Newly Discovered ‘Don’t Eat Me’ Signal May be a Target for Cancer Immunotherapy
, by NCI Staff
One property of cancer cells that can help them gain and maintain a foothold in the body is their ability to evade detection and destruction by the human immune system. Some tumor cells, for example, make higher-than-normal amounts of proteins called “don’t eat me” signals, which are found on the cell surface.
These “don’t eat me” proteins are a type of immune checkpoint. They are “like invisibility cloaks for the cancer,” preventing white blood cells called macrophages from detecting, engulfing, and devouring the tumor cells, explained Irving Weissman, M.D., of Stanford University School of Medicine.
In a new study, Dr. Weissman and his colleagues have discovered that a protein called CD24 is a new “don’t eat me” signal that they believe is a potential target for cancer immunotherapy.
The team’s findings suggest that ovarian cancer and triple-negative breast cancer, both of which are notoriously hard to treat, are among the cancers that could be targeted by blocking CD24, said Amira Barkal, an M.D., Ph.D. student at Stanford and lead researcher on the new study, which was published July 31 in Nature.
Further work is needed to better understand CD24’s role in human tumors and develop drugs that can block its activity, said Susan McCarthy, Ph.D., of NCI’s Division of Cancer Biology. But the study “provides real possibilities” that targeting CD24 might help a patient’s immune system act more strongly against certain cancers, including ovarian cancer and triple-negative breast cancer, for which immunotherapy has so far shown limited efficacy, Dr. McCarthy said.
Most types of immunotherapy already in use target white blood cells called T cells, which are key components of the body’s second line of immune defense, known as adaptive immunity. By contrast, macrophages, whose action CD24 suppresses, are part of the innate immune system—the body’s first line of defense against infections and abnormal cells.
The new findings, Dr. McCarthy said, are a reminder that macrophages, and not just T cells, can eliminate cancer cells.
Searching for New ‘Don’t Eat Me’ Signals
The usual purpose of “don’t eat me” signals is to keep macrophages from attacking normal cells in the body. In the past decade, Dr. Weissman’s lab has identified three other “don’t eat me” proteins, PD-L1, CD47, and B2M, that cancer cells use to evade macrophages.
In addition to serving as a “don’t eat me” signal to macrophages, PD-L1, which is present on some tumor cells, binds to the PD-1 immune checkpoint protein on T cells to turn down the adaptive immune response.
Treatments that target PD-L1 on tumor cells are already used to treat some types of cancer, and antibodies that block CD47 are in early-stage clinical trials. (Dr. Weissman is a co-founder, director, and consultant at a company that holds licenses for CD47-based discoveries.)
Because not all patients with cancers that express CD47 respond equally to CD47-blocking antibodies, Barkal and others in Dr. Weissman’s lab suspected that some tumor cells had additional, as-yet-unknown “don’t eat me” proteins.
To search for new “don’t eat me” proteins, the team took advantage of the fact that all three previously identified proteins of this type produce similar types of signals inside macrophages. These signals suppress the ability of macrophages to engulf cells, Dr. Weissman said.
“We started looking in a variety of human cancers to see whether there were additional molecules that used this same type of signaling mechanism and were present at high levels in different tumor types. And that’s how we homed in on CD24,” Barkal said.
Using data from The Cancer Genome Atlas program and NCI’s TARGET program, the Stanford team found that expression of the CD24 gene was higher in tumors than in the corresponding normal tissue. CD24 expression was elevated most dramatically in ovarian cancer. It also was markedly higher in triple-negative breast cancer than in healthy breast cells or in estrogen- and progesterone receptor–positive breast cancers.
In further experiments, the team showed that macrophages that infiltrate tumors interact with CD24 through a receptor called Siglec-10.
