Cancer immunotherapy has emerged as a powerful tool in cancer treatment. The key insight was that a patient’s own immune system could be harnessed to recognize and destroy cancer cells. Thanks to decades of NCI- and NIH-funded basic research, this insight has led to new treatments that have saved or extended the lives of many patients.
Scientists are working diligently to improve current immunotherapy approaches, and promising new areas of opportunity are being identified. For example, researchers are exploring the use of additional types of immune cells as cancer interventions and investigating how microbiomes, the communities of microbes that inhabit the gut and other tissues, shape the immune system and responses to cancer treatment.
Scientists envision this research leading to a future when a patient’s cancer cells, the cellular composition of their tumor, and the status of their immune system and gut microbiome will be molecularly characterized. This information will inform treatment decisions and monitoring of treatment responses. Such a comprehensive analysis of a patient and their cancer may point to combinations of treatments that target multiple factors and offer a better chance for a cure.
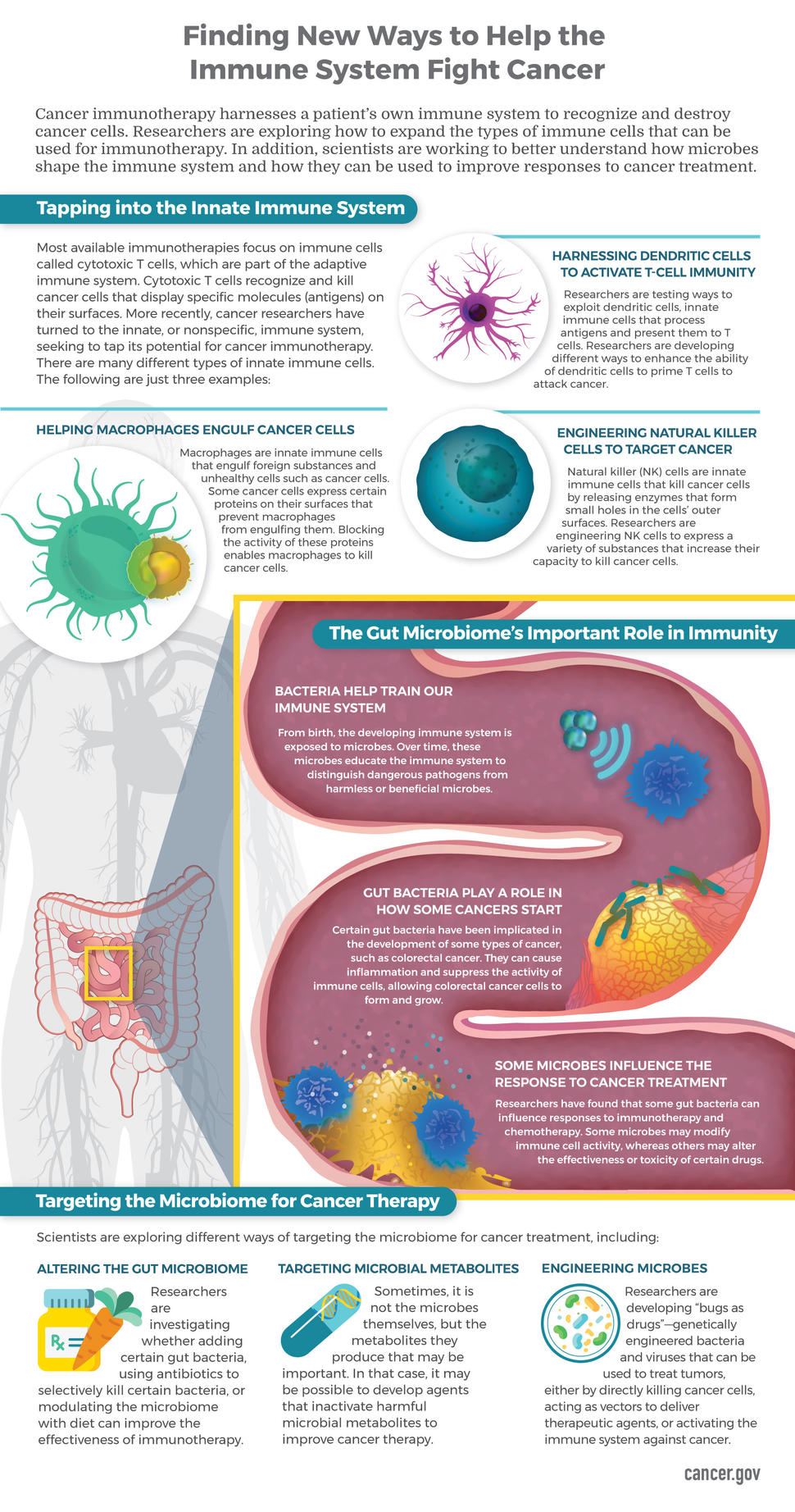
Cancer Immunotherapies: Tapping into Innate Immunity
Most available immunotherapies, such as immune checkpoint inhibitors and chimeric antigen receptor (CAR) T-cell therapies, focus on immune cells called cytotoxic T cells, which are part of the adaptive, or specific, immune system. Cytotoxic T cells recognize and kill cancer cells that display specific molecules (antigens) on their surfaces.
Cancer researchers have turned to the innate, or nonspecific, immune system, as well, seeking to tap its potential for cancer immunotherapy. The innate immune system provides the first line of defense against infections and abnormal cells. This defense does not require the recognition of antigens. However, once an innate immune response has been initiated, an adaptive immune response is stimulated, and both work together to eliminate infections or other threats to the body.
