brexucabtagene autoleucel
(brek-suh-KAB-tuh-jeen AW-toh-LOO-sel)
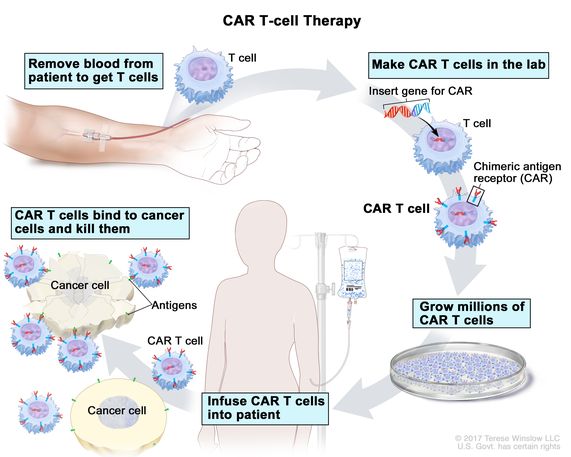
A drug used to treat adults with mantle cell lymphoma or B-cell acute lymphoblastic leukemia that came back or did not get better after other treatment. It is also being studied in the treatment of other types of cancer. Brexucabtagene autoleucel is made using a patient’s T cells (a type of immune system cell). A gene for a special receptor called chimeric antigen receptor (CAR) is added to the T cells in the laboratory. These changed T cells called CAR T cells are grown in large numbers in the laboratory and given to the patient by infusion. Brexucabtagene autoleucel binds to a protein called CD19, which is found on some lymphoma cells and leukemia cells. This helps the body’s immune system kill cancer cells. Brexucabtagene autoleucel is a type of CAR T-cell therapy. Also called Tecartus.