The Case for Blocking CD24
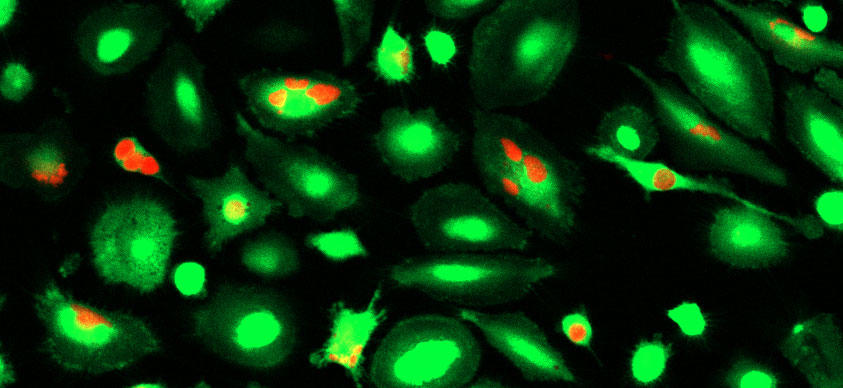
To find out whether CD24 is a new “don’t eat me” protein, the researchers used a gene-editing tool to remove the gene that makes the CD24 protein from a lab-grown human breast cancer cell line. They then mixed the CD24-deficient cells and normal breast cancer cells with human macrophages in lab dishes.
“We saw that cells that were deficient in CD24 were engulfed and eaten, or cleared, by the macrophages much more readily than the cancer cells that had CD24,” Barkal said.
Treating tumor cells that express CD24—including those from patients with metastatic ovarian cancer or triple-negative breast cancer—with an antibody that blocks the interaction between CD24 and its receptor on macrophages, Siglec-10, also boosted the ability of macrophages to clear the tumor cells. By contrast, the CD24-blocking antibody had no effect on macrophage clearance of tumor cells that lacked CD24.
Experiments in mice further linked CD24 to tumor growth and showed that it can stop macrophages from attacking tumor cells. In addition, in mice with already-established tumors, treatment with five doses of the CD24-blocking antibody over 2 weeks reduced tumor growth compared with treatment with a non-CD24–targeted (control) antibody.
Because some tumor cells may have elevated levels of both CD24 and CD47, the team also tested the effects of combined treatment with CD24- and CD47-blocking antibodies on various tumor cell types in laboratory experiments.
The findings suggest that blocking multiple “don’t eat me” proteins may be more effective in treating some cancers, the research team concluded, and that CD24 is the dominant “don’t eat me” signal in other cancers, including ovarian cancer and triple-negative breast cancer.
And because CD24 levels are markedly higher in some tumors than in the corresponding normal tissue, Barkal said, CD24-blocking therapies should be able to target and eliminate tumor cells without harming healthy cells.
“This study is important because we already had indications from earlier studies that if a tumor cell has multiple ‘don’t eat me’ proteins, then you’re going to have to block multiple proteins and figure out which ones are critical for each cancer type,” Dr. McCarthy said.
Plotting a Path Forward
To follow up on the new findings, Barkal said, “We will need to analyze more primary tumors from patients to figure out the extent to which CD24 is present—and found at elevated levels—in breast, ovarian, and other cancers.”
Future work will also need to determine whether there are additional “don’t eat me” signals that should be blocked, Dr. Weissman said. Identifying which ones are expressed in each cancer type, as well as in different patients with the same tumor type, he said, may help predict which patients will respond to specific therapies that block these signals, he said.
In the longer term, the team is hoping to develop a CD24-blocking antibody that is safe for use in people and begin testing it in patients, Barkal said.
“We still don’t know whether CD24 blockade will be effective on its own in some cancers or whether it will need to be combined with other immunotherapies or other anticancer drugs to optimize the effect,” Barkal said.
In addition, Dr. Weissman cautioned, while the team used a specific type of human macrophage in their laboratory experiments, the situation in patients is likely to be more complicated. The human immune system includes many macrophage subtypes, and individual human tumors may contain more than one of these, he said.