Researchers Create Modified Antibodies to Target RAS and p53 in Cancer
, by Nadia Jaber
Researchers have come up with a clever way to target two of the most notorious proteins involved in cancer, RAS and p53. In a series of studies, the scientists designed synthetic antibodies that, in mice, shrank tumors harboring these mutant proteins.
The team, led by researchers from the Johns Hopkins University School of Medicine, also used a similar approach to design antibodies that target a protein on blood cancer cells.
The newly designed antibodies act like a bridge, grabbing onto immune cells with one end and cancer cells with the other. By pulling them close together, the antibodies help immune cells find and kill the cancer cells. Three studies describing the approach were published on March 1 in different journals.
Mutations in the TP53 and RAS genes that promote tumor growth are quite common in cancer, being found in around 50% and 30% of all tumors, respectively. But the proteins made by these mutated genes have been hard to hit with the usual approaches to creating cancer drugs, so researchers have been exploring less conventional avenues. Although scientists have recently made headway against one mutant form of KRAS, they haven’t had any luck targeting mutant p53.
RAS and p53 “are high-value targets,” said study leader Shibin Zhou, Ph.D., of Hopkins. “People have been going after these targets for many years.”
It’s especially challenging to target mutant forms of p53 because they are generally inactive proteins, and most cancer drugs work by shutting off overactive proteins. Genes like TP53 that control cell growth and become inactive when mutated are called tumor suppressor genes.
“It’s a big deal that we were able to target a tumor suppressor because it’s so difficult to restore the activity of an inactive protein,” said study investigator Sandra Gabelli, Ph.D., of Hopkins.
Rather than blocking or restoring the activity of the mutant proteins, the newly designed antibodies use pieces of the mutant proteins as bullseyes, making it easier for immune cells to find and attack cancer cells.
“This approach could be applied to other common [cancer-related] mutations that are difficult to target by conventional means, allowing for more specific anticancer therapeutics,” the study authors wrote.
Searching Out Sparse Neoantigens
Lab-made antibodies are the basis of many different cancer treatments. They can distinguish between proteins that are remarkably similar and they’re easier and less expensive to make than cell-based immunotherapies.
However, a downside is that antibodies are typically too large to get inside cells, and most proteins—including many that drive cancer growth—are located inside the cell. But there is a loophole: Both healthy and cancer cells display snippets of inside proteins on their surfaces, cradled by a sort of receptacle known as HLA.
Cancer cell surfaces also display fragments of mutated proteins, known as neoantigens. Because these same protein snippets aren’t found on healthy cells, drugs targeting neoantigens may be less likely to harm healthy cells.
But it’s challenging to target neoantigens with antibodies because they tend to be scarce: There are less than 10 neoantigens from a mutant protein on the surface of a cancer cell, the Hopkins team found. Most antibody-based therapies require hundreds or thousands of targets to find and kill a cancer cell, they noted.
Until now, that was a major limitation to developing neoantigen-directed antibody treatments for cancer, Dr. Gabelli said. The antibodies the team designed appear to be able to search out and link to these sparse targets.
These studies are “proofs of concept that … we can target a low-density antigen on the surface of cancer cells and not kill healthy cells,” said Suman Paul, M.B.B.S., Ph.D., a medical oncology fellow at Hopkins.
