DCB Research Programs
The Division of Cancer Biology (DCB) supports research programs that foster emerging areas in cancer biology and the development of new experimental models for cancer research. These networks are funded through cooperative agreements and research project grants that promote collaborative interactions. These unique programs facilitate the development of important fields and are advancing basic cancer research.
Selected Programs from DCB:
-

Acquired Resistance to Therapy Network (ARTNet)
Focusing on the mechanistic basis of acquired resistance to cancer therapies and disease recurrence.
-
Cancer Immunoprevention Network (CIP-Net)
Advancing the understanding and development of immunoprevention strategies.
-

Cancer Systems Biology Consortium (CSBC)
Using systems biology to advance the understanding of mechanisms that underlie fundamental processes in cancer.
-

Cancer Target Discovery and Development (CTD²) Network
Bridging the knowledge gap between large-scale genomic datasets and the underlying etiology of cancer development, progression, and metastasis.
-
Cancer Tissue Engineering Collaborative (TEC)
Supporting the development and characterization of state-of-the-art biomimetic tissue-engineered technologies for cancer research.
-

Cellular Cancer Biology Imaging Research (CCBIR)
Developing and testing imaging technologies at the cellular and organ scales driven by questions in cancer biology.
-
Diet, Lipid Metabolism, and Tumor Growth and Progression (DLT) Program
Investigating mechanistic links between diet, lipid metabolism, and tumor growth/progression.
-
Epstein Barr Virus Associated Lymphoma Consortium (EALC)
Advancing the understanding of the role of Epstein Barr virus in non-Hodgkin Lymphoma and/or Hodgkin disease development with or without HIV infection.
-
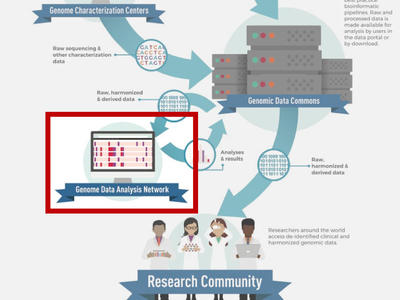
Genomic Data Analysis Network
Conducts key large-scale studies and generate genomic resources to support the genomic research community.
-

Human Tumor Atlas Network (HTAN)
Constructing 3D atlases of the cellular, morphological, molecular, and spatial features of human cancers (and their surrounding microenvironments) over time.
-

Human Cancer Models Initiative (HCMI)
Generating up to 1,000 patient-derived next-generation cancer models (NGCMs) as a community resource.
-

Metabolic Dysregulation and Cancer Risk Program: A Transdisciplinary Approach to Obesity-Associated Cancer Research
Enhancing our knowledge of the dynamics and underlying mechanisms that link obesity, metabolic dysregulation, and increased cancer risk.
-

Metastasis Research Network (MetNet)
Supporting research to advance the understanding towards a more comprehensive appreciation of metastasis as a whole-body, systems-level problem.
-

Onco-Aging Consortium (OAC)
Supporting research addressing key questions regarding how hallmarks of aging lead to impaired cellular activities and alterations in the microenvironment that contribute to the development of cancer-initiating cells.
-

Oncology Models Forum (OMF)
Advancing standard practices for modeling human cancers in mammalian models and facilitating collaborations, data sharing, and harmonization of mammalian models.
-

Pancreatic Ductal Adenocarcinoma Stromal Reprogramming Consortium (PSRC)
Focusing on the identification, integration, and mechanistic evaluation of tumor microenvironment elements driving pancreatic cancer progression and response to therapy.
-
Pediatric Immunotherapy Network (PIN)
Developing and advancing both basic and translational immunotherapy approaches for pediatric solid tumors.
-

Physical Sciences – Oncology Network (PS-ON)
Fostering the convergence of physical sciences approaches and perspectives with cancer research to advance our understanding of oncology through transdisciplinary team science.
-
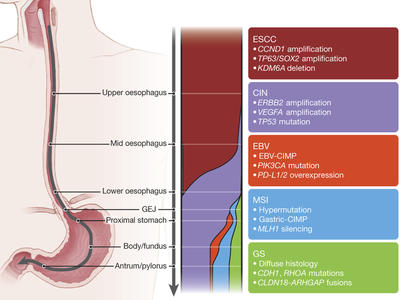
Program on the Origins of Gastroesophageal Cancers
Aiming to define how gastric and gastroesophageal junction (GEJ) adenocarcinomas initially evolve at the cellular level.
-

RNA Modifications Driving Oncogenesis (RNAMoDO) Program
Advancing fundamental studies in the emerging area of RNA modifications that underlie the oncogenic process.
-
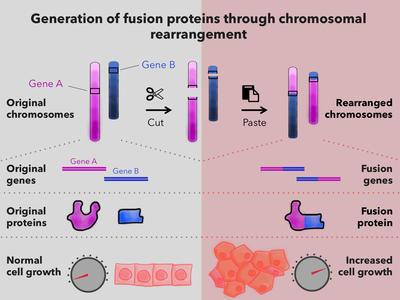
Targeting Fusion Oncoproteins in Childhood Cancers (TFCC)
Advancing our mechanistic understanding of fusion oncoproteins in pediatric cancers and accelerating preclinical development of treatments for fusion-driven childhood cancers.
-
Translational and Basic Science Research in Early Lesions (TBEL)
Aiming to understand the biological and pathophysiological mechanisms driving or restraining pre-cancers and early cancers to enable the development of biology-backed precision prevention approaches.