COVID-19, Vaccines, and the Immune System: Emerging Research from NCI’s SeroNet
, by Nadia Jaber
Since April 2020, NCI has led and funded numerous research studies and clinical trials of COVID-19. Part of that effort includes NCI’s Serological Sciences Network (SeroNet), which is leading studies to better understand how the immune system responds to the virus that causes COVID-19 (SARS-CoV-2) and to COVID-19 vaccines. In this Q&A, SeroNet leaders Samantha Finstad, Ph.D., and Juli Klemm, Ph.D., discuss emerging research findings from the network. Please note that many of the studies referenced in this article are “preprints,” meaning they have not been peer reviewed.
What are COVID-19 antibodies, and who might have them?
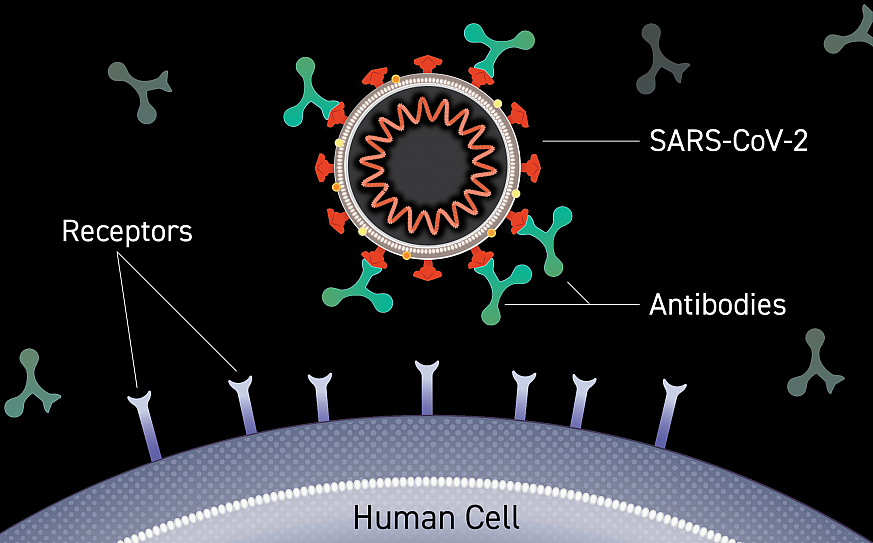
Dr. Klemm: Antibodies are small proteins in the blood that recognize and attach to viruses, bacteria, and other organisms that cause disease. Neutralizing antibodies are those that bind to a virus and interfere with its ability to infect a cell, so they are really important to understand in the context of COVID-19.
From SeroNet studies, we know that most people who have recovered from COVID-19 or who have been vaccinated against COVID-19 develop neutralizing antibodies against the virus. In one small study, all 25 study participants had detectable neutralizing antibodies 57 days after a single dose of the Janssen/Johnson & Johnson vaccine.
How long do those antibodies last in people who had COVID-19?
Dr. Finstad: It appears that antibodies to SARS-CoV-2 last for at least several months. One SeroNet study found that people who had mild to moderate COVID-19 had neutralizing antibodies for at least 5 months. Another found similar results—people who recovered from COVID-19 had neutralizing antibodies for 6 months.
A separate SeroNet study found that nearly all participants who recovered from COVID-19 had memory B cells targeted to SARS-CoV-2. Memory B cells are immune cells that can remain in our bodies for years and can rapidly produce more antibodies if we encounter the virus again. So this finding suggests that most people have a lasting immune response to COVID-19.
Do we need a certain level of COVID-19 antibodies to be protected from a future infection? Will we eventually need a booster shot for the COVID-19 vaccines?
Dr. Finstad: We don’t know that level yet. But given the evidence so far, public health experts are suggesting booster shots for the COVID-19 vaccines. This guidance is awaiting authorization from CDC and FDA.
More research is needed to determine what level of COVID-19 antibodies is protective, and this is an active area of investigation within SeroNet. For measles, for example, antibody testing is used as a surrogate measure to determine whether you're protected from a future infection—it’s called a correlate of protection. If you are above that antibody level, you're likely protected. And vice versa—if you fall below that level, your physician will likely give you a booster to raise your antibody levels again.
