Trial Yields Positive Data on Pembrolizumab for Lung Cancer, Potential Response Biomarker
, by NCI Staff
Findings from an early phase clinical trial may point to a biomarker that identifies patients with advanced non-small cell lung cancer most likely to respond to the immunotherapy drug pembrolizumab (Keytruda®).
In the trial, patients whose tumors expressed high levels of the protein PD-L1 were more likely to experience substantial reductions in their tumors following treatment with pembrolizumab than patients whose tumors had lower PD-L1 expression, according to results from an early phase clinical trial. These patients also lived longer without their cancer progressing.
These trial results were presented at the American Association for Cancer Research (AACR) annual meeting, and published simultaneously in the New England Journal of Medicine.
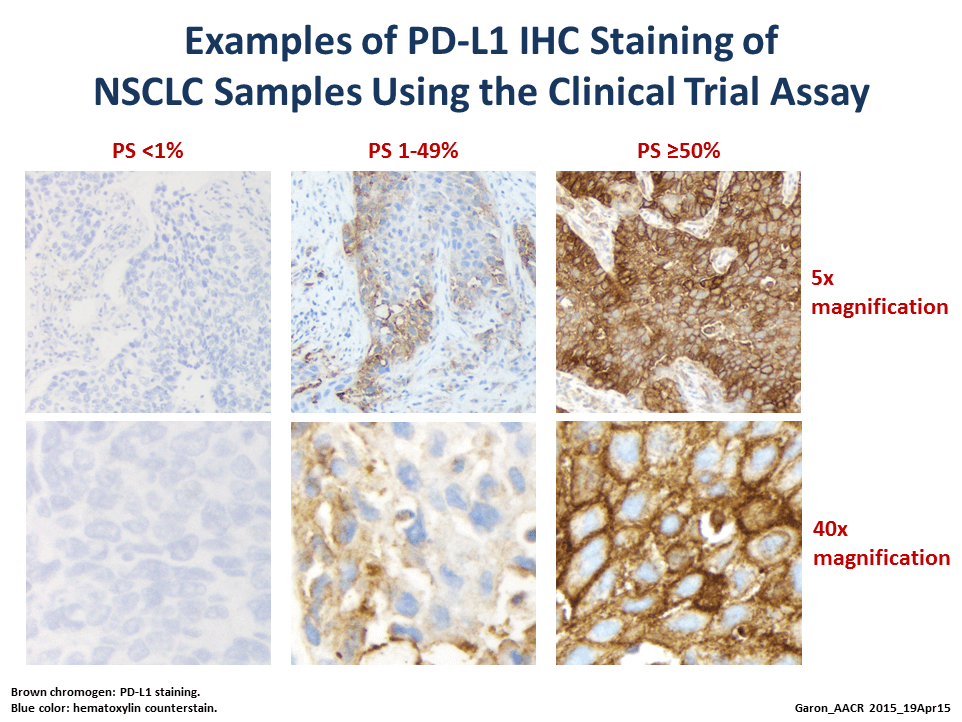
Called Keynote-001, the trial was initially designed to test the safety and efficacy of different dosing schedules of pembrolizumab, part of an emerging class of immune-based cancer therapies known as checkpoint inhibitors. But it was changed early on to also test whether PD-L1 expression levels—as measured by an investigational immunohistochemistry test—could identify patients who are most likely to respond to treatment.
Pembrolizumab targets a protein on immune cells called PD-1, one of a family of so-called checkpoint proteins that serve, in large part, to restrain the immune response. When PD-1 ligand, or PD-L1, on tumor cells binds to PD-1 on immune system cells, the PD-1 signaling pathway is activated, inhibiting an immune response. By blocking this interaction, pembrolizumab allows the immune system to recognize and attack tumor cells. It has already been approved by the FDA to treat some patients with advanced melanoma.
The results presented at the AACR meeting included those from a training set of 182 patients to establish a cut point of PD-L1 expression levels that appeared to correlate with tumor response, and an independent validation set of 313 patients to confirm that the cut point selected in the training set predicts patient response.
Based on several factors, the selected cut point was the presence of PD-L1 expression in at least 50 percent of sampled tumor cells, explained the study’s lead investigator, Edward Garon, M.D., from the UCLA Jonsson Comprehensive Cancer Center.
Of the 73 patients in the validation set whose tumors had this level of PD-L1 expression, he reported, the tumor response rate was 45.2 percent. The median progression-free survival of all patients in the trial with PD-L1 levels that exceeded this cut point was 6.3 months, and their median overall survival has not been reached.
By contrast, the response rate was 16.5 percent in patients in the validation set who had 1-49 percent of tumor cells positive for PD-L1 (103 patients) and 10.7 percent in patients with less than 1 percent tumor cells positive for PD-L1 (137 patients). The median progression-free and overall survival in these two groups was 3.3 and 8.8 months, and 2.3 and 8.8 months, respectively.
Generally, pembrolizumab was “very well tolerated,” Dr. Garon noted during a press briefing. The most common side effect was fatigue.
“It’s impressive that this assay appears to identify a group with prolonged [progression-free survival],” said Ross Camidge, Ph.D., of the University of Colorado following the presentation of the findings at an AACR plenary session.
“But it’s not a perfect assay,” he cautioned. Many patients, including those with high PD-L1 expression, still had rapid progression of their disease despite treatment with pembrolizumab, he said, and some patients with low PD-L1 expression also responded.
“But an imperfect [patient] enrichment strategy can still move the field forward,” Dr. Camidge said.
Merck has launched several randomized clinical trials to compare pembrolizumab with standard chemotherapy treatments in patients with advanced lung cancer, Dr. Garon said, including a trial enrolling previously untreated patients whose tumor cells have high PD-L1 expression.
Further Reading