NCTN Clinical Trials
NCTN clinical trials help to establish new standards of care, set the stage for approval of new therapies by the Food and Drug Administration, test new treatment approaches, and validate new biomarkers.
NCI has launched a number of trials through NCTN, including:
- ALCHEMIST: Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials
- DART: Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors Trial
- Lung-MAP: Phase II/III Biomarker-Driven Master Protocol for Second Line Therapy for all Advanced Stage Non-Small Cell Lung Cancers
- NCI-MATCH: Molecular Analysis for Therapy Choice for adults with advanced cancers
- NCI-COG Pediatric MATCH: Molecular Analysis for Therapy Choice for children and young adults with advanced cancers
- NCI-NRG ALK Master Protocol: A biomarker-driven trial for previously treated ALK-positive non-squamous NSCLC patients
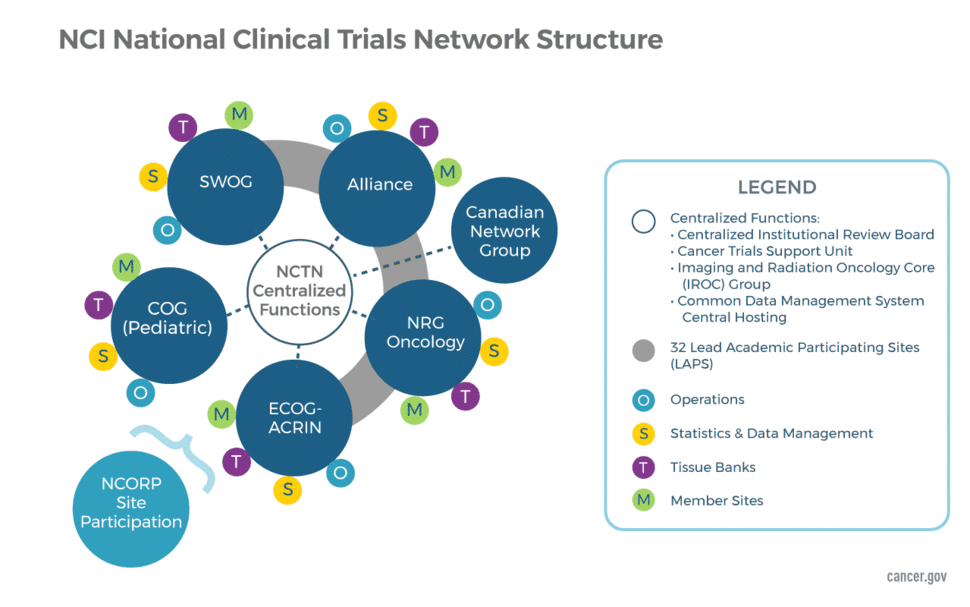
Network Groups and Their Support Components
The network's organizational structure is ideal for screening large numbers of patients to find those whose tumors exhibit the molecular features that give them the best chance of responding to new, targeted treatments. For physicians and their patients, a menu of important trials are widely available throughout the country, in large cities and small communities alike. NCTN offers access to the best approaches available for many common and, increasingly, even rare cancers.
Oversight of the NCTN—its organizational structure, funding, and long-term strategic direction—is under the purview of the Clinical Trials and Translational Research Advisory Committee (CTAC). This federal advisory committee is composed of clinical trials experts, industry representatives, and patient advocates from across the nation and provides recommendations to the NCI director.
Network Groups
The NCTN consists of four adult groups and one large group focused solely on childhood cancers. The structure also includes a Canadian Collaborating Clinical Trials Network. The five US Network Groups are:
- Alliance for Clinical Trials in Oncology
- ECOG-ACRIN Cancer Research Group
- NRG Oncology
- SWOG
- Children's Oncology Group (COG)
The US groups are each funded through two separate awards—one to support Network Operations and another to support the Statistics and Data Management Centers. The Operations Centers are responsible for developing new protocols and managing the regulatory, financial, membership and scientific committees of each group. The Statistical Centers are responsible for data management and analysis, manuscript preparation, and safety monitoring, in addition to assisting in trial design and development.
