Drug Combination Shrinks Duodenal Polyps in People with Familial Adenomatous Polyposis
, by NCI Staff
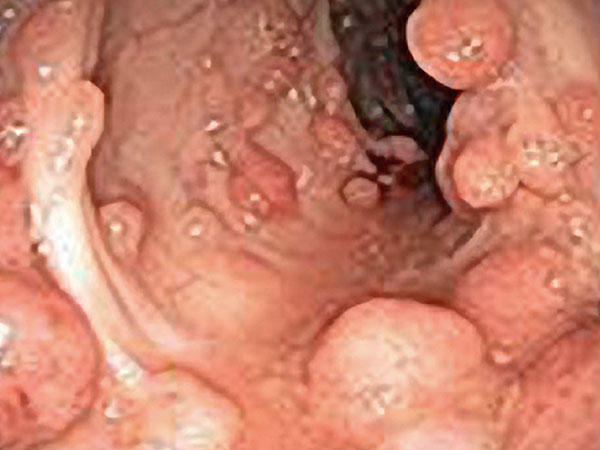
In a small clinical trial of people with an inherited condition that greatly increases the risk of developing gastrointestinal cancers, a two-drug combination has been shown to shrink duodenal polyps, precursor lesions for cancer, raising the possibility that the regimen could lower the risk of duodenal cancer.
In the randomized trial, patients with the condition, familial adenomatous polyposis (FAP), who took erlotinib (Tarceva®) and sulindac (Aflodac®) had far fewer precancerous polyps in the duodenum—the first section of the small intestine, just beyond the stomach—than patients who took placebo. The benefit was so pronounced that the trial was stopped early, after only two-thirds of the planned number of participants had completed treatment.
The trial results were published March 22 in the Journal of the American Medical Association.
First Study to Show Duodenal Polyp Reduction
Patients with FAP have inherited a mutation in a gene called APC, which normally suppresses abnormal cell growth in the digestive tract. As a result, they can develop hundreds to thousands of polyps in their colon, rectum, and duodenum during their lifetimes, explained Asad Umar, D.V.M., Ph.D., chief of the Gastrointestinal and Other Cancers Group in NCI’s Division of Cancer Prevention.
Some of these polyps can develop into cancer. Patients with FAP have a nearly 100 percent risk of developing colorectal cancer and often undergo colectomy—removal of the entire colon—once the polyp burden and associated risk of cancer grows too high.
Up to 12 percent of patients with FAP will also develop duodenal cancer. Non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and sulindac, given alone, have been shown to slow the growth of polyps in the colon of patients with FAP, but not in the duodenum.
Lead author N. Jewel Samadder, M.D., and his colleagues from the University of Utah recruited 92 patients with FAP into the study. Half were randomly assigned to receive erlotinib and sulindac daily for 6 months, and half to receive a placebo. The total polyp burden in the duodenum—calculated as the sum of the polyp diameters in a 10-centimeter length of the duodenum—was measured by endoscopy before and after the 6 months of treatment. Side effects were tracked by phone interviews every 2 weeks for the first 3 months.
Almost three-quarters of the patients given the drug combination required a reduction in erlotinib dose during the study, most often because they had developed a painful rash.
After 67 participants had completed 6 months of treatment, the trial’s data and safety monitoring board recommended that the study stop enrolling patients. In the placebo arm, there was an average of an 8 millimeter increase in total polyp burden (the sum of the diameters of all polyps in millimeters), while patients receiving erlotinib and sulindac had an average decrease of 8.5 millimeters. As a percentage of change, the polyp burden increased by 30.6 percent in the placebo group and decreased by 37.9 percent in the erlotinib/sulindac group.
This study “is the first time we’ve seen significant efficacy using any kind of intervention for advanced adenoma regression in duodenal polyps for FAP patients,” said Dr. Umar.
Unanswered Questions
The researchers picked this particular drug combination because it has the potential to target two different molecular pathways known to drive duodenal adenoma progression, explained Deborah Neklason, Ph.D., of the Huntsman Cancer Institute, one of the study’s lead authors.
Previous molecular studies have suggested that a combination of inactivation of the APC gene (as seen in FAP) and signaling by the EGFR pathway increases the activity of another molecular pathway—the COX-2 pathway—which promotes polyp formation. The COX-2 pathway is suppressed by sulindac and erlotinib targets the EGFR pathway.
“We talk a lot about precision medicine…this is really precision prevention,” she said. “We know these individuals have a mutation in the APC gene, and we know these pathways talk to [the APC] pathway.”
In a study within the trial that used tissue samples from seven patients, the researchers showed that the EGFR pathway was indeed suppressed as predicted.
One interesting question going forward, commented Dr. Umar, is whether erlotinib alone might be able to reduce the polyp burden to the same extent as erlotinib plus sulindac.
Researchers are interested in looking at this question, said Dr. Neklason. “But we believe that this trial was successful because we were able to block two separate [molecular] pathways that talk to each other.”
Dr. Neklason and her team are currently planning trials to try to answer two additional questions raised by their results: whether a lower dose of erlotinib, with potentially fewer side effects, would be as effective at suppressing polyp growth; and what sort of dosing schedule would best balance effectiveness and convenience for patients. They hope to test both 12 months of continuous administration and a dose-cycling schedule where the drugs are given once or twice a week instead of every day.
Longer-term studies may also help answer whether the drug combination prevents new polyps from forming in addition to shrinking existing polyps.
“And the long-term question that I think is probably the most important is clinical outcomes, including do [these drugs] truly prevent these individuals from getting cancer?” said Dr. Neklason.
An answer to that question could take follow-up of a decade or longer, she continued, so a more practical endpoint for future trials may be whether this approach helps patients with FAP avoid the repeated, debilitating surgeries that often have to be done to remove large duodenal adenomas.
“Those surgeries lead to a lot of scarring and a lot of complications…so it would be important to see whether we can actually prevent or delay those interventions.”