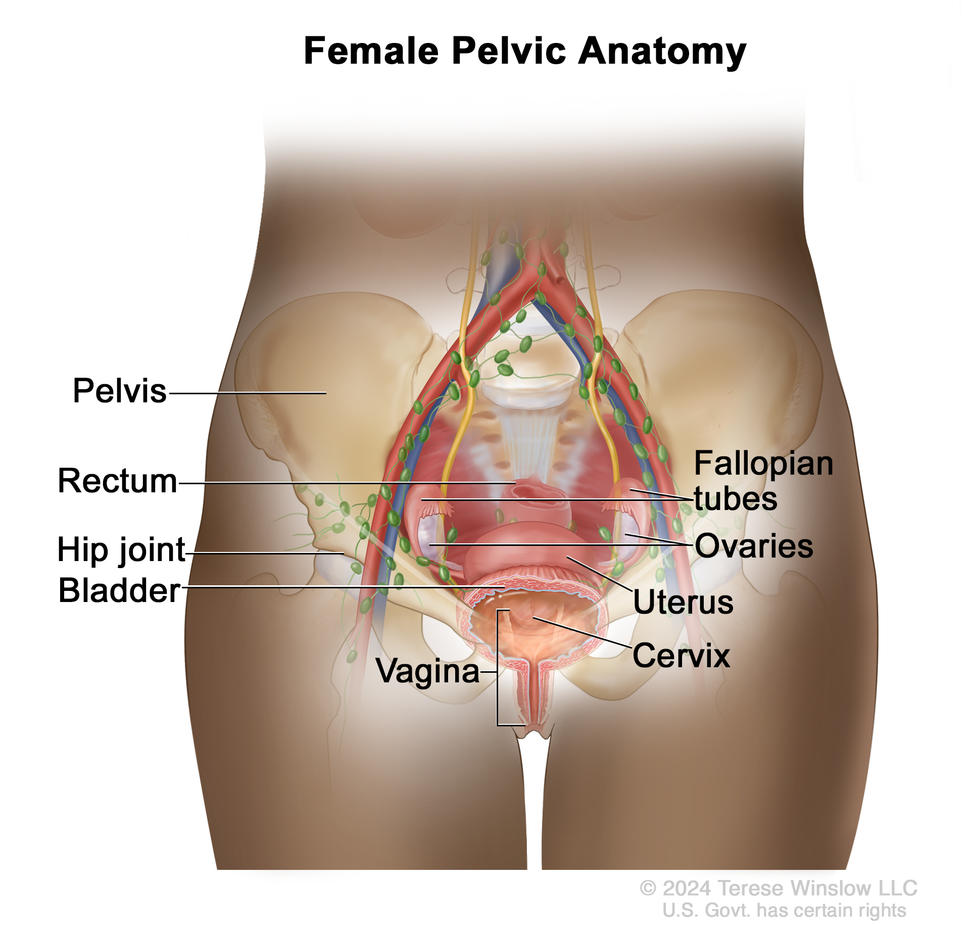
What is fertility?
Fertility is the ability to produce children. A female’s fertility depends on a functioning reproductive system and endocrine system. These systems work together to help a female conceive and carry a pregnancy to term.
Factors that may affect your fertility
Whether your fertility is affected by cancer or its treatment depends on:
- the type of cancer treatment(s)
- the amount (dose) of treatment
- the length (duration) of treatment
- your age at the time of treatment
- the amount of time that has passed since treatment
- the type of cancer and if the tumor is near reproductive organs
- your baseline fertility status, including any fertility problems in the past
- other personal health conditions and factors
Cancer treatments may cause infertility or lower your fertility
Cancer treatments may affect your fertility by harming reproductive organs and endocrine glands that control fertility. Changes to your fertility may be temporary or permanent. Treatments such as chemotherapy, radiation therapy, and hormone therapy can cause primary ovarian insufficiency, as explained in the section Primary ovarian insufficiency (POI) and cancer treatment.