The Canadian Network Group partners with the US Network Groups in the conduct of select, late-phase, multi-site clinical trials. The Canadian Network Group is:
The Network Operations and Statistical Centers for each NCTN group are geographically separate but work closely together. They are often located at an academic institution that has offered to "house" the group; however, in several cases, a center is located at a freestanding site that is funded via a nonprofit foundation. The only exception to the above is the Canadian Collaborating Clinical Trials Network, which received a single award for its Operations and Statistical Center.
Lead Academic Participating Sites (LAPS)
Thirty-two US academic institutions have been awarded a Lead Academic Participating Site (LAPS) grant, which is a source of funding created especially for the NCTN. The sites are academic research institutions with fellowship training programs, and most of the awardees are NCI-Designated Cancer Centers. To receive these awards, sites had to demonstrate their ability to enroll high numbers of patients onto NCTN trials, as well as scientific leadership in the design and conduct of clinical trials.
The 32 LAPS grantees are:
Case Western Reserve University – Case Comprehensive Cancer Center
Dana Farber/Harvard Cancer Center
Duke Cancer Institute at Duke University Medical Center
Emory University – Winship Cancer Institute
Fred Hutchinson Cancer Research Center
Johns Hopkins University - Sidney Kimmel Comprehensive Cancer Center
Memorial Sloan Kettering Cancer Center
Norris Cotton Cancer Center at Dartmouth Hitchcock Medical Center
Northwestern University – Robert H. Lurie Comprehensive Cancer Center
Ohio State University Comprehensive Cancer Center
Sidney Kimmel Cancer Center at Jefferson Health
University of Alabama at Birmingham
University of California Davis Comprehensive Cancer Center
University of Chicago Comprehensive Cancer Center
University of Colorado Cancer Center
University of Michigan Rogel Cancer Center
University of North Carolina Lineberger Comprehensive Cancer Center
University of Oklahoma – Stephenson Cancer Center
University of Pittsburgh Cancer Institute
University of Rochester Wilmot Cancer Institute
University of Southern California – Norris Comprehensive Cancer Center
University of Texas MD Anderson Cancer Center
University of Texas Southwestern Medical Center – Harold C. Simmons Cancer Center
University of Utah - Huntsman Cancer Institute
University of Wisconsin Carbone Cancer Center
Vanderbilt University Medical Center - Vanderbilt Ingram Cancer Center
Washington University at St. Louis - Siteman Cancer Center
Wayne State University Barbara Ann Karmanos Cancer Institute
Yale University – Yale Cancer Center
Higher levels of patient enrollment require a sustained level of data management work over several years, and the LAPS grants support the research staff required to manage this effort. The funds provided in the LAPS grants to cover this increased workload effectively raise the per-patient reimbursement level at the selected sites.
The LAPS awards also provide some funding for scientific and administrative leadership at the site itself, as the principal investigators at the site need to prioritize the clinical trials in which they participate, as well as educate and train staff at the sites in clinical research and develop strategies to promote patient enrollment.
Community Hospitals and Medical Centers
Many other investigators at community hospitals and medical centers can participate in NCTN trials, even if they are at sites that did not receive a LAPS award. These sites, as well as a number of international sites, either receive research reimbursement directly from one of the network groups with which they are affiliated or they receive awards from the NCI Community Oncology Research Program (NCORP).
Site membership in the individual NCTN groups is based on criteria that are specific to each group. Sites conducting clinical trials can belong to more than one group, and membership in at least one group allows a site to participate in the trials led by any NCTN group for which their investigators are qualified. Consequently, researchers from the LAPS, NCORP, other academic centers, community practices, and international members associated with the Network groups may all enroll patients into NCTN trials.
Imaging and Radiation Oncology Core Group (IROC)
To help monitor and ensure quality in trials that involve new imaging modalities and/or radiation therapy, NCTN established an Imaging and Radiation Oncology Core (IROC) Group that assists all of the NCTN groups that use these modalities in their trials.
Integrated Translational Science Awards (ITSA)
The final component of the NCTN are the Integrated Translational Science Awards (ITSAs). The five academic institutions that received ITSAs include teams of translational scientists who use innovative genetic, proteomic, and imaging technologies to help identify and qualify potential predictive biomarkers of response to therapy that the network groups can incorporate into future clinical trials.
These awards are used to leverage work already underway in these investigators' laboratories, often supported in part by other NCI grants, with the expectation that these researchers will help the network groups bring new laboratory discoveries into clinical trials. These labs all employ cutting-edge technologies that enable better characterization of tumors and help to identify changes in tumor biology in response to treatment that may help explain how treatment resistance can develop.