NCI-funded investigators have recently found new ways to leverage the innate immune system against cancer and manipulate it to improve cancer immunotherapy. For example:
Harnessing Dendritic Cells to Activate T-Cell Immunity
A group of NCI-funded researchers at the University of Pennsylvania has found a way to exploit dendritic cells, innate immune cells that process antigens and present them to T cells. Dendritic cells often express a protein called CD40, which triggers a cascade of biochemical reactions that prime T cells to attack tumor cells. In a mouse model of pancreatic cancer, activating CD40 in dendritic cells altered the microenvironment of the tumor, caused an expansion of T cells within it, and led to tumor destruction. Based on this work, clinical trials are underway in patients with pancreatic cancer, a difficult-to-treat disease that has thus far been resistant to immunotherapy approaches.
Helping Macrophages Engulf Cancer Cells
Macrophages are innate immune cells that engulf and digest cancer cells, cell debris, bacteria, and other foreign substances. Normal cells are protected from being eaten by macrophages because they display a protein called CD47 on their surface. In effect, CD47 is a “don’t eat me” signal to macrophages. Many cancer cells, however, also display CD47 on their surface, protecting them from macrophages. NCI-funded researchers at Stanford University and their collaborators have developed an antibody, now being tested in clinical trials, that blocks CD47, making cancer cells susceptible to attack and engulfment by macrophages. Read how Allen from Maryland benefited from this experimental drug. In addition, another group of University of Pennsylvania researchers recently demonstrated that the metabolism of macrophages can be “rewired,” enabling them to eat cancer cells even if they express CD47.
Engineering Natural Killer Cells to Target Cancer
Natural killer (NK) cells are yet another type of innate immune cell that recently have been used to treat cancer. For example, researchers are engineering CAR NK cells to improve their ability to kill cancer cells. Using NK cells overcomes a limitation of CAR T-cell therapy: the fact that personalized CAR T cells have to be made from the patient’s own cells. CAR NK cells can be made from another person’s blood cells and, so far, seem to cause fewer side effects than CAR T cells. A trial testing CAR NK cells in patients with B-cell lymphoma has just begun at the University of Texas MD Anderson Cancer Center.
Microbes: An Army of Helpers?
In the past several years, research on the microbiome has grown exponentially. Scientists have found that the microbiome is essential in shaping the development of innate and adaptive immunity and, in turn, the immune system shapes the microbiome.
Now, NCI-funded researchers are working to gain a better understanding of how the microbiome influences cancer development and the response to therapy. Recent findings show the promise of this emerging area of research:
Removing Microbes That Suppress Immunity in Liver Cancer
Metabolites produced by gut microbes appear to play an important role in antitumor immunity. For instance, a recent study conducted by scientists in NCI’s intramural research program showed that, in mice, the modification of bile acids by a particular type of gut bacteria species (Clostridium) can suppress innate immune cells called natural killer T (NKT) cells and inhibit their ability to control the growth of liver tumors. When the investigators used antibiotics to selectively kill the bacteria, NKT cells accumulated in the liver and inhibited liver tumor growth. Based on this laboratory research, a clinical trial initiated at the NIH Clinical Center is testing a combination of the antibiotic vancomycin, which kills Clostridium species, with other drugs that enhance antitumor immune responses.
Identifying Microbes That Enhance (or Suppress) the Response to Cancer Therapy
NCI-funded researchers have revealed associations between the gut microbiome and responses to cancer immunotherapy. For example, investigators at MD Anderson and the University of Chicago have found that certain types of gut bacteria in patients with cancer are associated with clinical responses to immune checkpoint inhibitors. Research is shedding light on how these microbes might exert their effects, including by influencing the function of dendritic cells and their ability to initiate an attack by the adaptive immune system.
Disrupting Microbes to Prevent and Treat Colon Cancer
Researchers have also discovered that certain microbes are associated with the development of cancer. For instance, the bacterium Fusobacterium nucleatum is strongly associated with colorectal cancer. NCI-funded research indicates that this bacterial species affects the activity of both innate and adaptive immune cells, leading to the development of an immunosuppressive tumor microenvironment and promoting colorectal cancer progression. Scientists are using this knowledge to develop cancer prevention and treatment strategies aimed at disrupting the effects of this bacterium.
The Path to New Therapies: First, Answer Fundamental Questions
NCI-funded research on innate immunity and the interactions between resident microbial species and the immune system is revealing many new opportunities for additional progress against cancer. Achieving a better understanding of how bacteria interact with immune cells in patients with cancer will lead to entirely new therapeutic approaches, as well as improvements in existing treatments. In the future, it may even be possible to develop "bugs as drugs," using genetically engineered microbes to promote potent antitumor immune responses.
To build on the progress that has been made, additional research is necessary to answer many fundamental questions: What bacterial species positively or negatively influence antitumor immune responses? What are the mechanisms by which bacteria exert their effects on the immune system in the context of cancer? How does altered composition of the bacterial species found in the gut influence susceptibility to cancer? What is the role of diet in these processes? Can other components of the innate immune system be harnessed for cancer therapy?
In addition, new resources, including better cancer models, are needed to support additional basic research and preclinical drug development. Technologies that enable analyses of single tumor and immune cells and advanced tumor imaging will drive progress in this emerging area of research. In addition, ongoing collaborative efforts such as the Human Tumor Atlas Network will provide researchers with dynamic, detailed information about tumors and the components of their microenvironments.
Researchers have only scratched the surface in understanding the complexity of the immune system and microbiomes in the context of cancer. With continued investment in these areas, scientists will discover new strategies to prevent cancer and improve the lives of people who develop it.