Chemotherapy and fertility
Chemotherapy destroys cancer cells but can also harm healthy cells, such as those in the ovarian follicles, which contain oocytes (egg cells). Some types of chemotherapy pose a high risk to your fertility. For example, alkylating agents present a high risk to your fertility because they can cause the ovaries to stop developing mature eggs and producing estrogen. This can lead to primary ovarian insufficiency. You may be at greater risk of infertility if you receive high doses of chemotherapy or several chemotherapy drugs at the same time.
Hormone therapy and fertility
Hormone therapy, also called endocrine therapy, adds, blocks, or removes hormones (estrogen and progesterone). It may be used to stop or slow the growth of cancer that uses hormones to grow. This can cause primary ovarian insufficiency. Fertility-related side effects depend on the specific type of hormone therapy and may include symptoms such as those listed in the section Primary ovarian insufficiency (POI) and cancer treatment.
Research has found that some women being treated for breast cancer can stop hormone therapy while trying to become pregnant without raising the risk of recurrence in the short-term, according to initial results from the POSITIVE clinical trial.
Immunotherapy and fertility
Immunotherapy stimulates or suppresses your immune system to fight cancer. Immune checkpoint inhibitors are a type of immunotherapy drug used to treat some types of cancer. The effects of immunotherapy on fertility and pregnancy are still being studied. If your doctor recommends immunotherapy, ask what is known about the type of immunotherapy you will be receiving and how it may affect your fertility.
Radiation therapy and fertility
Radiation therapy destroys cancer cells or slows their growth. Radiation to your reproductive organs, pelvic region, or central nervous system can affect your fertility. The dose of radiation, the part of your body that received radiation, and your age all play a role in whether your fertility is affected. The type of radiation therapy used is also a factor. For example, proton beam radiation therapy and intensity-modulated radiation therapy may have less of an effect on your fertility than standard radiation therapy.
Pelvic region: Radiation therapy to or near reproductive organs (such as your cervix, fallopian tubes, ovaries, uterus, vagina, and vulva) in your pelvic region can damage ovaries or destroy eggs and may cause primary ovarian insufficiency. Radiation therapy near the uterus can affect the blood flow or cause scarring, which may cause infertility or increase the risk of pregnancy-related complications.
Central nervous system: Radiation therapy to your brain may affect glands that send signals to the ovaries to make the hormones estrogen and progesterone that are needed for ovulation. Learn about procedures that can help protect your fertility during radiation therapy in the section Fertility preservation methods for females.
Stem cell transplant and fertility
Before having a stem cell transplant, also called a bone marrow transplant or hematopoietic cell transplant, you may receive high doses of chemotherapy, radiation therapy, or both. Because these treatments can damage the ovaries, they may cause primary ovarian insufficiency or infertility. Researchers are studying reduced-intensity conditioning regimens to determine if they can lower the risk of primary ovarian insufficiency or infertility.
Surgery and fertility
The type, size, and location of the tumor are important factors in whether surgery affects your fertility. Some cancers may require surgeries that could affect your fertility:
- Gynecologic cancers (cancers of the female reproductive system). Cervical, ovarian, and uterine cancers may require hysterectomy, which is surgery to remove the uterus. You may no longer be able to carry a pregnancy. An oophorectomy, which is surgery to remove one or both ovaries, may cause infertility if both ovaries are removed.
- Cancers in the abdomen or pelvis. Anal, bladder, colon, and rectal cancers may require surgery that can harm nearby organs. These surgeries may cause adhesions, which are scar-like tissues that form between organs and tissues. Adhesions can prevent eggs from travelling from the fallopian tubes to the uterus or prevent implantation. Surgeries, such as a radical cystectomy, also sometimes remove reproductive organs, which can cause infertility.
Talk with your doctor to learn whether the advised surgery may affect your fertility.
Targeted therapy and fertility
Targeted therapy uses drugs or other substances to block proteins or other molecules that control how cancer cells grow, divide, and spread. While the effects of targeted therapies on fertility and pregnancy are still being studied, some studies have found that tyrosine kinase inhibitors (TKIs) may affect fertility. Research has also found that targeted therapy may affect organs in the endocrine system, such as the thyroid, which may lower your fertility. If targeted therapy is recommended, talk with your doctor about how the specific type of targeted therapy may affect your fertility.