Going After p53 and RAS
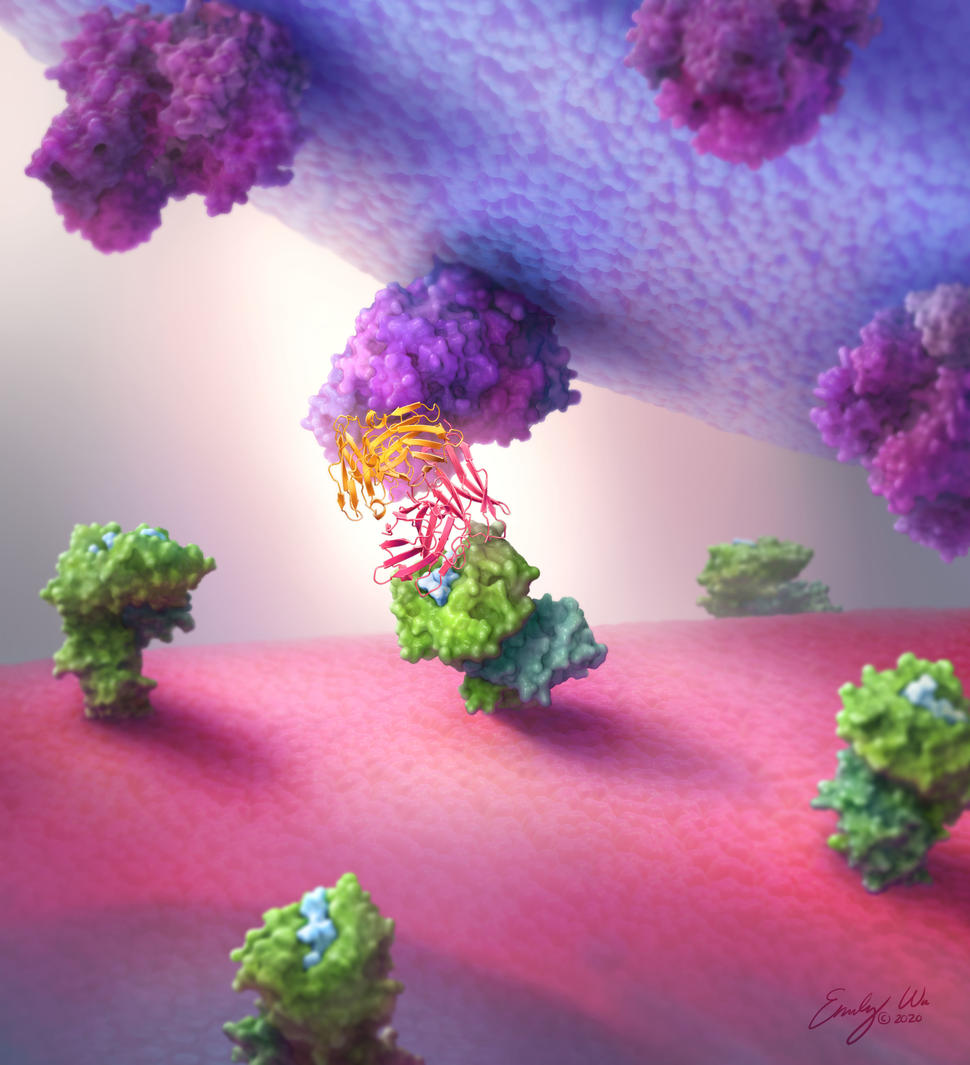
Antibodies made by the human body are Y-shaped proteins with two “arms” that bind to the same target. But researchers have gotten creative, engineering "bispecific" antibodies that bind to a different target with each arm.
Bispecific antibodies come in different configurations. The Hopkins team tested six different types and found that the “single-chain diabody” format consistently worked best. Single-chain diabodies look like the tips of two different antibodies fused together end-to-end, explained Dr. Gabelli, a structural biologist.
It “is a smaller, more compact, and more globular protein than [a regular] antibody,” she added.
The team created single-chain diabodies that grab onto protein fragments of the most common TP53 and RAS mutations with one end and immune cells with the other. In lab experiments, the p53- and RAS-specific diabodies attached to their respective neoantigen targets but not to fragments from normal p53 and RAS proteins or from other, closely related proteins.
Drugs that bind to something other than the intended target can cause serious side effects, the researchers noted.
In lab dishes, the single-chain diabodies directed immune cells to kill several different kinds of cancer cells harboring either a TP53 or RAS mutation, including ovarian cancer, pancreatic cancer, and leukemia.
