With Two FDA Approvals, Prostate Cancer Treatment Enters the PARP Era
, by NCI Staff
UPDATE: According to results from a large clinical trial called TRITON3, treatment with rucaparib (Rubraca) improves how long some people with metastatic prostate cancer live without their cancer getting worse. The trial’s results were published February 23, 2023, in the New England Journal of Medicine.
The approximately 400 participants in TRITON3 had metastatic, castration-resistant prostate cancer and tumors with mutations in the BRCA1, BRCA2, or ATM genes. Among patients whose tumors had BRCA mutations, progression-free survival was 11.2 months in those treated with rucaparib and 6.4 months in those who were treated with any of three other commonly used drugs for this form of prostate cancer.
TRITON3 was a follow-up trial to TRITON2, the results of which formed the basis for FDA’s 2020 approval of rucaparib for people with metastatic, castration-resistant prostate cancer with BRCA mutations. The TRITON2 results and details of the initial FDA approval are described in the story below.
Two recent approvals by the Food and Drug Administration (FDA) have opened a new avenue of treatment for some men with prostate cancer: an expanded role for targeted therapies.
The approvals are for the drugs olaparib (Lynparza) and rucaparib (Rubraca). They cover the use of the drugs in men whose prostate cancer has spread, or metastasized, and whose disease has stopped responding to standard hormone treatments, often called castration-resistant disease. To receive either drug, men must also have specific genetic alterations that prevent their cells from repairing damage to their DNA.
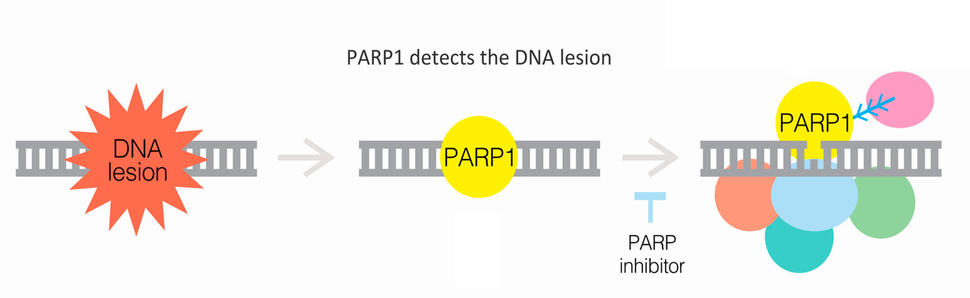
Many treatments of metastatic prostate cancer are centered around therapies that block the ability of hormones to fuel the cancer’s growth and spread. But olaparib and rucaparib, which are taken as pills, work differently. They block the activity of a protein known as PARP, which helps cells mend specific types of damage to DNA.
Studies have shown that 20%–30% of men with metastatic prostate cancer have genetic alterations that impair cells’ DNA repair mechanisms. So, to now have two new approved therapies for these men, and in such rapid succession, “is good news for patients,” said Oliver Sartor, M.D., medical director of the Tulane Cancer Center and a prostate cancer expert.
Fatima Karzai, M.D., clinic chief of the Genitourinary Malignancies Branch in NCI’s Center for Cancer Research, whose research focuses on developing new therapies for prostate cancer, agreed. The past decade has seen a boom in new treatments for prostate cancer, Dr. Karzai said. But few of them are genomic targeted therapies, those intended to work on cells with specific genetic alterations, which are now commonly used to treat other types of cancer.
“In prostate cancer, we’re now starting to see the benefits of these targeted therapies,” she continued. “I think it’s exciting.”
PARP: Prime Treatment Target for Prostate Cancer
Over the past decade, olaparib and rucaparib have become important treatments for women with ovarian and breast cancer, in whom genetic alterations that affect DNA repair processes are common. Among the most frequent such alterations are those in the BRCA1 and BRCA2 genes.
It’s no accident that researchers have identified people who have alterations in BRCA genes as ideal candidates for treatment with PARP inhibitors.
BRCA proteins and some PARP proteins are both integral components of cells’ response to DNA damage. If that response is already dysfunctional because of BRCA1 or BRCA2 mutations, then researchers reasoned that blocking the activity of PARP proteins could further hamper any chance of repair—akin to punching a hole in a tire that already has a slow leak. If the cancer cells can’t fix the DNA damage, they will die.
Prostate cancer emerged as another strong candidate for PARP inhibitors after studies suggested that alterations in BRCA1 and BRCA2, as well as other genes involved in a cell’s ability to respond to DNA damage, may be present in approximately one-quarter of men with the disease. Other studies linked these genetic changes to an increased risk of prostate cancer, as well as more aggressive disease.
Those findings, Dr. Karzai explained, led to a series of clinical trials of PARP inhibitors in men with metastatic prostate cancer, laying the foundation for the new FDA approvals.
PROFOUND Trial: Most Benefit in Men with BRCA2 Alterations
Olaparib’s approval, announced on May 19, was based on the results of a large clinical trial called PROFOUND.
The trial enrolled men with mutations in DNA repair genes and divided them into two cohorts. Cohort A included men with alterations in the BRCA1, BRCA2, or ATM genes, each of which plays an important role in DNA repair. Cohort B included men who had alterations in a group of 12 other genes that have some involvement in repairing DNA.
