February 2020 - Cancer Currents Blog
-
New Approach Uses Much Less Tissue to Analyze Tumor Proteins and Genes
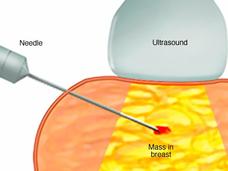
Researchers have developed a “microscaled” approach to analyze the proteins and genetic changes (proteogenomics) of a tumor that uses tissue from a core needle biopsy. The analyses can provide important information that may help guide treatment.
-
Mouse Study Points to Strategy for Preserving Bone During Chemotherapy
Bone loss associated with chemotherapy appears to be induced by cells that stop dividing but do not die, a recent study in mice suggests. The researchers tested drugs that could block signals from these senescent cells and reverse bone loss in mice.
-
Remodeled CAR T-Cell Therapy Reduces Side Effects in First Clinical Trial
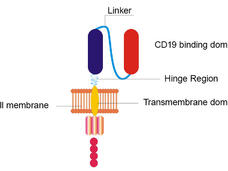
A remodeled CAR T-cell therapy causes fewer neurologic side effects and is equally effective as the original form of the treatment, according to results from the first clinical trial testing the approach in patients with B-cell lymphomas.
-
VA Study Finds No Disparities in Prostate Cancer Deaths with Equal Access to Care
In the Veterans Affairs health care system—where all patients have equal access to care—African American men did not appear to have more-aggressive prostate cancer when diagnosed or a higher death rate from the disease than non-Hispanic white men.
-
Artificial Intelligence Expedites Brain Tumor Diagnosis during Surgery
A method that combines artificial intelligence with an advanced imaging technology can accurately diagnose brain tumors in fewer than 3 minutes during surgery, a new study shows. The approach can also accurately distinguish tumor from healthy tissue.
-
Is Proton Therapy Safer than Traditional Radiation?
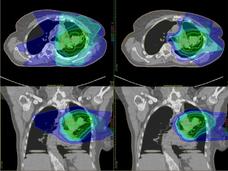
Some experts believe that proton therapy is safer than traditional radiation, but research has been limited. A new observational study compared the safety and effectiveness of proton therapy and traditional radiation in adults with advanced cancer.
-
Avapritinib Approved to Treat GIST with a Rare Gene Alteration
Avapritinib (Ayvakit) has been approved for adults with gastrointestinal stromal tumors (GIST) whose tumors have an alteration in a portion of the PDGFRA gene called exon 18. The approval applies to those whose tumors cannot be removed with surgery or have spread.