In mice, treatment with the p53-specific diabody shrank TP53-mutant tumors compared with a “blank” diabody that doesn’t bind to p53 fragments. And in mice with RAS-mutant tumors, treatment with RAS-specific diabodies modestly slowed tumor growth.
A Diabody for Blood Cancers
Some blood cancers start when immune cells called B cells or T cells turn cancerous.
Antibody-based therapies have drastically changed how B-cell cancers are treated, Dr. Paul said. For example, the bispecific antibody blinatumomab (Blincyto) is an effective treatment for B-cell acute lymphoblastic leukemia. Immunotherapies like blinatumomab, which bind to proteins expressed by all B cells, can kill both cancerous and healthy B cells. Fortunately, patients can withstand losing healthy B cells.
On the other hand, drugs that hit both healthy and cancerous T cells would severely damage the person’s immune system, Dr. Paul explained. So, there are few antibody therapies for T-cell cancers (such as T-cell acute lymphoblastic leukemia or T-cell lymphoma) because it’s been challenging to find molecules that are present on cancerous T cells but not healthy T cells.
With that in mind, the Hopkins team zeroed in on the T-cell receptor (TCR). Each T cell, normal or cancerous, expresses 1 of 30 TCR variable region gene families. These genes are more or less randomly expressed by healthy T cells. But for an individual with T-cell cancer, all of their cancerous T cells express the same TCR variable gene, meaning they all have an identical TCR.
So, as a proof of concept, the researchers created single-chain diabodies that target two TCR variable genes. Testing in the lab showed that the diabodies prompted healthy T cells to specifically kill cancerous T cells with the matching TCR variable gene.
In mice implanted with leukemic T cells and healthy human T cells, treatment with either of the two diabodies drastically lowered the amount of cancer in the mice. In addition, mice treated with the leukemia-specific diabody lived longer than mice treated with a diabody targeting an unrelated cancer. Importantly, the treatment spared enough healthy T cells to preserve a functioning immune system.
Potential as Cancer Treatments
The newly designed diabodies are not yet ready to be tested in human studies. But the research team is hopeful about their potential.
In a commentary on the three studies, Jon Weidanz, Ph.D., of the University of Texas at Arlington, noted several considerations for using these diabodies as cancer treatments.
One is that a diabody-based treatment could be an off-the-shelf immunotherapy, which would be a major advantage. Such treatments could be manufactured in bulk and stored as a readily available treatment, whereas CAR T-cell therapy must be tailor-made for each patient.
But the diabodies would still have to be tailored slightly, the research team noted, because patients would need to have the matching mutation and HLA type, or matching TCR variable gene, for a particular diabody. Different people can have different forms of HLA, although some forms are more common than others.
The Hopkins team hopes to create diabodies for the ten most common mutated cancer proteins and HLAs. “That would apply to about 1 million patients per year, worldwide.” Dr. Gabelli noted.
How such diabodies would be given to patients is another consideration, Dr. Weidanz wrote. Bispecific antibodies are small, so they are filtered out of the blood within minutes to hours, Dr. Paul explained. To treat mice, the researchers used a small pump that continuously releases the diabodies.
Blinatumomab, the bispecific antibody treatment for B-cell leukemia, is delivered to patients the same way. And it’s “showing remarkable success in the clinic. That makes us hopeful that our molecules will translate well into the clinic as well,” Dr. Paul said.
Patients who get biomarker testing often find out that they have a TP53 mutation in their cancer and ask about possible drugs to target it, Dr. Paul said. “With these techniques, we may be able to intervene and offer a useful treatment instead of just staring at the [testing] report,” he said.
TP53 was identified as a tumor suppressor gene in 1989 by Bert Vogelstein, M.D., a senior author of the studies, and others. Having a way to finally target the protein “is a lifelong dream for him,” Dr. Zhou said.
“This was a true interdisciplinary effort” between experts in cancer genetics, immunology, structural biology, and medical oncology, Dr. Zhou added.
“When we all come together, good things happen,” he said.