By following individuals over time through SeroNet studies, we'll be able to take a look at the antibody levels that correlate with protection. For COVID-19 vaccines, protection means you are protected from developing severe disease, but it doesn’t mean you can’t get infected. One SeroNet group actually wrote a very helpful article that explains how the vaccines can protect against illness but don’t completely prevent you from getting infected.
A huge challenge with finding correlates of protection for SARS-CoV-2 is that we haven’t had a way to compare measurements of antibody levels between studies. That's a big gap in the field that NCI is trying to address with the SARS-CoV-2 serology standard. The standard is a pooled sample of plasma from four donors who have COVID-19 antibodies. Researchers who use the standard can start to compare people’s antibody levels across different studies, even if they’re using different antibody tests.
Dr. Klemm: Recently, researchers at the Frederick National Laboratory for Cancer Research calibrated the standard to a unit of measure approved by the World Health Organization. And now we're advocating for everyone to report their results in these units so that we have a way to compare the measurement of antibody levels across international studies.
[Editor’s note: The Serology Lab at Frederick National Lab, a component of SeroNet, provides expertise and leadership on the development, validation, and standardization of serology tests for HPV and COVID-19 studies.]
What has SeroNet learned about the effects of COVID-19 vaccines on people with cancer?
Dr. Klemm: There’s evidence coming out about some cancer patients not mounting a strong response to the COVID-19 vaccines. Several SeroNet groups are following vaccinated cancer patients to measure their immune response over time. The CDC is now recommending that moderately to severely immunocompromised people who received an mRNA vaccine get an additional dose of the same vaccine. That includes people who are actively getting treatment for blood cancer, those who have received a stem cell transplant within the last 2 years, and those who are taking medicine to suppress the immune system.
Dr. Finstad: I want to mention that the effectiveness of COVID-19 vaccines is not the same for everyone with cancer. We’ve seen that some people with blood cancers and some people undergoing immunosuppressive therapies don't mount a robust antibody response. But if you're a cancer survivor—if you’ve finished treatment and are in remission—you're more likely to be able to mount a robust immune response to the vaccine.
The other thing is, the pandemic has really opened up a lot of questions, not just about COVID-19 vaccines but also about other vaccines: When would be the best time for people with cancer to be vaccinated—while they're undergoing active therapy, or should they wait until some of their treatments are done? Does vaccine response depend on what type of treatment they’re getting?
All of these questions are coming up through the research that SeroNet is doing, especially in our large population studies.
In addition to antibodies, do other parts of the immune system play a role in the response to SARS-CoV-2?
Dr. Klemm: We do talk a lot about antibodies, but SeroNet actually studies the immune response to SARS-CoV-2 more broadly. Several lines of evidence suggest that T cells play an important role in the response to the coronavirus. For example, one SeroNet study found T cells that recognize SARS-CoV-2 in blood samples obtained from people who had recovered from COVID-19.
And it appears that T cells contribute to protection from infection, at least in lab animals. In one study, nonhuman primates that lacked T cells were more susceptible to reinfection with SARS-CoV-2.
But we're just scratching the surface of understanding the T-cell response to SARS-CoV-2 here. There’s still a lot of important research to be done to answer questions about the role of T cells in fighting COVID-19.
Dr. Finstad: One of the things that makes T-cell studies more difficult to do in large populations is that tests of T cell functionality take a lot of time, are expensive, and require a larger blood sample than antibody tests.
What has SeroNet research shown about whether COVID-19 vaccines protect against variants of the virus?
Dr. Finstad: For the most part, antibodies isolated from vaccinated individuals can react with SARS-CoV-2 variants in lab tests. For example, plasma from vaccinated people or from people who recovered from COVID-19 neutralized the alpha variant. And people who received the Johnson & Johnson vaccine appear to produce antibodies that can neutralize several different variants, but at varying levels.
Another team found that T cells from people who have recovered from an infection with the original strain of SARS-CoV-2, or who are vaccinated, react to several variants of the virus.
But the delta variant is what’s on everyone’s mind right now. A new SeroNet study suggests that people who recovered from COVID-19 or who were vaccinated with the Moderna or Pfizer vaccines are still protected against the delta and kappa variants. But that protection is less than that of the original virus.
Does the immune system respond differently to a SARS-CoV-2 infection than to a vaccine?
