Edgar Engleman, M.D., Ph.D., a CSBC Investigator at Stanford University, has been researching the immune system and its role in diseases for forty years.
After studying immunology as a post-doctoral fellow, Dr. Edgar Engleman became a faculty member in the Department of Pathology. This position also involved creating and directing the Stanford Blood Center, and allowed him to access immune cells (or white blood cells) for his immunological research.
He moved into the field of cancer biology, since, in his view, cancer represents a failure of the immune system to attack a tumor.
In this interview, he discusses his cancer immunotherapy studies, the role of mentorship in research, and the importance of cancer systems biology.
Can you describe your early cancer immunology research?
I was taught that the immune system was incapable of recognizing cancer because cancers are comprised almost entirely of the same substances as normal tissues. The immune system is trained to avoid attacking “self”.
As recently as 10 years ago, there was almost total skepticism about the prospects of immune based therapy for cancer. However, I disagreed with these beliefs and thought the immune system could potentially be exploited to treat cancer. I hypothesized that we could manipulate dendritic cells, which are powerful immune stimulating cells, to induce an anti-tumor immune response.
Back in the early 1990s, my lab did an experiment where we isolated dendritic cells from cancer patients with advanced disease. We loaded the cells with tumor antigens in vitro, and then injected them back into patients. This not only stimulated a strong immune response, but also resulted in dramatic tumor shrinkage in some of the patients. It provided the first evidence that a cancer patient’s immune system can be trained to attack and kill tumor cells.
These studies ultimately led to the first cancer immunotherapy that was approved by the FDA in 2010. The initial product was a dendritic cell vaccine for prostate cancer. Now, other types of immunotherapy are becoming standard-of-care for many types of cancers.
How has mentoring trainees contributed to your cancer research?
Trainees inspire me. Students and post-docs are generally fearless, and they are the reason why my lab has a long history of undertaking high-risk projects. I’m committed to teaching students and post-docs, because trainees go on to make the next great leaps in terms of understanding and treating disease.
How do you define cancer systems biology?
In a New Yorker article, a prominent cancer biologist described the “seed and soil” concept for cancer.
For many years, the focus of cancer research was on studying only the tumor cell, or the “seed,” rather than the “soil” environment where it grew. However, cancer results from complex interactions between tumor cells and the host environment, including immune cells.
We need to understand the various forces in the “soil” to optimally treat cancer, which requires a systems biology approach. Systems biology attempts to analyze the effects of multiple components at the same time rather than focus on a single cell or gene.
Why do you study the role of the lymph node in cancer metastasis?
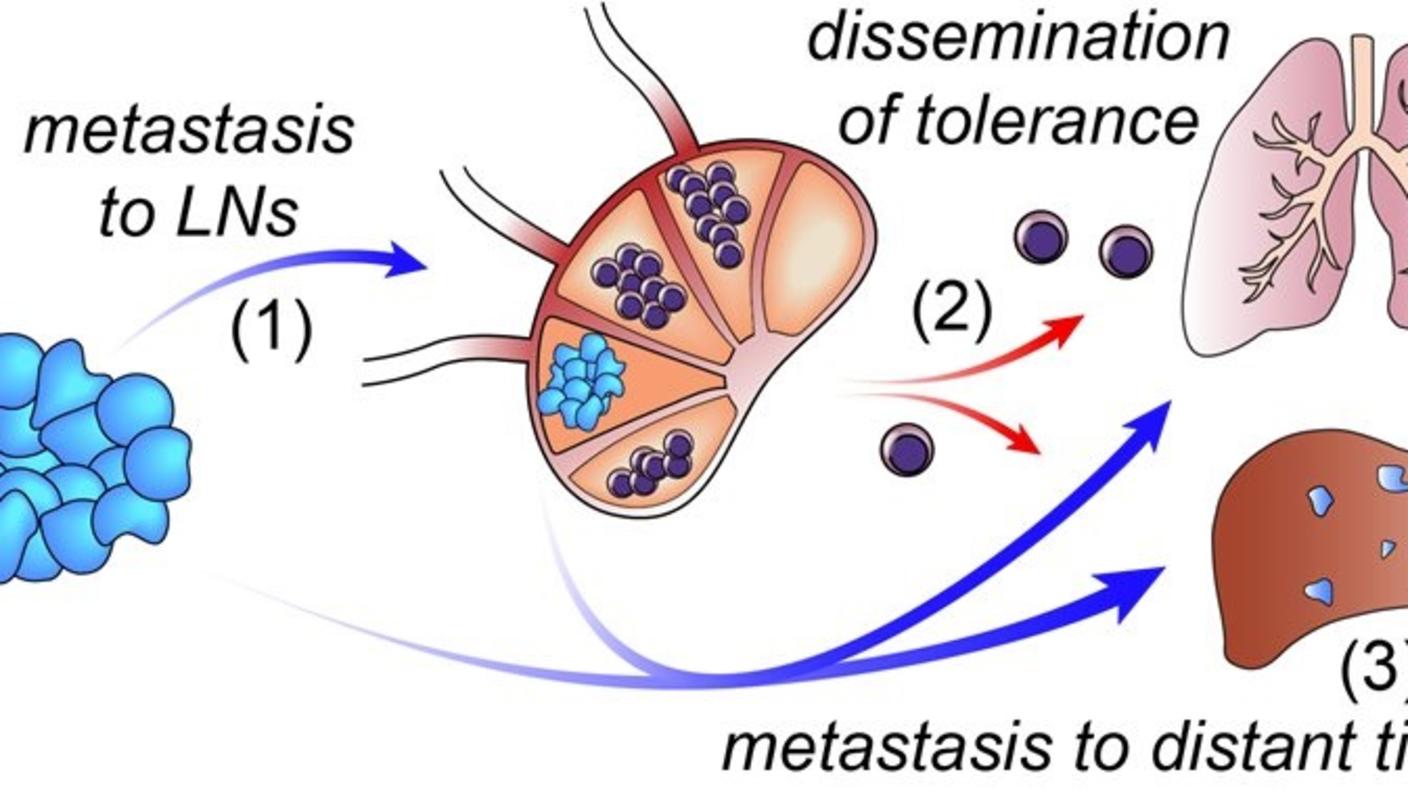
A post-doctoral fellow, Nate Reticker-Flynn, joined my lab in 2013. After spending several months, he came to me with an idea.
He knew that when cancer spreads, it typically goes first to the “draining” lymph node. It has been known for many years that lymph nodes serve as the main sites where unwanted substances, including infectious pathogens, are deposited for processing and destruction by the immune system. For this reason, it has been widely assumed that lymph nodes serve to slow cancer spread. However, Nate asked, “Is it possible that the tumor takes advantage of this draining system to enable it to spread to other sites?”
He hypothesized that tumor cells in the lymph node hijack immune cells and reprogram them to aid and abet cancer progression. This was the basis for our CSBC program with Sylvia Plevritis, Garry Nolan, and our other collaborators.
What are advantages of using system-wide models of cancer immunity?
The immune system is comprised of multiple cell types present throughout the body that stay in close communication with one another. When challenged, even in one location, the system responds in a coordinated system-wide manner.
We found in studies of experimental mouse models of cancer that to effectively treat tumors, you need a potent, systemic immune response. Thus, analyzing the immune response throughout the body is critical to understanding tumor-immune cell interactions and developing truly effective immunotherapies.