Primary ovarian insufficiency (POI) and cancer treatment
Cancer treatments can cause the ovaries to stop working properly, which can affect hormone levels and the functioning of follicles. This can lead to a condition called primary ovarian insufficiency. Sometimes females diagnosed with primary ovarian insufficiency still ovulate and have irregular or occasional menstrual periods after cancer treatment. Other times, damage to your ovaries is permanent and you experience early menopause. Your doctor can help you understand what to expect based on your specific cancer treatment.
Symptoms of primary ovarian insufficiency may be more intense than in natural menopause and may include:
- lack of concentration
- hot flashes and night sweats; learn more about hot flashes and night sweats
- irregular or no menstrual periods
- joint pain (arthralgia) and muscle aches
- mood swings or disorders, feeling sad or irritable
- sleep problems and disorders; learn more about sleep problems related to cancer treatment
- vaginal dryness
- loss of libido
Primary ovarian insufficiency can also cause long-term health problems such as low bone mineral density, weakening of the bones (osteoporosis), and increased risk of heart and cardiovascular problems. Learn more in Late Effects of Cancer Treatment, for adults, and Late Effects of Treatment for Childhood Cancer, for children who have been treated for cancer.
Cancers that may affect your fertility
Some cancers pose a risk to your fertility. Gynecologic cancers such as ovarian cancer and cervical cancer as well as endometrial, fallopian tube, uterine, and vaginal cancers can impact your fertility because treatment often involves removing all or part of a reproductive organ.
Cancers that affect young people, including children, adolescents, and young adults, are also associated with fertility-related problems. These cancers include breast cancer, lymphoma, leukemia, and central nervous system tumors. Learn about cancer in adolescents and young adults and cancers in children.
Fertility preservation methods for females
Fertility preservation is the process of saving or protecting your eggs, embryos, or reproductive tissue in order to have biological children in the future. Fertility treatments and procedures that help you achieve pregnancy are also called assisted reproductive technology. These procedures may be available at the hospital or cancer center where you receive treatment or at a fertility clinic. You can also learn about fertility clinics in the Oncofertility Consortium’s Clinic Finder.
The success rate, financial cost, and availability of these procedures vary. Learn about states that require insurance companies to cover fertility preservation methods.
If you choose to preserve your fertility, your doctor and a fertility specialist will work together to help you develop a cancer treatment plan that includes fertility preservation. Your health care team will advise you on the timing of fertility procedures and advise you on the impact, if any, of delaying the start of cancer treatment to undergo a fertility preservation procedure.
Embryo freezing
Embryo freezing, also called embryo banking or embryo cryopreservation, is the process of freezing and storing embryos for use in a future pregnancy. Embryo banking often starts with hormone level tests and an ultrasound. You then receive fertility drugs that stimulate your ovaries to mature eggs, which are harvested, fertilized with sperm in the lab to form embryos, and frozen for future use. When you are ready to try to become pregnant, the frozen embryos are thawed and placed in the uterus. This process is called in vitro fertilization (IVF). IVF is the most common type of assisted reproductive technology.
Egg freezing
Egg freezing, also called egg banking, egg cryopreservation, or oocyte cryopreservation, is a procedure which removes mature eggs from the ovary and freezes them. Later, when you are ready to try to become pregnant, the eggs can be thawed, fertilized with sperm in the lab to form embryos, and placed in your uterus.
Gonadotropic-releasing hormone agonist (GnRHa)
Gonadotropin-releasing hormone agonists (GnRHa) are drugs that cause the ovaries to shut down and stop making estradiol, a form of estrogen. GnRHa is sometimes used as a hormone therapy for breast cancer in premenopausal females.
Ovarian shielding
Ovarian shielding, also called gonadal shielding, is a procedure used to protect the ovaries during radiation therapy. Lead shields, also called lead aprons, are protective garments that placed over the ovaries and other reproductive organs to reduce the risk of scatter radiation.
