There are many similarities between science and music, including quantitative patterns within elaborate compositions. Like many scientists who have a connection to music, Nathan Reticker-Flynn, Ph.D., plays the drums. Before he became a CSBC junior investigator at Stanford University, he opened for Arcade Fire and planned on becoming an acoustical engineer based on his interests in math, science, and music.
Although he moved away from creating musical instruments, his pursuit of engineering led to his Master’s work studying the design and function of microelectromechanical systems at the Massachusetts Institute of Technology. Eventually, he decided to bring his design mentality, which considers the effects of individual parameters on entire systems, to cancer biology. This perspective prompted him towards systems biology approaches investigating the complex system of cancer.
In this interview, Nathan discusses his views on systems biology and its application to understanding cancer metastasis and immunotherapy.
How do you define systems biology?
I think of it as an interdisciplinary field that seeks to combine biological experiments with computational, engineering, and mathematical approaches to understand multiscale systems.
In contrast, reductionist biology only investigates one element, such as a single gene or mutation, in normal or pathophysiological states. Systems biology seeks to interrogate the entire system and examines interactions between different networks.
Why do you think systems biology is important for cancer research?
I have a mathematician friend who described cancer as “uncontrolled chaos.” It is the inherent diversity and complexity of cancer that enables it to affect multiple organ systems, evade therapies, and spread throughout the body. Systems biology embraces the complexity of cancer while most fields try to evade it.
What do you think the biggest challenge in cancer systems biology research will be in the next 10 years?
In many ways, the interdisciplinary nature is both its strength and its weakness. Systems biology involves many different scientists from diverse backgrounds, and communication is not always straight forward between these groups. A mathematician may develop a model to address cancer biology. However, a clinician treating cancer patients may have no idea how to turn that mathematical model into a clinical approach.
How do you think young investigators can overcome this cancer systems biology challenge?
I think young investigators see the merit in combining mathematical modeling and experimental strategies. The lines of scientific fields are blurring and they are gaining aptitude in multiple areas. Young investigators are embracing new technologies and techniques that involve computational approaches, such as next generation sequencing, single-cell sequencing, and high dimensional analyses, to answer their research questions.
Can you describe your research and how it utilizes systems biology?
My main interest is understanding how metastatic cancer interacts with the immune system. This research plays an important role in preventing and treating metastases, which kill most cancer patients. I use systems biology for my cancer immunology studies because the immune system requires orchestration across multiple cell types, organ systems, and signaling molecules and networks.
When thinking about effective immune responses against cancer, you need to know what’s going on with the entire immune system. For my experiments, I employ a variety of systems biology tools.
In a study, my research group used mass cytometry and the Statistical Scaffold framework (which gives a comprehensive view of immune cells in a tissue) to compare immune cell profiles during immunotherapy and found that responses in lymphoid organs are essential for eliciting effective responses.
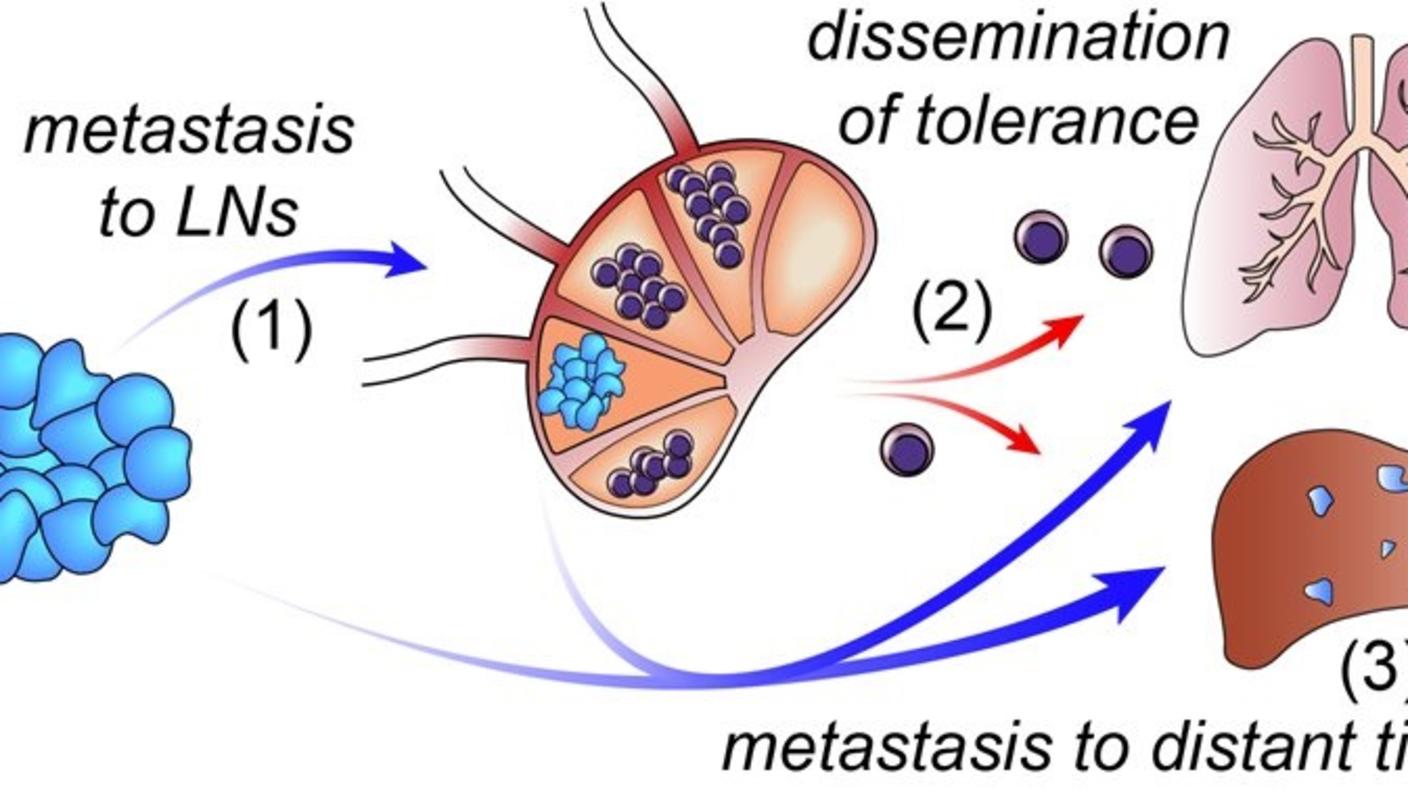
My current work uses a variety of sequencing approaches to investigate how cancer metastasis to lymph nodes effects immune responses.
Based on your recent experimental findings, how do you think scientists and clinicians can improve cancer immunotherapies?
Something that is immediately actionable from our cancer research is to examine systemic immune responses during immunotherapy treatment. Currently, it takes a long time to observe many clinical indicators of effective immunotherapies, such as reduced tumor size and increased survival.
For example, during some of the first immunotherapy clinical trials with a checkpoint inhibitor, tumor size initially increased due to an influx of immune cells.
Our recent data, however, showed that systemic immunity could potentially discriminate effective from ineffective responses to immunotherapies. We also discovered that immune tolerance often occurs at the lymph nodes.
Thus, querying these tissues may provide a better marker of effective immune responses during cancer treatments. Similarly, scientists and clinicians could potentially target immunotherapies to the lymph nodes to prevent the induction of tolerance and a pro-tumor immune phenotype.