The ITSA grantees are:
Children’s Hospital of Philadelphia
Emory University – Winship Cancer Institute
Memorial Sloan Kettering Cancer Center
Ohio State University Comprehensive Cancer Center
University of North Carolina Lineberger Comprehensive Cancer Center
NCTN Tissue Banks
Each NCTN group also collects and stores tissue from patients in NCTN trials in a harmonized network of tissue banks. Standard protocols have been developed to ensure that the tissue collected is of the highest quality. Computerized records of the stored samples have important clinical details, such as the treatments received by the patients from whom the tissue was taken, treatment response, and patient outcome. Participants in NCTN trials may also consent to the use of their tissue specimens for studies beyond the NCTN trial in which they are enrolled. The NCTN tissue bank program includes a web-based system that any researcher can use. Researchers, including those who are not affiliated with the NCTN, can query the system about the availability of tissue that meets certain criteria and track the review and approval process of any requests to use samples.
Scientific Oversight Committees
The NCTN groups propose concepts for new clinical trials to the NCI Disease/Imaging Steering Committees. These committees are organized by NCI to evaluate and prioritize new clinical trials and recommend to NCI those most likely to have the highest scientific and clinical impact. Each committee is led by nongovernmental co-chairs who are not permitted to hold leadership positions in the NCTN groups, although they can be group members. The remainder of the committee membership consists of NCTN group members selected by each group, other disease experts not involved in leadership positions in the groups, representatives of NCI-funded SPORE and Consortia, biostatisticians, patient advocates, and NCI disease experts.
NCTN Budget
The overall NCTN budget is $171 million, distributed to the various components of the network. This system provides for an annual enrollment of about 17,000-20,000 participants on cancer treatment and imaging trials.
Efficiencies in Collaboration
NCTN groups are able to reduce the costs of conducting trials by sharing resources. This collaborative approach allows members of one NCTN group to support trials led by other groups and affords NCTN members the ability to conduct a full portfolio of trials in the most common cancers.
Because the NCTN has only four US adult groups, with fewer Operations and Statistical Centers that require financial support, there has been a net cost savings. All of the groups use a common data management system (Medidata Rave) and an integrated IT system for the tissue banks, which translates into cost savings.
Additional Support
Clinical trials are complex undertakings that require a host of support organizations and funding streams. The network includes a number of other features that are not included in the NCTN awards but that are essential to carrying out the NCTN mission.
The additional support includes:
- Central Institutional Review Boards, an important component of NCI's clinical trials system that adds speed, efficiency, and uniformity to ethics review.
- The Cancer Trials Support Unit (CTSU), an NCI-funded contract that provides clinical investigators and their staff with one-stop online access to NCTN trials and allows investigators to register new patients.
- A dedicated tissue bank for each Network group funded through a separate NCI award mechanism.
- The Biomarker, Imaging, and Quality of Life Studies Funding Program (BIQSFP), a separate funding stream for NCTN trials that supports correlative science studies on group trials. NCTN groups compete for funds that are specifically reserved annually for this purpose. The availability of dedicated funds greatly facilitates coordination, as clinical trials must meet stringent deadlines.
- In addition, approximately one-quarter of patient accrual on NCTN treatment trials is paid for by the NCORP program. The community hospitals and medical centers participating in the NCORP program are reimbursed for accruing patients to NCTN treatment trials by their NCORP awards, not via the NCTN Group Operations award.
Finally, in addition to these substantial annual expenditures, NCI also subsidizes the NCTN by paying for many other essential clinical trial functions, thereby further reducing costs borne by the Network groups:
- NCI pays for the licenses and hosting fees of the electronic, common data management system, called Medidata Rave, used by all of the NCTN groups.
- NCI oversees a national audit system for NCTN trials.
- NCI manages Investigational New Drug applications to the Food and Drug Administration along with the distribution of these drugs for many NCTN trials.
Collaboration among the groups is viewed as critical to success at all organizational levels and is now specifically rewarded at the time of grant review. Efficiency is also stressed, and mandatory timelines are now in place for protocol development. Although these changes are viewed as vital for the health of the public system, they also come at an opportune moment, because exciting changes in oncologic science are offering new avenues for rapid advances, particularly for the development of new systemic treatment