All of the men in the trial had cancer that had worsened despite treatment with either abiraterone (Zytiga) or enzalutamide (Xtandi), which work in different ways to block hormones in prostate cancer cells.
The 387 men in the trial were randomly assigned to either the treatment group, which received olaparib, or the control group, which received either abiraterone or enzalutamide (as selected by each patient’s oncologist).
In cohort A, men treated with olaparib lived more than twice as long without evidence of their cancer getting worse (as measured by standard imaging procedures) than men treated with abiraterone or enzalutamide: a median of 7.4 months versus 3.6 months. The treatment group in cohort A also lived longer overall, with olaparib improving survival by more than 4 months (19.1 months versus 14.7 months).
In addition, men treated with olaparib were far more likely to see their tumors shrink (a tumor response) than men treated with one of the other two drugs (33% versus 2%).
Prostate cancer tends to spread to the bones, so reducing the size of those particular tumors can have a meaningful impact on patients, according to the trial’s lead investigator, Maha Hussain, M.D., of Northwestern Medicine. “Metastases that are poorly controlled in the bone can be quite painful,” Dr. Hussain said when she presented the trial results late last year at the European Society for Medical Oncology (ESMO) annual meeting.
FDA’s approval covers the use of the drug in men with alterations in any of the DNA repair genes analyzed in the trial. But Dr. Sartor, who also was an investigator on the trial, noted that men with alterations in BRCA2 seemed to respond best to the treatment, experiencing the largest improvement in progression-free survival. And these men accounted for about one-third of trial participants. Men with ATM alterations, on the other hand, didn’t do any better than those in the control group.
In cohort B, olaparib did appear to provide some benefit in men whose tumors had alterations in some “unusual genes” involved in DNA repair, Dr. Sartor continued, including RAD54L and PALB2.
FDA also simultaneously approved two tests, BRACAnalysis CDx and FoundationOne CDx, for identifying patients with metastatic castration-resistant prostate cancer who have the appropriate genetic alterations to receive olaparib.
TRITON2 Leads to Accelerated Approval for Rucaparib
FDA’s approval for rucaparib, announced on May 15, is slightly different than what was granted to olaparib.
To begin with, it was an accelerated approval. That means the approval was granted based on results from a clinical trial that strongly suggests rucaparib could be beneficial for patients—such as an improvement in progression-free survival—although that level of proof is not yet available.
In addition, the approved use is only for men with mutations in BRCA1 or BRCA2 and only for cancer that has progressed despite earlier treatment with both a hormone-blocking treatment as well as chemotherapy.
The approval was based on the results of a 115-patient clinical trial, called TRITON2. Similar to the PROFOUND trial, TRITON2 enrolled men with alterations in a host of DNA repair genes, the largest group of which was those with BRCA2 mutations. All the men in the trial were treated with rucaparib.
According to data presented at the ESMO meeting late last year—and similar to what was seen in PROFOUND—men with BRCA2 alterations were most likely to respond to the PARP inhibitor. Of the 62 men with BRCA2 alterations, nearly 45% had a tumor response. And, in more than half of these men, the response lasted for at least 6 months.
Overall, Dr. Karzai said, it does appear that BRCA2 alterations “really do drive the benefit” of PARP inhibitors among men with metastatic prostate cancer. “I think we’re really seeing that in these trials.”
Making Treatment Choices: Olaparib or Rucaparib?
Often, when multiple drugs are approved for the same—or in this case, a very similar—use, the side effects associated with each drug can help doctors decide which therapy is best for each patient.
Overall, Dr. Sartor explained, there aren’t notable differences in the types or severity of the side effects caused by olaparib and rucaparib.
And although most patients seem to handle the side effects caused by both drugs relatively well, he continued, they can cause substantial problems, including anemia, severe drops in white blood cell count, nausea, and vomiting.
Dr. Karzai also pointed to the risk of myelodysplastic syndrome, a disorder that affects the formation of blood cells in the bone marrow and that has been seen in a very small percentage of patients treated with PARP inhibitors.
“These drugs definitely require close monitoring [of patients],” Dr. Sartor said.
One potential advantage of rucaparib over olaparib could be the eventual availability of a blood test, called a liquid biopsy, that can identify men with BRCA1 or BRCA2 alterations (as well as other genetic alterations) who are candidates for the drug. This liquid biopsy, called FoundationOne Liquid CDx, is currently being evaluated by FDA and a decision on its approval is expected soon, according to a spokesperson for Foundation Medicine, which manufactures the test.
One reason that’s important, Dr. Karzai explained, also comes back to the fact that prostate cancer so often spreads to the bones. Because bone metastases are often hard and dense, she said, biopsies of these sites are “notorious for not having enough tissue to do standard genetic sequencing.”
In addition to tissue quantity, there are also issues with the type of tissue that comes from the biopsy and its quality. “There a lot of variables that can make it difficult,” she added.
The PROFOUND trial provides a case in point: In nearly one-third of tissue samples collected from more than 4,000 men screened as possible participants for the trial, the genetic test used wasn’t able to determine whether the specific genetic alterations were present in 31% of patients, according to data presented at the 2019 ESMO meeting.
Even with a liquid biopsy, ensuring the routine testing of patients for the presence of alterations in DNA repair genes will likely be the biggest barrier to oncologists’ adoption of these drugs in everyday patient care, Dr. Sartor believes.
“I think that’s where there will be a real learning curve among practicing physicians,” he said.