Dr. Klemm: Studies are suggesting that the immune response to the virus may be similar to the immune response to your first dose of a vaccine. One SeroNet study found that antibody responses to a single vaccine dose in people who had COVID-19 were similar to the responses seen after two doses among people who hadn’t had COVID-19. And another study found that nursing home residents who previously had COVID-19 had a better immune response to the vaccine than those who hadn’t been infected. So, CDC’s recommendation is that people who've had COVID-19 still get vaccinated. Then the vaccine becomes almost like a booster shot, enhancing the immune response that’s already present against the virus.
Why do some people have severe COVID-19 and others have milder symptoms?
Dr. Klemm: A handful of SeroNet studies have found that the way the immune system responds to the virus correlates with disease severity. One study found that people with high levels of COVID-19 antibodies tended to have milder disease. And among children, immune responses to the virus differed between those with mild and severe COVID-19. The immune response was also different among kids who did and didn’t develop multisystem inflammatory syndrome (MIS-C) in response to SARS-CoV-2 infection, a serious condition where inflammation develops in multiple body parts. Specifically, there were differences in the levels of certain kinds of immune cells and antibodies.
While there are important findings emerging in these research studies, there's still a lot of work to be done to fully understand specific causes of disease severity.
What can antibody studies tell us about how the coronavirus spread last year and about herd immunity?
Dr. Klemm: Antibody studies can help paint a picture of the dynamics of the pandemic. With an antibody test, we can see if someone was previously infected with the virus, even if they didn’t know it at the time. And if we do that on a large scale, we can find out the percentage of people who were infected during a certain time period—that’s called the seroprevalence.
So, for example, the Serology Lab at the Frederick National Lab studied blood samples that had been previously collected from 24,000 participants of NIH’s All of Us Research Program. And they found signs of SARS-CoV-2 infections in 5 different states as far back as January 2020.
Along similar lines, a SeroNet team in New York City—where one of the first COVID-19 cases in the United States was found—did serology testing of blood samples from 10,000 people who had visited the hospital from February to April of 2020. And they found evidence that SARS-CoV-2 was circulating in New York City by mid-February 2020—earlier than originally reported.
Serology studies have also confirmed disparities in the spread of COVID-19. A study of North Carolina residents from April to October of 2020 found that people who are Latinx, Black, or who lacked health insurance had the highest odds of getting COVID-19. Racial differences in COVID-19 infection rates have been attributed to underlying inequalities in areas such as housing and access to health care.
Dr. Finstad: Serology studies of big groups can also help tell us whether we have reached herd immunity. For some other diseases, herd immunity is achieved when the seroprevalence is above 70%, but for COVID-19 we don't yet know what that number would be.
Has SeroNet research revealed any insights into possible COVID-19 treatments?
Dr. Finstad: SeroNet itself does not study treatments for COVID-19, but as we understand the immune response better, that will certainly inform treatment strategies and targets for drugs.
There’s preliminary evidence from SeroNet research that new kinds of lab-engineered antibodies and even some cancer drugs may have some potential activity as COVID-19 treatments. We're also starting to see that some immunosuppressed individuals don't mount a good immune response to the vaccines, even after additional vaccination boosts. That suggests that these people may benefit from treatment with lab-made antibodies or convalescent plasma.
What social science research is SeroNet doing?
Dr. Klemm: In addition to supporting basic science research around COVID-19 serology, SeroNet also supports social science research, with a focus on engaging groups of people who might have some hesitancy about vaccination or participation in COVID-19 research.
We have a team at the University of Massachusetts Medical School in Worcester that is using a storytelling approach to help communicate the importance of participation in COVID-19 research. Another team, at the University of Arkansas, has developed a tool for evaluating the various reasons why someone might be concerned about COVID-19 vaccines. Arkansas has relatively low vaccine uptake right now. So, they’re using the tool to study vaccine hesitancy in different social and ethnic groups, and they’re getting a more comprehensive view of this very complicated question of vaccine hesitancy.
Dr. Finstad: And what’s great is that a number of the other SeroNet investigators said, “Can we use that tool too?” And the Arkansas team said, “Absolutely!” So, the network enables rapid dissemination of research and tools so that other scientists can quickly adopt them.