Ovarian tissue freezing
Ovarian tissue freezing, also called ovarian tissue banking and ovarian tissue cryopreservation, refers to surgical removal and cryopreservation of egg-containing ovarian tissue. This tissue is later thawed and placed back into your body, for hormone production and egg release to resume. Females who have undergone ovarian tissue freezing and reimplantation have conceived with or without assistance.
Ovarian tissue freezing is a fertility preservation method for:
- girls who have not yet gone through puberty
- females who are advised not to delay cancer treatment for a fertility preservation procedure
- females who are advised not to receive hormonal treatments that are needed for some types of fertility preservation procedures
Ovarian transposition (oophoropexy)
Ovarian transposition, also called oophoropexy, is an operation to move the ovaries, and sometimes the fallopian tubes, away from the area receiving radiation. This can lower their exposure to radiation. This procedure may be done during surgery to remove the cancer.
Radical trachelectomy (radical cervicectomy) for cervical cancer
Radical trachelectomy (also called radical cervicectomy) is surgery used to treat women with early-stage cervical cancer who would like to become pregnant in the future. This operation removes the cervix, nearby tissue, the upper part of the vagina, and sometimes lymph nodes. The uterus, fallopian tubes, and ovaries remain in place. The uterus is then attached to the lower part of the vagina, with a special band that helps keep the uterus closed during pregnancy.
Using birth control during cancer treatment
Even though cancer and cancer treatments can lower your fertility, there may still be a chance you could become pregnant. Some cancer treatments may be harmful or cause a miscarriage. Your doctor may advise using a method of birth control during treatment for cancer.
Questions to ask your doctor about fertility
Before starting treatment, talk with your doctor about whether the recommended cancer treatment may affect your fertility:
- Could the proposed cancer treatment make it more difficult to become pregnant or carry a pregnancy in the future? Are there other cancer treatments that might not cause infertility or might cause fewer fertility problems?
- Would you recommend a fertility specialist, such as a reproductive endocrinologist, that I could talk with to learn more about methods to preserve my fertility?
- Would you recommend a social worker who could help me understand issues related to insurance coverage and cost of fertility preservation methods?
- Which fertility preservation method(s) do you advise for me? What fertility preservation methods are available at this hospital? At a fertility clinic?
- What methods of birth control should I use during treatment?
After completing treatment, ask your doctor:
- Should I use a method of birth control after treatment? If so, for how long?
- What are the chances that people who have this treatment become pregnant in the future?
- If fertility changes are temporary, how long might it take for my fertility to return?
Getting personalized care and support
It’s important to make decisions that reflect what is important to you. If having biological children is important, talk with your health care team about how the proposed cancer treatment may affect your ability to become pregnant. Keep in mind that a growing number of hospitals have cancer patient navigator programs to help you learn about fertility-related resources, including fertility specialists and financial support.
If you are the parent of a young girl or teen with cancer, this video of fertility options for young female cancer patients from the Children's Hospital of Philadelphia may help you talk with your daughter’s health care team.
While many people want to have children at some point in their life, others don’t or choose to build families in different ways. For support reach out to your health care team, as well as to professionally led cancer support groups. Learn more about coping with cancer at Emotional Support for Young People with Cancer.
Finding resources, financial support, and clinical trials
These organizations have information about fertility preservation options for people with cancer, as well as access to patient navigators and information about the costs of these procedures:
- Oncofertility Consortium: Learn about fertility preservation options, connect with a patient navigator, and find community resources.
- Alliance for Fertility Preservation: Comprehensive information on fertility preservation, including answers to commonly asked questions and laws and legislation in some states that require insurance companies to cover the cost of fertility preservation for people with cancer.
- Livestrong Fertility: Access financial support and find a fertility clinic in your area. Learn about a financial discount program for qualifying cancer patients.
Use the clinical trials search to find NCI-supported clinical trials related to fertility and cancer treatment. Clinical trials supported by other organizations can be found at ClinicalTrials.gov.