTD.
Funded Projects
Projects Investigating Mechanisms of Fusion-Driven Oncogenesis in Childhood Cancers (U01s)
| Institution | Principal Investigator(s) | Center Title |
|---|---|---|
| Brigham and Women's Hospital | Christopher French, Kyle Eagen | Overcoming limitations of BET inhibition in NUT carcinoma |
| Memorial Sloan Kettering Cancer Center | Marc Ladanyi, Andrea Ventura | Desmoplastic small round cell tumor: Harnessing new insights and new models |
| St. Jude Children's Research Hospital | Stephen Mack, Richard Kriwacki | Discovering the mechanisms underlying oncogenesis by ZFTA-RELA and pinpointing therapeutic targets |
Next Generation Chemistry Centers for Fusion Oncoproteins (UM1)
| Institution | Principal Investigator(s) | Center Title |
|---|---|---|
| Massachusetts Institute of Technology | Angela Koehler, Alex Burgin, Alexandra Gould, Corinne Linardic, Daniel Nomura | Chemical approaches to modulate PAX3-FOXO1 in fusion-positive alveolar rhabdomyosarcoma |
| University of Texas Southwestern Medical Center | David McFadden, Joseph Ready | Targeting transcriptional addiction in fusion-driven sarcoma |